Abstract
The alternate cofactor (7004 cofactor) for Escherichia coli adenosine 3'-phosphate 5'-phosphosulfate (PAPS) reductase originally discovered in an E. coli mutant (tsnC 7004) lacking thioredoxin activity has now been purified and characterized. The tryptic peptide map of the 7004 cofactor is totally different from that of thioredoxin, indicating that the two proteins are unrelated in their primary structure. The 7004 cofactor has an amino acid composition different from that of thioredoxin but similar to that of glutaredoxin, a protein required for the glutathione-dependent deoxyribonucleotide formation by ribonucleotide reductase. Thus, the 7004 cofactor could not be a mutated form of thioredoxin, as was suspected earlier. Thioredoxin but not glutaredoxin is a substrate for thioredoxin reductase, but both thioredoxin and glutaredoxin can catalyze the dithiothreitol- or glutathione-dependent reduction of PAPS. On a molar basis, the dithiothreitol-coupled cofactor activity of thioredoxin is three- to fourfold higher that that of glutaredoxin. Comparison of the cofactor activities in the glutathione-coupled and the dithiothreitol-coupled PAPS reductase reaction shows that the cofactor activity of thioredoxin in the glutathione-coupled reaction is only 23% of that observed in the dithiothreitol-coupled reaction. However, in the case of glutaredoxin, cofactor activities are approximately the same in both the dithiothreitol- and glutathione-coupled reactions.
Full text
PDF
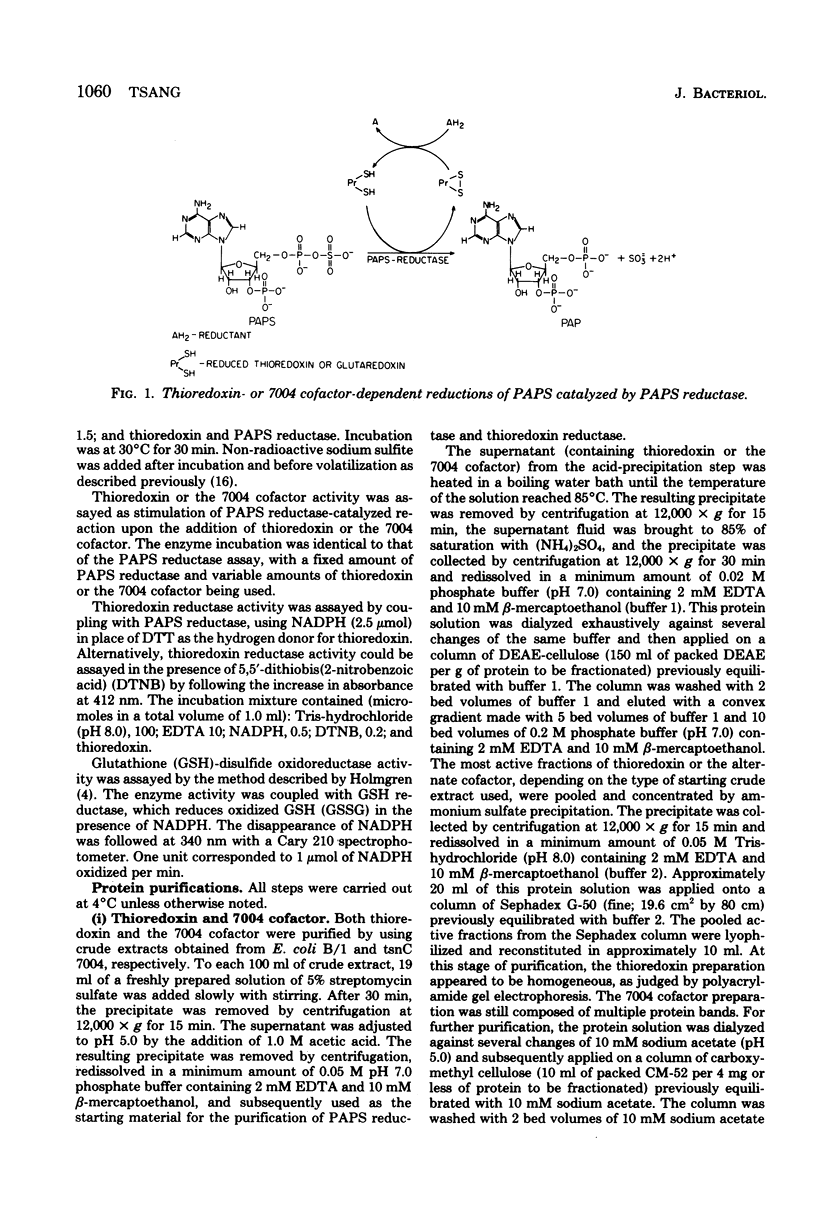




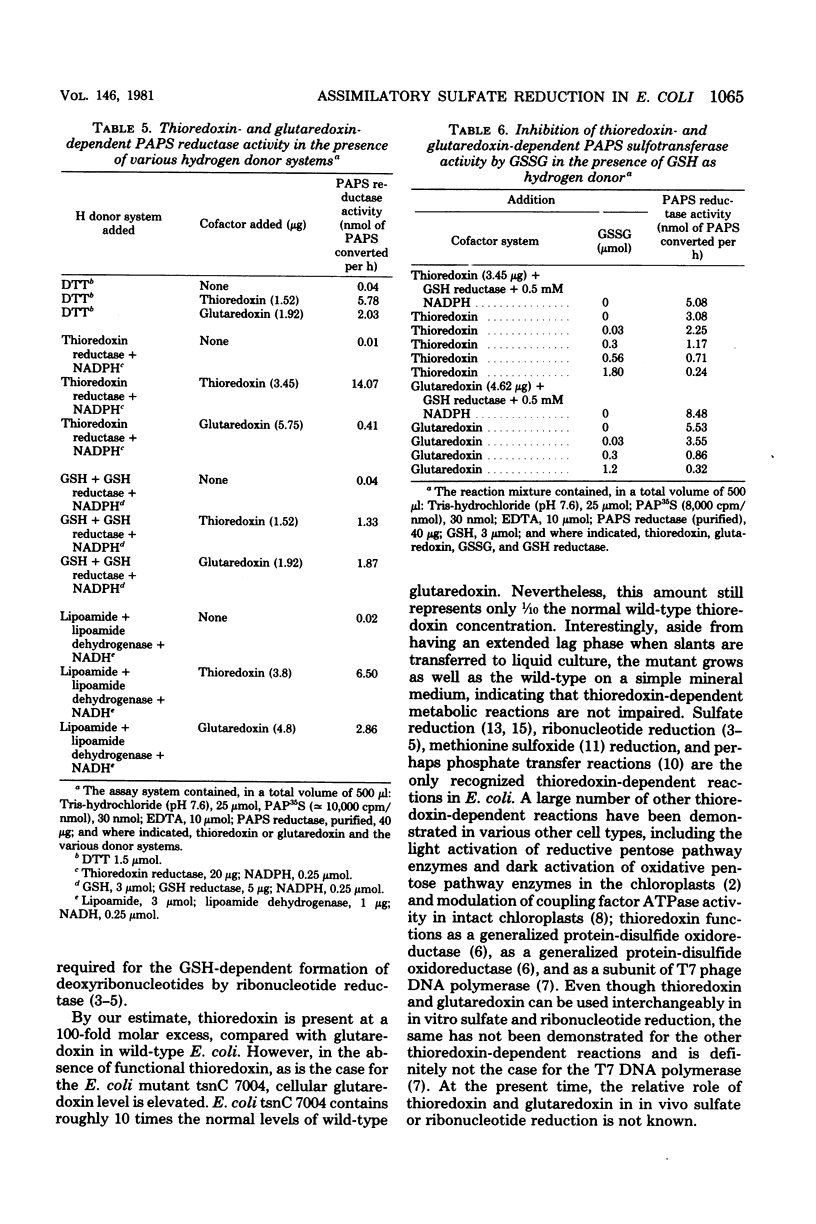

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez Porqué P., Baldesten A., Reichard P. The involvement of the thioredoxin system in the reduction of methionine sulfoxide and sulfate. J Biol Chem. 1970 May 10;245(9):2371–2374. [PubMed] [Google Scholar]
- Holmgren A. Glutathione-dependent synthesis of deoxyribonucleotides. Characterization of the enzymatic mechanism of Escherichia coli glutaredoxin. J Biol Chem. 1979 May 10;254(9):3672–3678. [PubMed] [Google Scholar]
- Holmgren A. Glutathione-dependent synthesis of deoxyribonucleotides. Purification and characterization of glutaredoxin from Escherichia coli. J Biol Chem. 1979 May 10;254(9):3664–3671. [PubMed] [Google Scholar]
- Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2275–2279. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Reduction of disulfides by thioredoxin. Exceptional reactivity of insulin and suggested functions of thioredoxin in mechanism of hormone action. J Biol Chem. 1979 Sep 25;254(18):9113–9119. [PubMed] [Google Scholar]
- Lik-Shing Tsang M., Schiff J. A. Properties of enzyme fraction A from Chlorella and copurification of 3' (2'), 5'-biphosphonucleoside 3' (2')-phosphohydrolase, adenosine 5'phosphosulfate sulfohydrolase and adenosine-5'-phosphosulfate cyclase activities. Eur J Biochem. 1976 May 17;65(1):113–121. doi: 10.1111/j.1432-1033.1976.tb10395.x. [DOI] [PubMed] [Google Scholar]
- Mark D. F., Richardson C. C. Escherichia coli thioredoxin: a subunit of bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1976 Mar;73(3):780–784. doi: 10.1073/pnas.73.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigiet V., Conley R. R. Isolation and characterization of phosphothioredoxin from Excherichia coli. J Biol Chem. 1978 Mar 25;253(6):1910–1920. [PubMed] [Google Scholar]
- Schiff J. A., Levinthal M. Studies of sulfate utilization by algae. 4. Properties of a cell-free sulfate-reducing system from chlorella. Plant Physiol. 1968 Apr;43(4):547–554. doi: 10.1104/pp.43.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Stephens R. E. Fluorescent thin-layer peptide mapping for protein identification and comparison in the subnanomole range. Anal Biochem. 1978 Jan;84(1):116–126. doi: 10.1016/0003-2697(78)90490-6. [DOI] [PubMed] [Google Scholar]
- Tsang M. L., Lemieux J., Schiff J. A., Bojarski T. B. Preparation of adenosine 5'-phosphosulfate (APS) from adenosine 3'-phosphate 5'-phosphosulfate (PAPS) prepared by an improved procedure. Anal Biochem. 1976 Aug;74(2):623–626. doi: 10.1016/0003-2697(76)90249-9. [DOI] [PubMed] [Google Scholar]
- Tsang M. L., Schiff J. A. Assimilatory sulfate reduction in an Escherichia coli mutant lacking thioredoxin activity. J Bacteriol. 1978 Apr;134(1):131–138. doi: 10.1128/jb.134.1.131-138.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang M. L., Schiff J. A. Sulfate-reducing pathway in Escherichia coli involving bound intermediates. J Bacteriol. 1976 Mar;125(3):923–933. doi: 10.1128/jb.125.3.923-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]




