Abstract
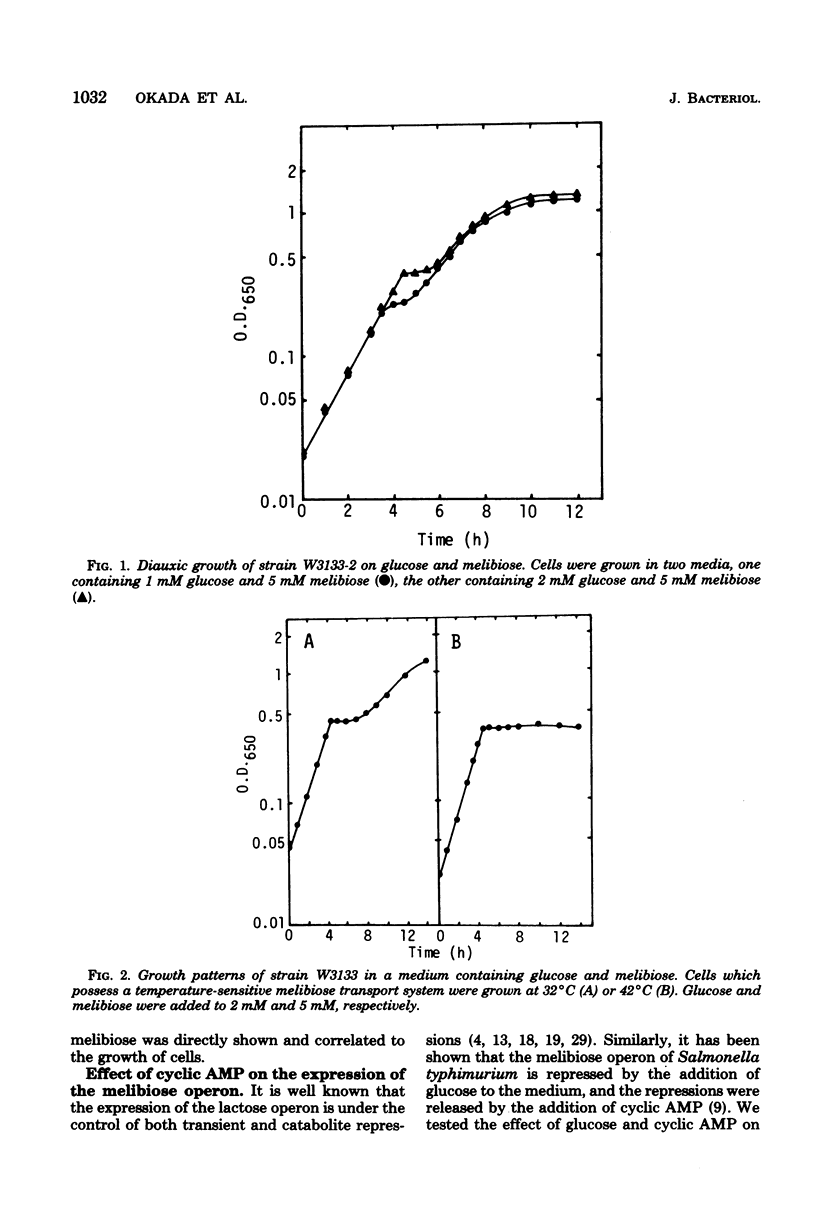
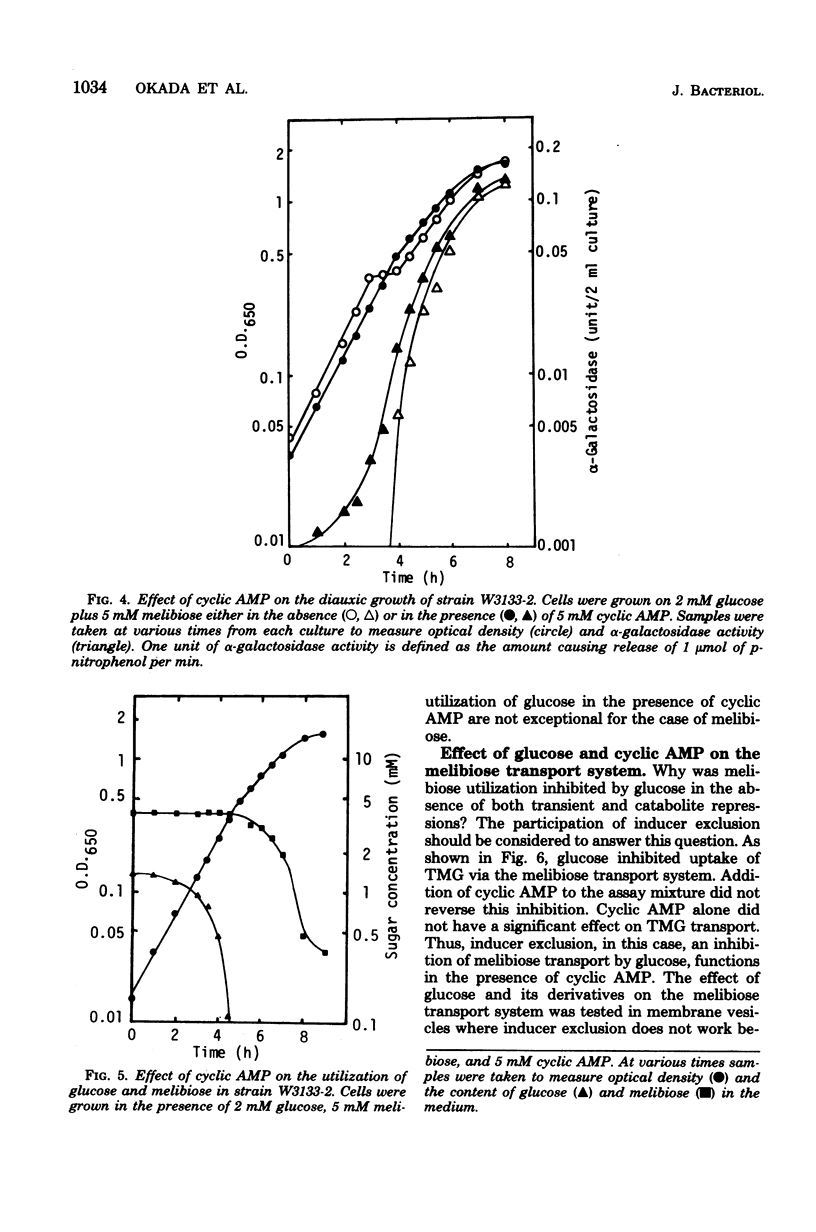
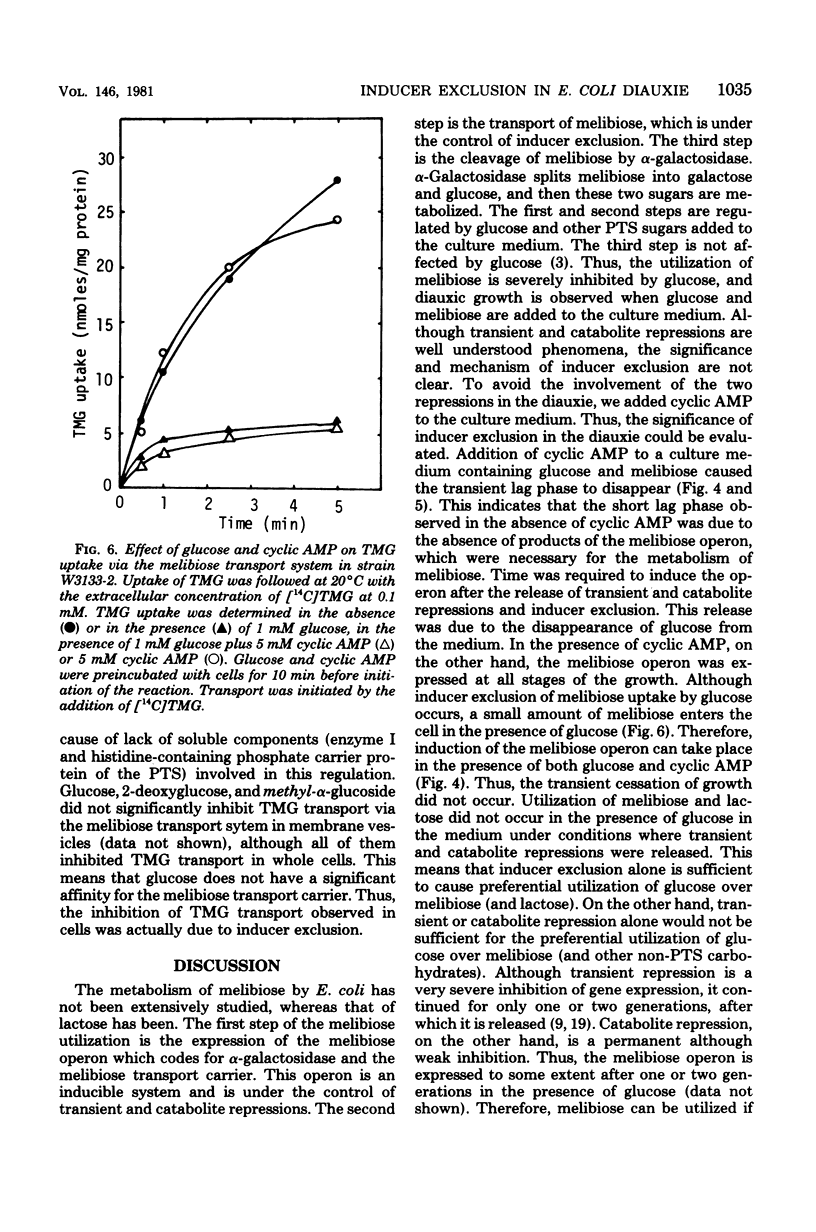
The role of inducer exclusion in diauxic growth of Escherichia coli on glucose and melibiose was investigated. The amounts of glucose and melibiose in the culture medium were determined during the diauxie. Glucose was consumed during the first growth cycle of the diauxie, and melibiose was consumed during the second cycle. The addition of adenosine 3',5'-cyclic monophosphate to the culture medium released both transient and catabolite repressions on the melibiose operon by glucose. Biphasic growth without a transient lag phase was observed in the presence of adenosine 3',5'-cyclic monophosphate. Preferential utilization of glucose over melibiose was observed even under such conditions. Thus, it is clear that inducer exclusion alone is sufficient to ensure the preferential utilization of glucose over melibiose. Similar results were obtained from a glucose-lactose diauxie. Inducer exclusion itself was not affected by adenosine 3',5'-cyclic monophosphate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASENSIO C., AVIGAD G., HORECKER B. L. PREFERENTIAL GALACTOSE UTILIZATION IN A MUTANT STRAIN OF E. COLI. Arch Biochem Biophys. 1963 Dec;103:299–309. doi: 10.1016/0003-9861(63)90419-3. [DOI] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Burstein C., Kepes A. The alpha-galactosidase from Escherichia coli K12. Biochim Biophys Acta. 1971 Jan 26;230(1):52–63. doi: 10.1016/0304-4165(71)90053-5. [DOI] [PubMed] [Google Scholar]
- Chambers D. A., Zubay G. The stimulatory effect of cyclic adenosine 3'5'-monophosphate on DNA-directed synthesis of beta-galactosidase in a cell-free system. Proc Natl Acad Sci U S A. 1969 May;63(1):118–122. doi: 10.1073/pnas.63.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaro C. Genetics of the bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Genet. 1976;10:341–359. doi: 10.1146/annurev.ge.10.120176.002013. [DOI] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- HAYASHI S., LIN E. C. CAPTURE OF GLYCEROL BY CELLS OF ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Mar 29;94:479–487. doi: 10.1016/0926-6585(65)90056-7. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Anraku Y. Transient repression of beta-galactosidase synthesis by glucose-6-phosphate in a mutant of Escherichia coli lacking enzyme II specific for glucose in the phosphoenolpyruvate-sugar phosphotransferase system. J Biochem. 1978 May;83(5):1337–1343. doi: 10.1093/oxfordjournals.jbchem.a132041. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levinthal M. Biochemical studies of melibiose metabolism in wild type and mel mutant strains of Salmonella typhimurium. J Bacteriol. 1971 Mar;105(3):1047–1052. doi: 10.1128/jb.105.3.1047-1052.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopilato J., Tsuchiya T., Wilson T. H. Role of Na+ and Li+ in thiomethylgalactoside transport by the melibiose transport system of Escherichia coli. J Bacteriol. 1978 Apr;134(1):147–156. doi: 10.1128/jb.134.1.147-156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Niiya S., Moriyama Y., Futai M., Tsuchiya T. Cation coupling to melibiose transport in Salmonella typhimurium. J Bacteriol. 1980 Oct;144(1):192–199. doi: 10.1128/jb.144.1.192-199.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Regulation of beta-galactosidase synthesis in Escherichia coli by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1968 Oct 25;243(20):5420–5427. [PubMed] [Google Scholar]
- Perlman R., Pastan I. Cyclic 3'5-AMP: stimulation of beta-galactosidase and tryptophanase induction in E. coli. Biochem Biophys Res Commun. 1968 Mar 27;30(6):656–664. doi: 10.1016/0006-291x(68)90563-9. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Interaction of enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system with adenylate cyclase of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2920–2924. doi: 10.1073/pnas.72.8.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROE J. H. The determination of dextran in blood and urine with anthrone reagent. J Biol Chem. 1954 Jun;208(2):889–896. [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Hofstadter L. J. Regulation of carbohydrate uptake and adenylate cyclase activity mediated by the enzymes II of the phosphoenolpyruvate: sugar phosphotransferase system in Escherichia coli. J Biol Chem. 1976 Feb 10;251(3):883–892. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., McCaman M. T. Regulation of intracellular adenosine cyclic 3':5'-monophosphate levels in Escherichia coli and Salmonella typhimurium. Evidence for energy-dependent excretion of the cyclic nucleotide. J Biol Chem. 1975 Oct 10;250(19):7593–7601. [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Lopilato J., Wilson T. H. Effect of lithium ion on melibiose transport in Escherichia coli. J Membr Biol. 1978 Jul 21;42(1):45–59. doi: 10.1007/BF01870393. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Wilson T. H. Cation-sugar cotransport in the melibiose transport system of Escherichia coli. Membr Biochem. 1978;2(1):63–79. doi: 10.3109/09687687809063858. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Monod J. Cyclic AMP as an antagonist of catabolite repression in Escherichia coli. FEBS Lett. 1968 Nov;2(1):57–60. doi: 10.1016/0014-5793(68)80100-0. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. Inhibition of beta-galactoside transport by substrates of the glucose transport system in Escherichia coli. Biochim Biophys Acta. 1967;135(5):1030–1051. doi: 10.1016/0005-2736(67)90073-9. [DOI] [PubMed] [Google Scholar]


