Abstract
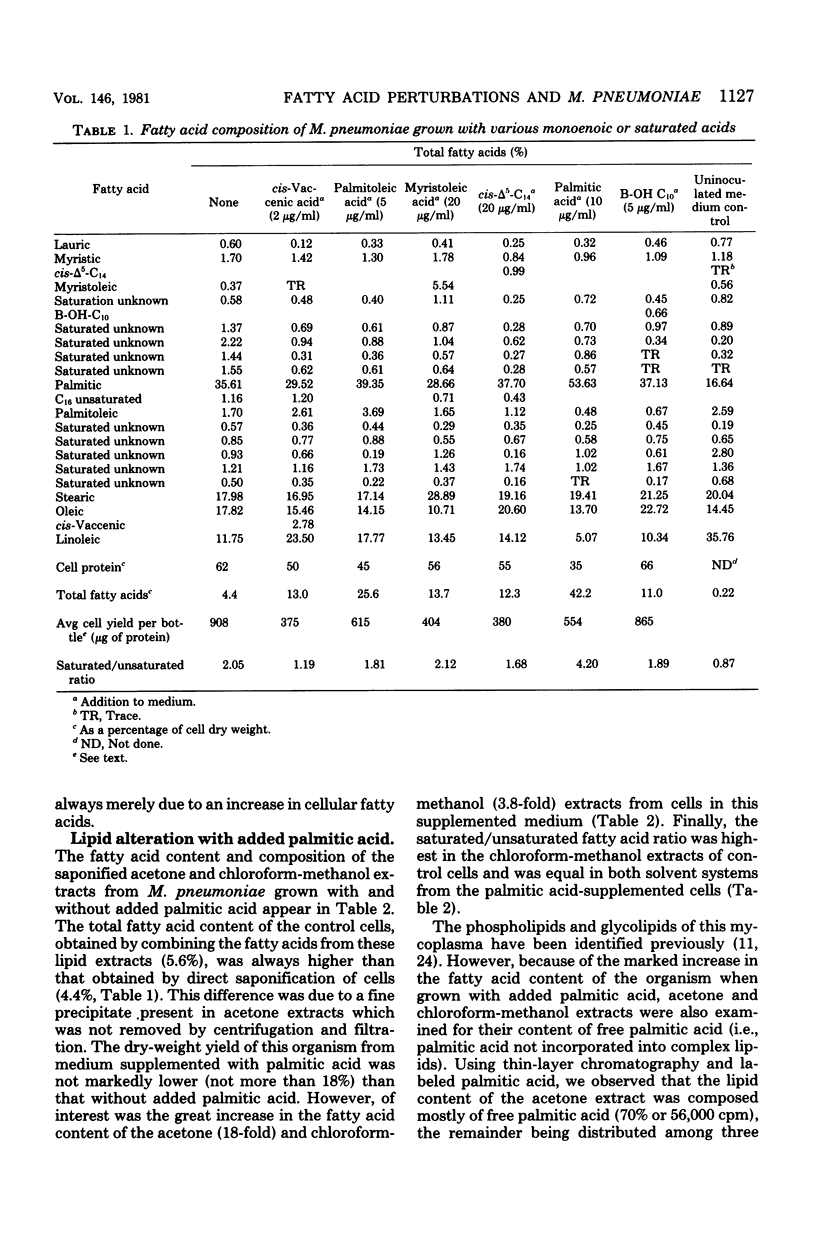
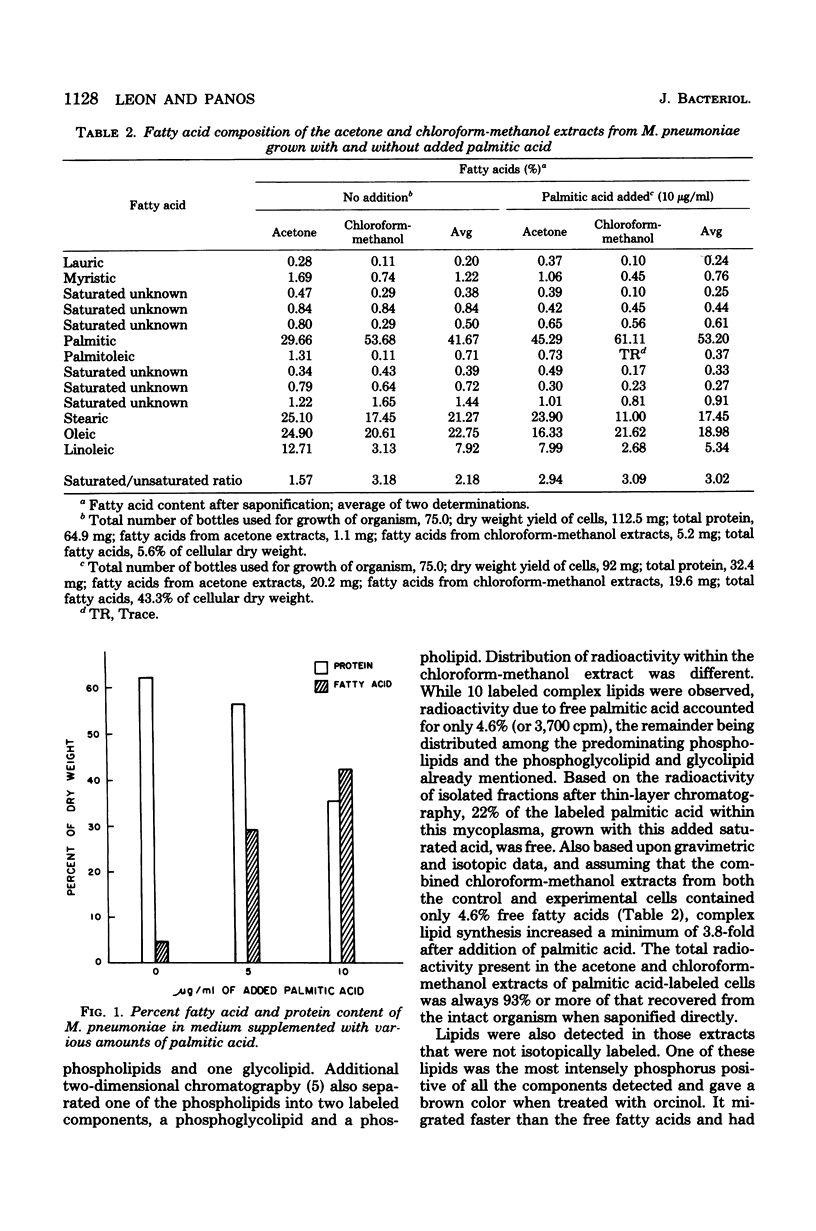
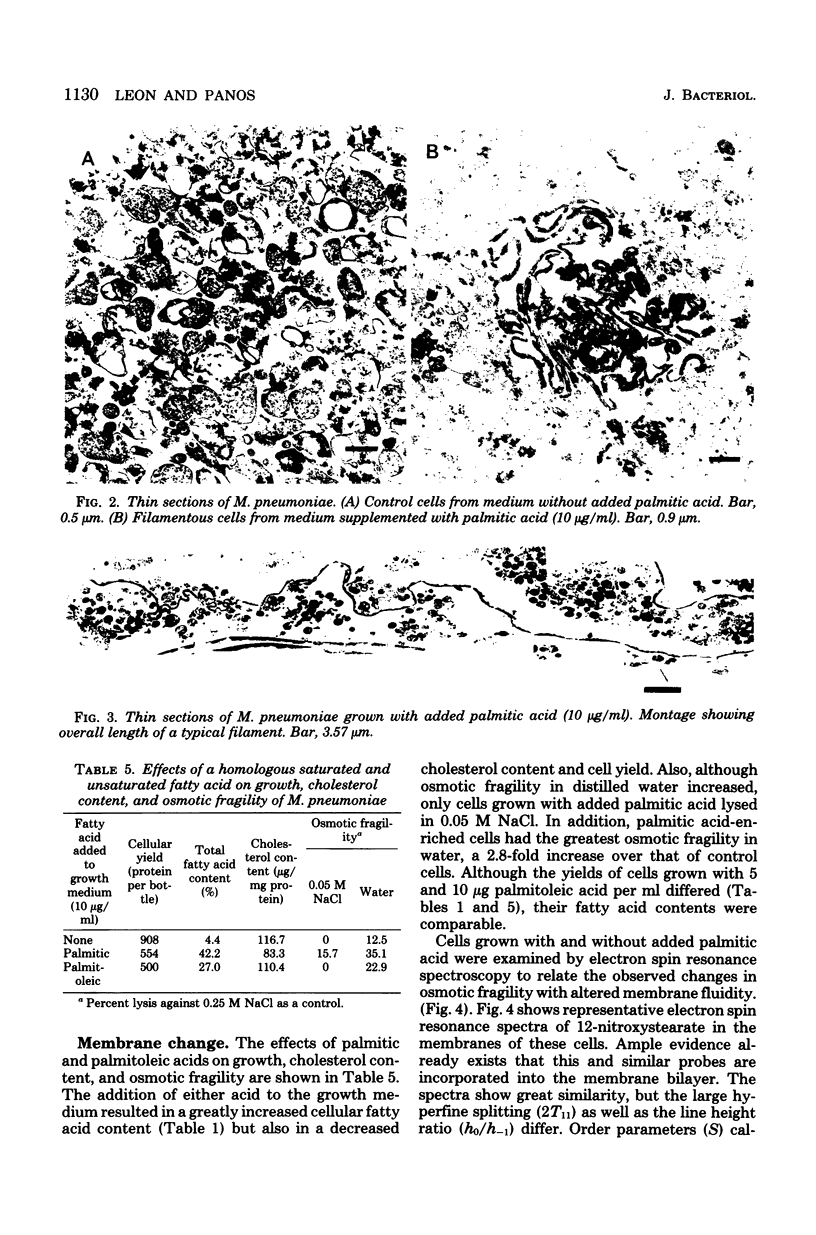
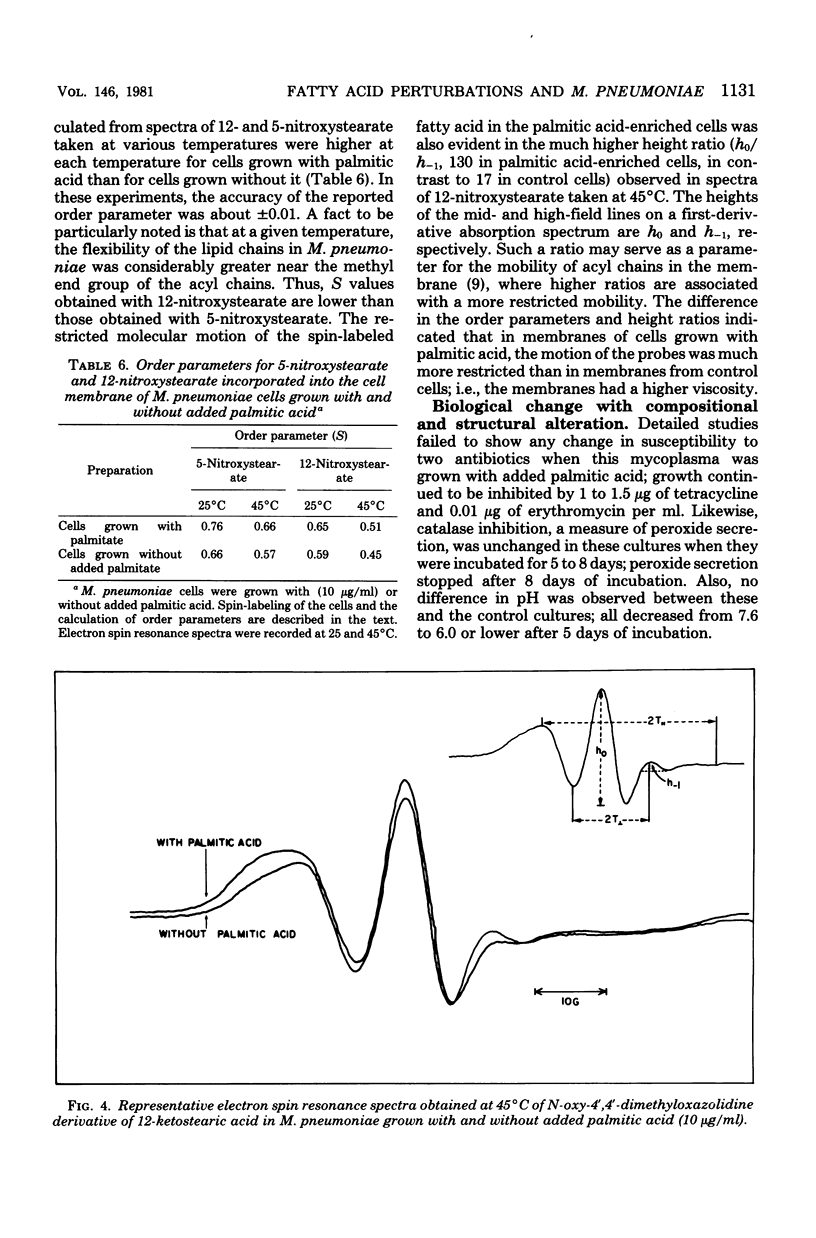
The fatty acid content of Mycoplasma pneumoniae increased 2.5- to 9.6-fold when the growth medium was supplemented with a saturated, unsaturated, or beta-hydroxy fatty acid, the greatest increase occurring with palmitic acid. The amount of each supplemented fatty acid found within this organism was 2.8 to 5.5% of the total fatty acid content; the exception was palmitic acid. Up to 57% of the palmitic acid was utilized from the supplemented medium, whereas only 0.2 to 10% of the other fatty acids was utilized. Chromatographic and isotopic analyses revealed that 22% of the labeled palmitic acid incorporated from the palmitic acid-supplemented medium remained free in this organism. Also, even though complex lipid synthesis increased a minimum of 3.8-fold under these conditions, this mycoplasma continued to incorporate intact complex lipids from the growth medium. Bacteriostatic and bactericidal studies which used high concentrations of various long-chain fatty acids showed that only palmitic, myristic, and beta-hydroxydecanoic acids were not bactericidal. The addition of palmitic acid to the growth medium resulted in the formation of exceedingly long, filamentous cells in approximately 25% of the population. Osmotic fragility and electron spin resonance spectroscopy studies showed a correlation among this increased fatty acid content, decreased membrane fluidity, and the increased osmotic fragility of palmitic acid-grown cells. In addition, these cells had a lowered cholesterol content. The effect of such compositional changes on osmotic fragility is discussed in this paper. Finally, the profound increase in the total fatty acid content of palmitic acid-grown cells altered neither sensitivity to tetracycline or erythromycin nor the amount of hydrogen peroxide secreted.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldassare J. J., Breneckle G. M., Hoffman M., Silbert D. F. Modification of membrane lipid. Functional properties of membrane in relation to fatty acid structure. J Biol Chem. 1977 Dec 25;252(24):8797–8803. [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Glucose-dependent secretion and destruction of hydrogen peroxide by Mycoplasma pneumoniae. J Bacteriol. 1969 May;98(2):547–551. doi: 10.1128/jb.98.2.547-551.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiVecchia L., Somerson N. L. Tetrazolium reduction as a measure of metabolic activity for glass-adherent Mycoplasma pneumoniae. Appl Microbiol. 1973 Sep;26(3):298–302. doi: 10.1128/am.26.3.298-302.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Ishizuka I., Landgraf H. R., Herrmann J. Glycerophosphoryl diglucosyl diglyceride, a new phosphoglycolipid from Streptococci. Biochim Biophys Acta. 1973 Mar 8;296(3):527–545. doi: 10.1016/0005-2760(73)90113-6. [DOI] [PubMed] [Google Scholar]
- Gaffney B. J. Fatty acid chain flexibility in the membranes of normal and transformed fibroblasts. Proc Natl Acad Sci U S A. 1975 Feb;72(2):664–668. doi: 10.1073/pnas.72.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morrisett J. D., Pownall H. J., Plumlee R. T., Smith L. C., Zehner Z. E. Multiple thermotropic phase transitions in Escherichia coli membranes and membrane lipids. A comparison of results obtained by nitroxyl stearate paramagnetic resonance, pyrene excimer fluorescence, and enzyme activity measurements. J Biol Chem. 1975 Sep 10;250(17):6969–6976. [PubMed] [Google Scholar]
- PARSONS J., WYCOFF H. D. Chromatographic microassay for cholesterol and cholesterol esters. Science. 1957 Feb 22;125(3243):347–348. doi: 10.1126/science.125.3243.347. [DOI] [PubMed] [Google Scholar]
- Panos C., Leon O. Replacement of the octadecenoic acid growth-requirement for Acholeplasma laidlawii A by cis-9,10-methylenehexadecanoic acid, a cyclopropane fatty acid. J Gen Microbiol. 1974 Jan;80(1):93–100. doi: 10.1099/00221287-80-1-93. [DOI] [PubMed] [Google Scholar]
- Pollack J. D., Somerson N. L., Senterfit L. B. Isolation, Characterization, and Immunogenicity of Mycoplasma pneumoniae Membranes. Infect Immun. 1970 Sep;2(3):326–339. doi: 10.1128/iai.2.3.326-339.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raccach M., Rottem S., Razin S. Survival of frozen mycoplasmas. Appl Microbiol. 1975 Aug;30(2):167–171. doi: 10.1128/am.30.2.167-171.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Cosenza B. J., Tourtellotte M. E. Filamentous growth of mycoplasma. Ann N Y Acad Sci. 1967 Jul 28;143(1):66–72. doi: 10.1111/j.1749-6632.1967.tb27645.x. [DOI] [PubMed] [Google Scholar]
- Razin S. Physiology of mycoplasmas. Adv Microb Physiol. 1973;10:1–80. doi: 10.1016/s0065-2911(08)60086-7. [DOI] [PubMed] [Google Scholar]
- Romano N., Smith P. F., Mayberry W. R. Lipids of a T strain of Mycoplasma. J Bacteriol. 1972 Feb;109(2):565–569. doi: 10.1128/jb.109.2.565-569.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Hubbell W. L., Hayflick L., McConnell H. M. Motion of fatty acid spin labels in the plasma membrane of mycoplasma. Biochim Biophys Acta. 1970;219(1):104–113. doi: 10.1016/0005-2736(70)90065-9. [DOI] [PubMed] [Google Scholar]
- Rottem S., Muhsam-Peled O., Razin S. Acyl carrier protein in mycoplasmas. J Bacteriol. 1973 Feb;113(2):586–591. doi: 10.1128/jb.113.2.586-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Panos C. The effect of long chain fatty acid isomers on growth, fatty acid composition and osmotic fragility of Mycoplasma laidlawii A. J Gen Microbiol. 1969 Dec;59(3):317–328. doi: 10.1099/00221287-59-3-317. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Membrane lipids of Mycoplasma hominis. J Bacteriol. 1973 Feb;113(2):565–571. doi: 10.1128/jb.113.2.565-571.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaskovsky V. E., Kostetsky E. Y. Modified spray for the detection of phospholipids on thin-layer chromatograms. J Lipid Res. 1968 May;9(3):396–396. [PubMed] [Google Scholar]




