Abstract
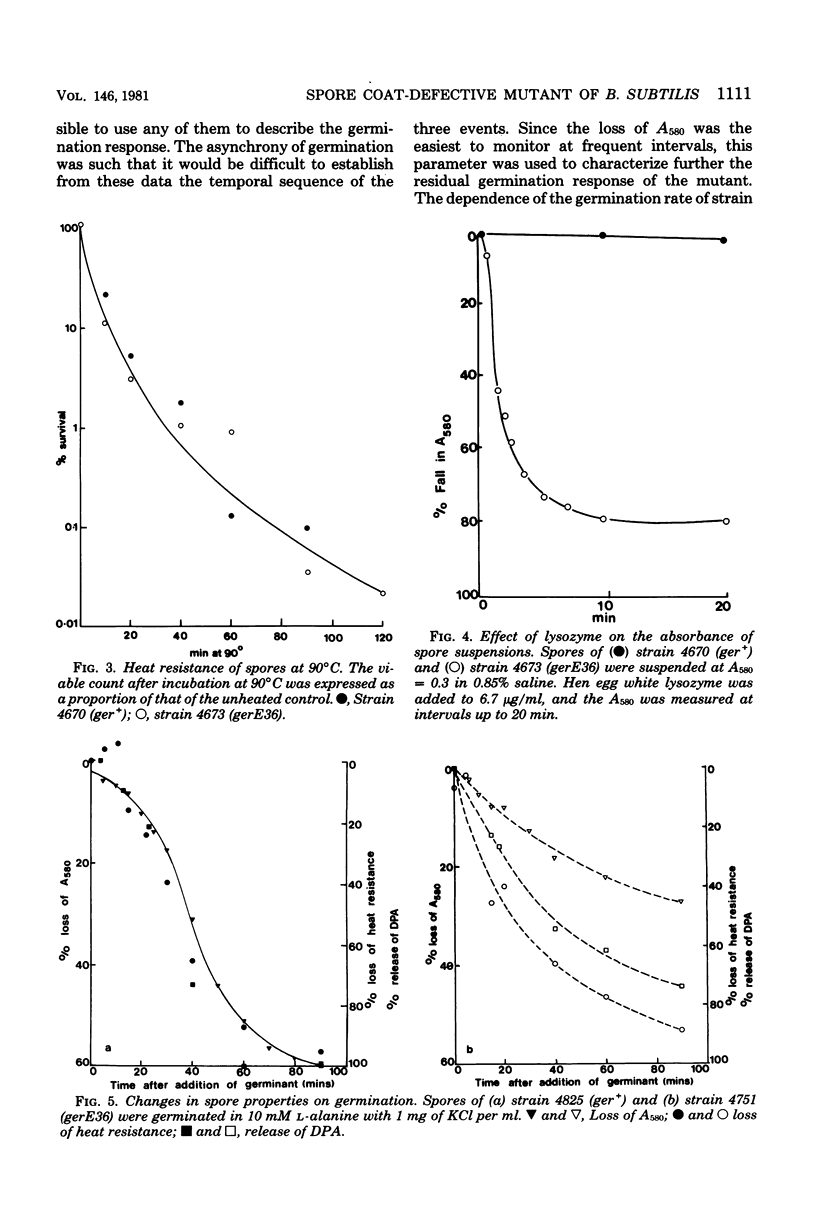
The presence of the gerE36 mutation in strains of Bacillus subtilis 168 resulted in poor germination of their spores in a range of germinants, as measured by the fall in absorbance of spore suspensions. Although resistant to heat and organic solvents, spores were sensitive to lysozyme; electron microscopy revealed that their coat structure was incomplete. These spores responded to germinants by losing heat resistance and changing from phase bright to phase gray. The release of dipicolinic acid and the fall in absorbance of spore suspensions reached only 75 and 50% of wild-type levels, respectively, but followed the same time course as the loss of heat resistance. Although the germination response was incomplete, the concentration of L-alanine required to elicit it was the same for the mutant as for the wild type. The properties of mutant spores suggest that an intact spore coat is not required for the initial interaction between germinant and spore, but that the coat layers may contain molecules important in later stages of germination. In transduction with phage SPP1, the gerE36 mutation mapped between citF and ilvB and was 90% cotransduced with citF2. The gerE mutation identifies the location of a gene important for the progress of late stages of spore formation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. C. Properties of Bacillus cereus spore coat mutants. J Bacteriol. 1975 Jul;123(1):354–365. doi: 10.1128/jb.123.1.354-365.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal N., Kleinschmidt A. K., Spatz H. C., Trautner T. A. Physical properties of the DNA of bacteriophage SP50. Mol Gen Genet. 1967;100(1):39–55. doi: 10.1007/BF00425774. [DOI] [PubMed] [Google Scholar]
- Cassier M., Ryter A. Sur un mutant de Clostridium perfringens donnant des spores sans tuniques à germination lysozyme-dépendante. Ann Inst Pasteur (Paris) 1971 Dec;121(6):717–732. [PubMed] [Google Scholar]
- Cheng Y. S., Aronson A. I. Alterations of spore coat processing and protein turnover in a Bacillus cereus mutant with a defective postexponential intracellular protease. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1254–1258. doi: 10.1073/pnas.74.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Fitz-James P., Aronson A. I. Characterization of a Bacillus cereus protease mutant defective in an early stage of spore germination. J Bacteriol. 1978 Jan;133(1):336–344. doi: 10.1128/jb.133.1.336-344.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari E., Canosi U., Galizzi A., Mazza G. Studies on transduction process by SPP1 phage. J Gen Virol. 1978 Dec;41(3):563–572. doi: 10.1099/0022-1317-41-3-563. [DOI] [PubMed] [Google Scholar]
- HILLS G. M. Chemical factors in the germination of spore-bearing aerobes: observations on the influence of species, strain and conditions of growth. J Gen Microbiol. 1950 Jan;4(1):38–47. doi: 10.1099/00221287-4-1-38. [DOI] [PubMed] [Google Scholar]
- Hageman J. H., Carlton B. C. Effects of mutational loss of specific intracellular proteases on the sporulation of Bacillus subtilis. J Bacteriol. 1973 May;114(2):612–617. doi: 10.1128/jb.114.2.612-617.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Frieben W. R., Conti S. F. Microgermination of Bacillus cereus spores. J Bacteriol. 1969 Dec;100(3):1385–1392. doi: 10.1128/jb.100.3.1385-1392.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- Kerjan P., Keryer E., Szulmajster J. Characterization of a thermosensitive sporulation mutant of Bacillus subtilis affected in the structural gene of an intracellular protease. Eur J Biochem. 1979 Aug 1;98(2):353–362. doi: 10.1111/j.1432-1033.1979.tb13194.x. [DOI] [PubMed] [Google Scholar]
- Levinson H. S., Hyatt M. T. Sequence of events during Bacillus megaterim spore germination. J Bacteriol. 1966 May;91(5):1811–1818. doi: 10.1128/jb.91.5.1811-1818.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A., Lafferty E., Smith D. A. Genetics analysis of spore germination mutants of Bacillus subtilis 168: the correlation of phenotype with map location. J Gen Microbiol. 1979 Mar;111(1):165–180. doi: 10.1099/00221287-111-1-165. [DOI] [PubMed] [Google Scholar]
- Ohné M., Rutberg B., Hoch J. A. Genetic and biochemical characterization of mutants of Bacillus subtilis defective in succinate dehydrogenase. J Bacteriol. 1973 Sep;115(3):738–745. doi: 10.1128/jb.115.3.738-745.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad C., Srinivasan V. R. Methyl anthranilate, an inhibitor for thegermination of spores of aerobic bacilli. Appl Microbiol. 1969 Jul;18(1):131–132. doi: 10.1128/am.18.1.131-132.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg B., Hederstedt L., Holmgren E., Rutberg L. Characterization of succinic dehydrogenase mutants of Bacillus subtilis by crossed immunoelectrophoresis. J Bacteriol. 1978 Oct;136(1):304–311. doi: 10.1128/jb.136.1.304-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott I. R., Ellar D. J. Study of calcium dipicolinate release during bacterial spore germination by using a new, sensitive assay for dipicolinate. J Bacteriol. 1978 Jul;135(1):133–137. doi: 10.1128/jb.135.1.133-137.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa J. C., Silva M. T., Balassa G. An exosporium-like outer layer in Bacillus subtilis spores. Nature. 1976 Sep 2;263(5572):53–54. doi: 10.1038/263053a0. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C. Germination of Bacillus megaterium spores after various extraction procedures. J Bacteriol. 1973 Nov;116(2):797–802. doi: 10.1128/jb.116.2.797-802.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXI. Temperature-sensitive mutants for initiation of germination. J Bacteriol. 1970 Jan;101(1):327–329. doi: 10.1128/jb.101.1.327-329.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARTH A. D., OHYE D. F., MURRELL W. G. Location and composition of spore mucopeptide in Bacillus species. J Cell Biol. 1963 Mar;16:593–609. doi: 10.1083/jcb.16.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B., Jr, Zahler S. A. Genetic studies of leucine biosynthesis in Bacillus subtilis. J Bacteriol. 1973 Nov;116(2):719–726. doi: 10.1128/jb.116.2.719-726.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Young F. E. Transduction in Bacillus subtilis by bacteriophage SPP1. J Virol. 1974 Dec;14(6):1343–1348. doi: 10.1128/jvi.14.6.1343-1348.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]




