Abstract
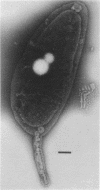
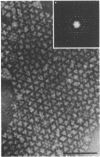
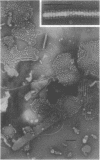
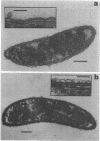
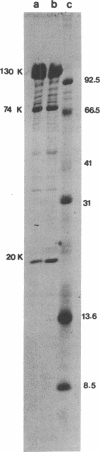
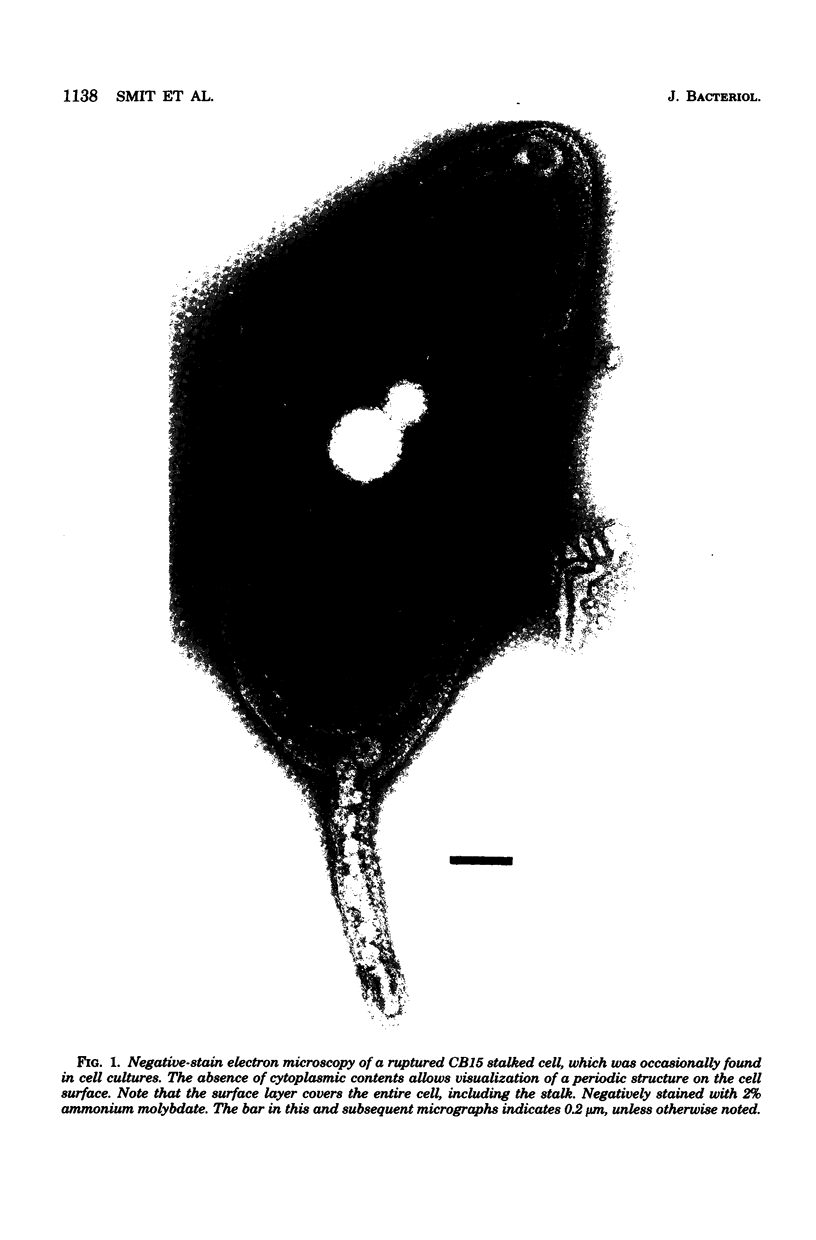
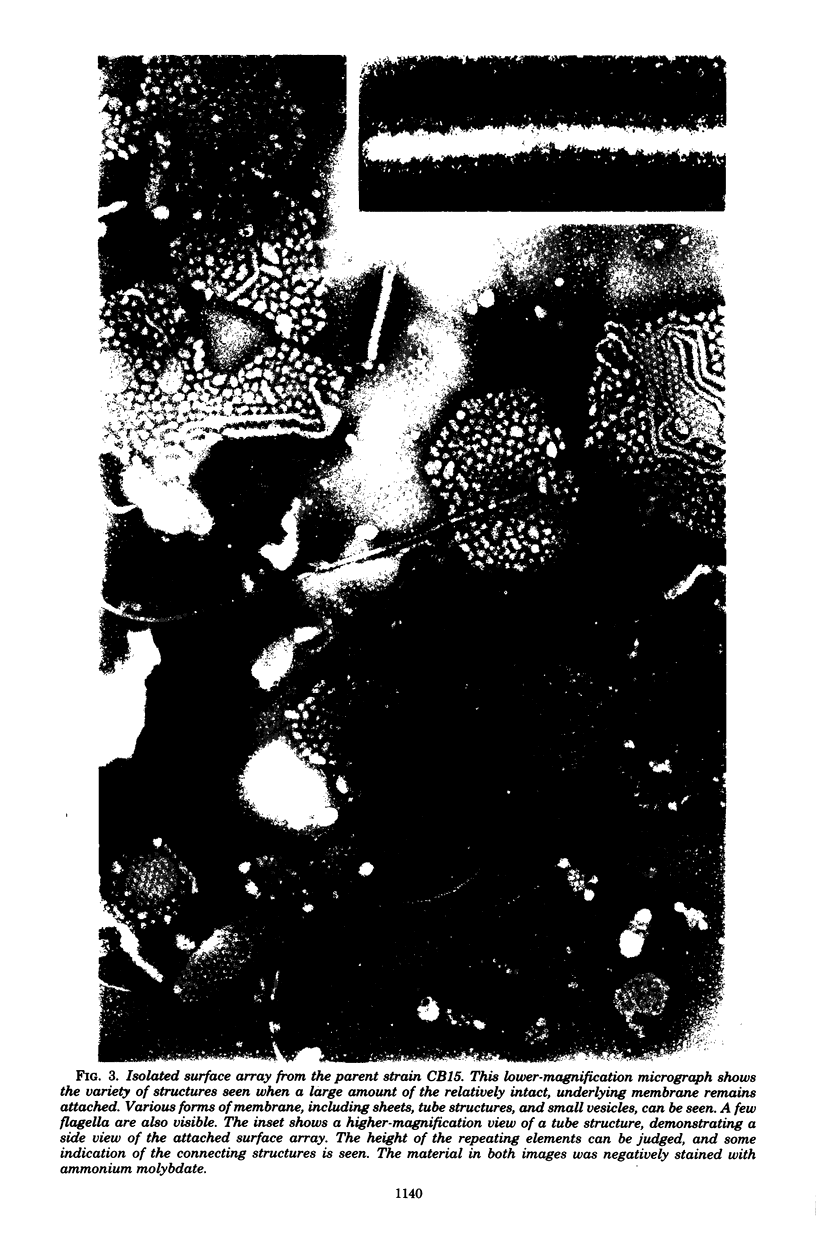
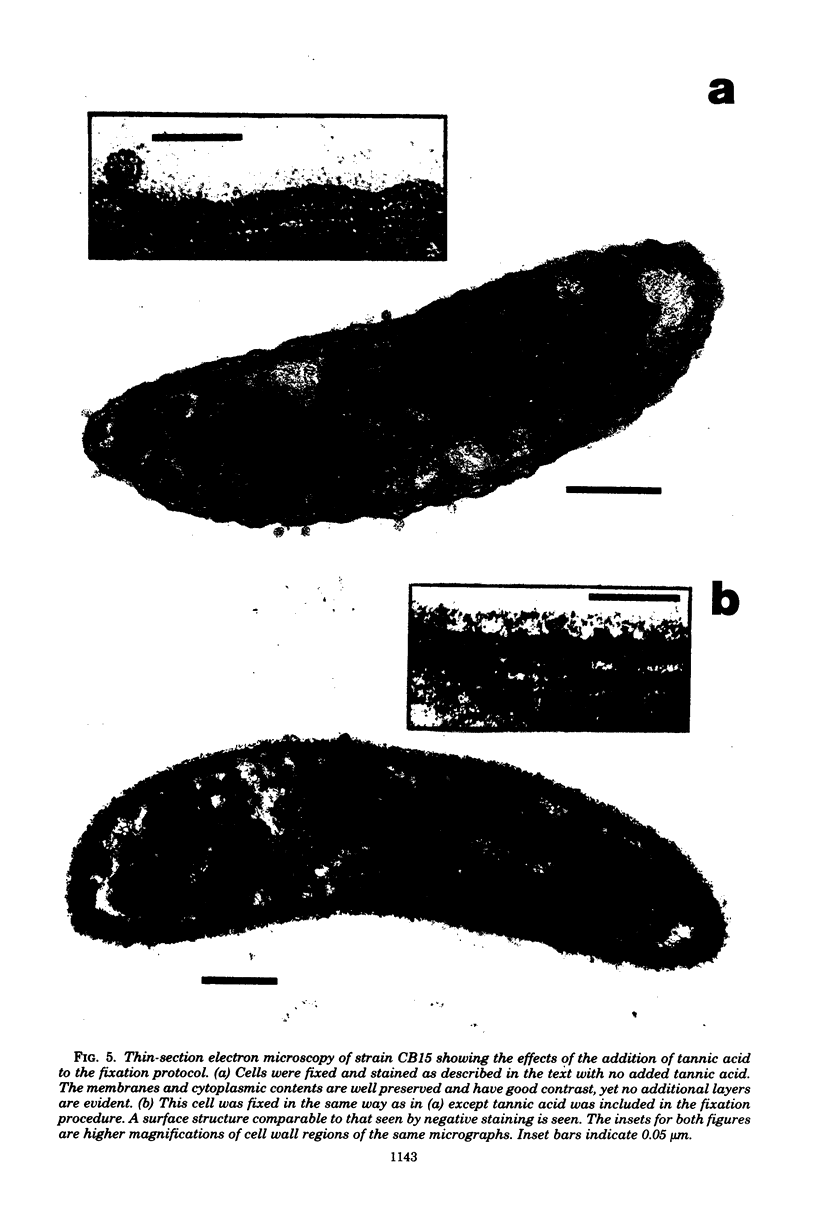
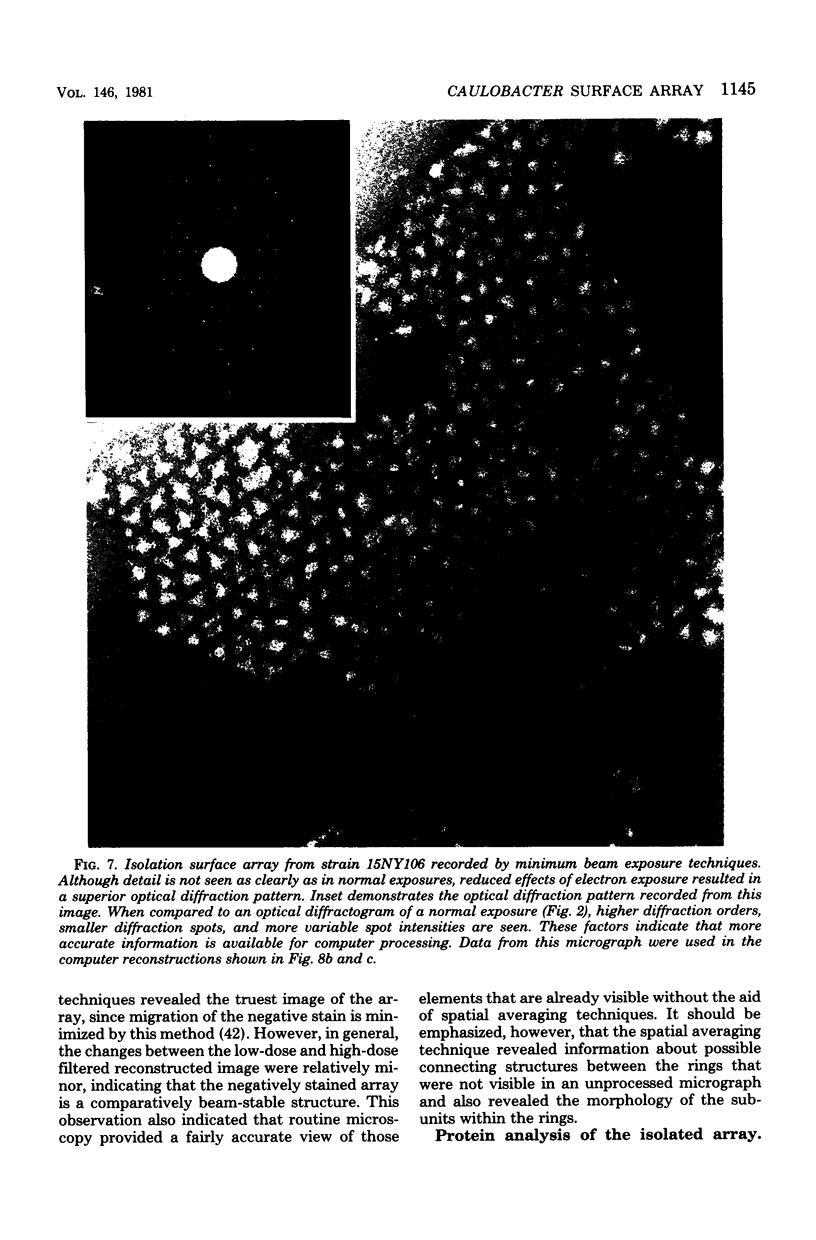
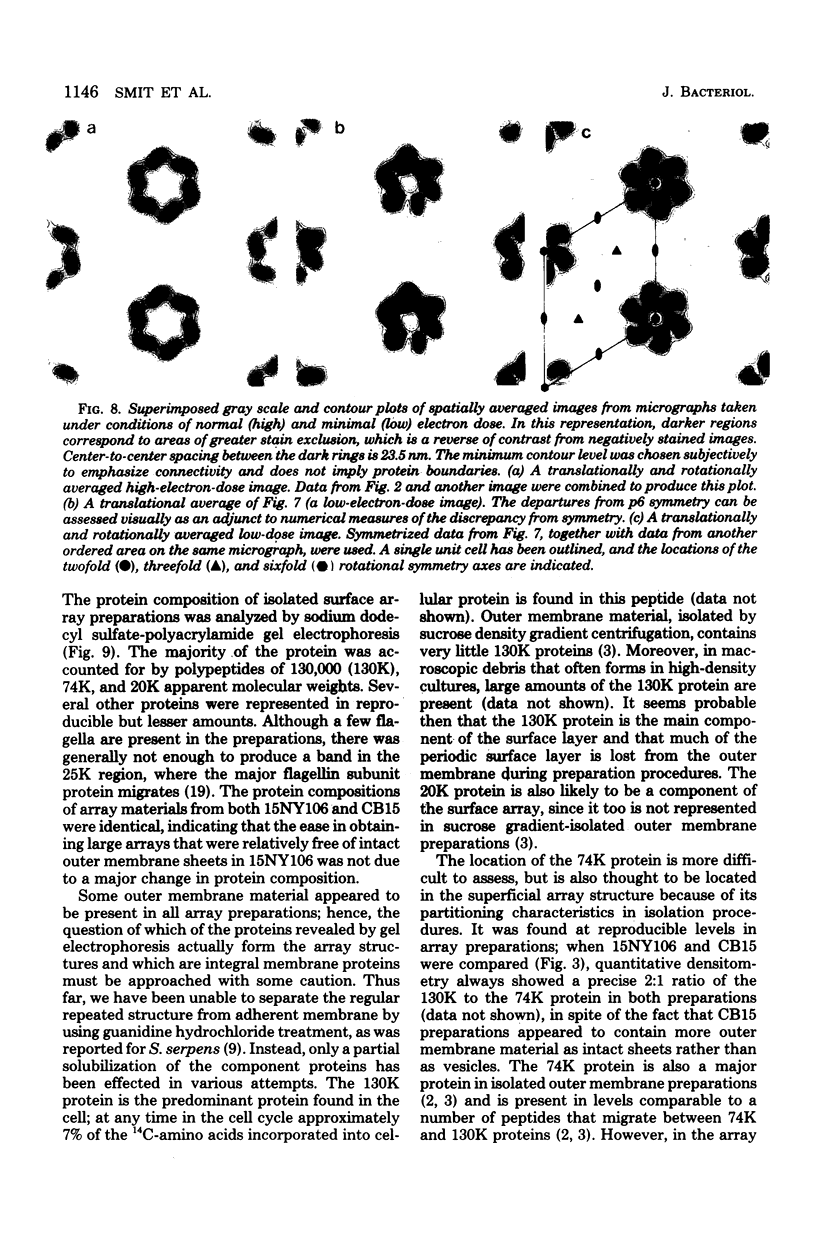
A periodic array structure on the cell surface of Caulobacter crescentus CB15 was revealed by electron microscopy of the cell envelope, using negative staining, thin-sectioning, and freeze-etching. This structural layer has been isolated from liquid cultures, in which large pieces of the two-dimensional array are shed by cells grown to high density. Often areas of intact array corresponding to the entire cell surface could be found. The hexagonally arranged structure was highly ordered and had an unusual degree of complexity, as determined by optical diffraction and computer processing of micrographs of negatively stained, isolated surface array. Filtered, reconstructed images were obtained from both normal and low-electron-dose micrographs demonstrating resolutions of 2.9 and 25 nm, respectively. Comparison by optical diffraction and image filtering of micrographs recorded by using either normal or minimal beam exposure techniques suggested that the lower-resolution features of the image are very stable to electron exposure. Gel electrophoresis indicated that isolated array preparations contain a number of polypeptides. It appears likely that more than one of these proteins are structural components of the array, in contrast to a single protein found in many bacterial surface arrays. The Caulobacter surface array is also unusual in that the repeated units are widely spaced with no apparent direct connection. Computer spatial averaging provided information about the shape and complexity of the connecting elements, and this was compared with some additional electron microscopic evidence of linking structures. Thin-sectioning studies confirmed the image features seen by other techniques, but the addition of tannic acid in the fixation procedure was required to visualize the structure. A comparison of these results with out current knowledge of the Caulobacter cell envelope suggests interesting questions about the biogenesis of this membrane structure and its involvement in the cell development process of this organism.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., ten Heggeler B., Onorato L., Kistler J., Showe M. K. New method for localizing proteins in periodic structures: Fab fragment labeling combined with image processing of electron micrographs. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5514–5518. doi: 10.1073/pnas.74.12.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agabian-Keshishian N., Shapiro L. Bacterial differentiation and phage infection. Virology. 1971 Apr;44(1):46–53. doi: 10.1016/0042-6822(71)90151-6. [DOI] [PubMed] [Google Scholar]
- Agabian N., Evinger M., Parker G. Generation of asymmetry during development. Segregation of type-specific proteins in Caulobacter. J Cell Biol. 1979 Apr;81(1):123–136. doi: 10.1083/jcb.81.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agabian N., Unger B. Caulobacter crescentus cell envelope: effect of growth conditions on murein and outer membrane protein composition. J Bacteriol. 1978 Feb;133(2):987–994. doi: 10.1128/jb.133.2.987-994.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister W., Kübler O. Topographic study of the cell surface of micrococcus radiodurans. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5525–5528. doi: 10.1073/pnas.75.11.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Reassembly in vitro of the superficial cell wall components of Spirillum putridiconcyhylium. J Ultrastruct Res. 1976 Apr;55(1):105–118. doi: 10.1016/s0022-5320(76)80086-x. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Superficial cell-wall layers on Spirillum "Ordal" and their in vitro reassembly. Can J Microbiol. 1976 Apr;22(4):567–582. doi: 10.1139/m76-085. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Surface arrays on the cell wall of Spirillum metamorphum. J Bacteriol. 1975 Dec;124(3):1529–1544. doi: 10.1128/jb.124.3.1529-1544.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. 1. Isolation and partial purification of the outermost cell wall layer. Can J Microbiol. 1970 Oct;16(10):1011–1022. doi: 10.1139/m70-171. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. II. Chemical characterization of the outer structured layer. Can J Microbiol. 1973 Jan;19(1):59–66. doi: 10.1139/m73-009. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Chester I. R., Murray R. G. Protein-lipid-lipopolysaccharide association in the superficial layer of Spirillum serpens cell walls. J Bacteriol. 1978 Feb;133(2):932–941. doi: 10.1128/jb.133.2.932-941.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser R. M., Chiu W., Grano D. Structure of the surface layer protein of the outer membrane of Spirillum serpens. J Ultrastruct Res. 1979 Mar;66(3):235–242. doi: 10.1016/s0022-5320(79)90121-7. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Hollaus F., Sleytr U. On the taxonomy and fine structure of some hyperthermophilic saccharolytic Clostridia. Arch Mikrobiol. 1972;86(2):129–146. doi: 10.1007/BF00413368. [DOI] [PubMed] [Google Scholar]
- Horne R. W., Ronchetti I. P. A negative staining-carbon film technique for studying viruses in the electron microscope. I. Preparative procedures for examining icosahedral and filamentous viruses. J Ultrastruct Res. 1974 Jun;47(3):361–383. doi: 10.1016/s0022-5320(74)90015-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Physical characterization of Caulobacter crescentus flagella. J Bacteriol. 1976 Oct;128(1):435–444. doi: 10.1128/jb.128.1.435-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller L. L., Jacks T. J. Rapid chemical dehydration of samples for electron microscopic examinations. J Histochem Cytochem. 1975 Feb;23(2):107–110. doi: 10.1177/23.2.1117127. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Murray R. G. Ultrastructure of the cell wall of Bacillus polymyxa. J Bacteriol. 1967 Jun;93(6):1949–1965. doi: 10.1128/jb.93.6.1949-1965.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter J. S., Hornack P. R., Armstrong P. A. Intracellular development of a large DNA bacteriophage lytic for Caulobacter crescentus. Arch Mikrobiol. 1967;59(1):237–246. doi: 10.1007/BF00406337. [DOI] [PubMed] [Google Scholar]
- Poindexter J. S. Selection for nonbuoyant morphological mutants of Caulobacter crescentus. J Bacteriol. 1978 Sep;135(3):1141–1145. doi: 10.1128/jb.135.3.1141-1145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway H. F., Wagner R. M., Dawsey W. T., Lewin R. A. Fine structure of the cell envelope layers of Flexibacter polymorphus. Can J Microbiol. 1975 Nov;21(11):1733–1750. doi: 10.1139/m75-254. [DOI] [PubMed] [Google Scholar]
- STOVEPOINDEXTER J. L., COHEN-BAZIRE G. THE FINE STRUCTURE OF STALKED BACTERIA BELONGING TO THE FAMILY CAULOBACTERACEAE. J Cell Biol. 1964 Dec;23:587–607. doi: 10.1083/jcb.23.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L., Agabian-Keshishian N., Hirsch A., Rosen O. M. Effect of dibutyryladenosine 3':5'-cyclic monophosphate on growth and differentiation in Caulobacter crescentus. Proc Natl Acad Sci U S A. 1972 May;69(5):1225–1229. doi: 10.1073/pnas.69.5.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. Differentiation in the Caulobacter cell cycle. Annu Rev Microbiol. 1976;30:377–407. doi: 10.1146/annurev.mi.30.100176.002113. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. J Cell Biol. 1976 Sep;70(3):608–621. doi: 10.1083/jcb.70.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Glauert A. M. Ultrastructure of the cell walls of two closely related clostridia that possess different regular arrays of surface subunits. J Bacteriol. 1976 May;126(2):869–882. doi: 10.1128/jb.126.2.869-882.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B. Heterologous reattachment of regular arrays of glycoproteins on bacterial surfaces. Nature. 1975 Oct 2;257(5525):400–402. doi: 10.1038/257400a0. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Smit J., Nikaido H. Outer membrane of gram-negative bacteria. XVIII. Electron microscopic studies on porin insertion sites and growth of cell surface of Salmonella typhimurium. J Bacteriol. 1978 Aug;135(2):687–702. doi: 10.1128/jb.135.2.687-702.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M., Beveridge T. J., Murray R. G. Structure of the regular surface layer of Spirillum putridiconchylium. J Mol Biol. 1980 Feb 15;137(1):1–8. doi: 10.1016/0022-2836(80)90153-9. [DOI] [PubMed] [Google Scholar]
- Stewart M., Beveridge T. J. Structure of the regular surface layer of Sporosarcina ureae. J Bacteriol. 1980 Apr;142(1):302–309. doi: 10.1128/jb.142.1.302-309.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. A., Glaeser R. M. Hydrophilic support films of controlled thickness and composition. Rev Sci Instrum. 1973 Oct;44(10):1546–1547. doi: 10.1063/1.1685999. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Oliver R. C., Heath M. F. Phospholipase A2 activity of the regularly arranged surface protein of Acinetobacter sp.199A. Biochim Biophys Acta. 1976 Dec 20;450(3):335–341. doi: 10.1016/0005-2760(76)90006-0. [DOI] [PubMed] [Google Scholar]
- Thornley M. J., Thorne K. J., Glauert A. M. Detachment and chemical characterization of the regularly arranged subunits from the surface of an Acinetobacter. J Bacteriol. 1974 May;118(2):654–662. doi: 10.1128/jb.118.2.654-662.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Remsen C. C. Cell envelope of Nitrosocystis oceanus. J Ultrastruct Res. 1970 Oct;33(1):148–160. doi: 10.1016/s0022-5320(70)90122-x. [DOI] [PubMed] [Google Scholar]
- West D., Lagenaur C., Agabian N. Isolation and characterization of Caulobacter crecentus bacteriophage phi Cd1. J Virol. 1976 Feb;17(2):568–575. doi: 10.1128/jvi.17.2.568-575.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Fisher H. W. Electron microscopy of tobacco mosaic virus under conditions of minimal beam exposure. J Mol Biol. 1970 Aug 28;52(1):121–123. doi: 10.1016/0022-2836(70)90181-6. [DOI] [PubMed] [Google Scholar]