Abstract
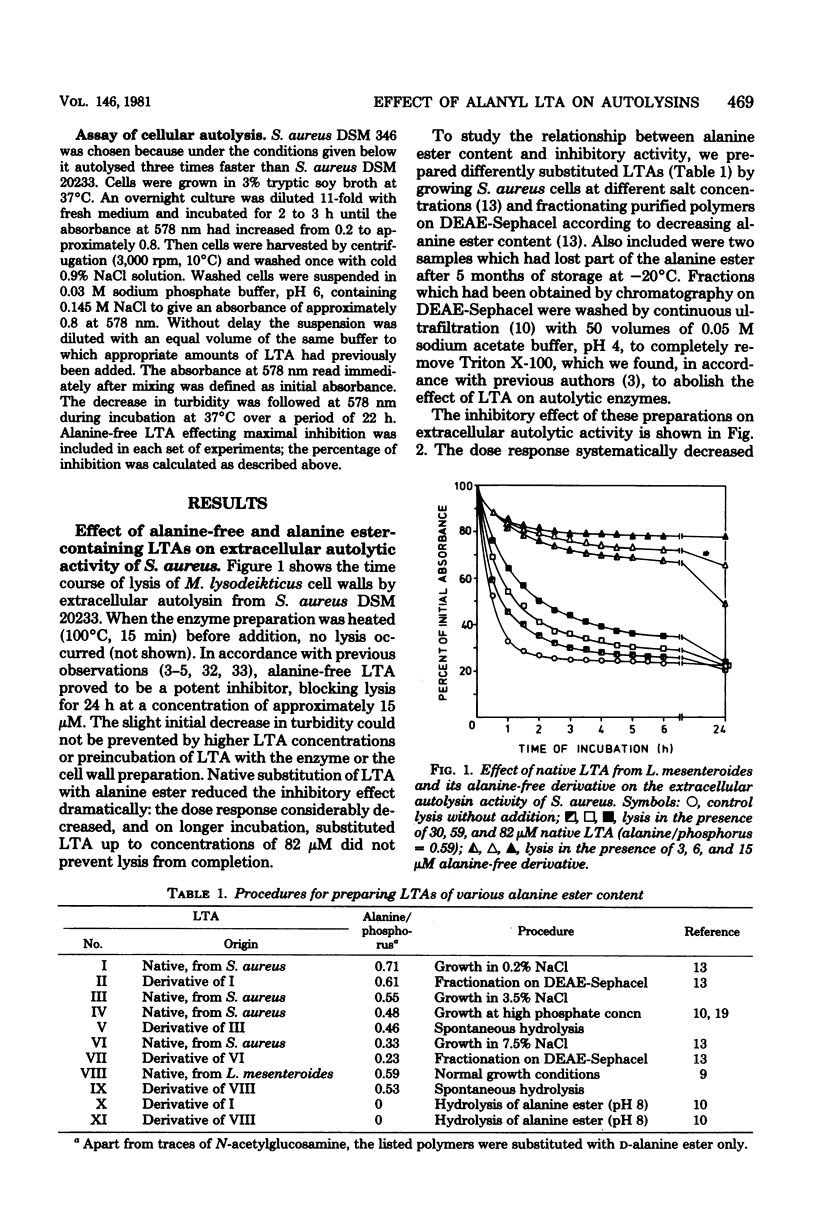
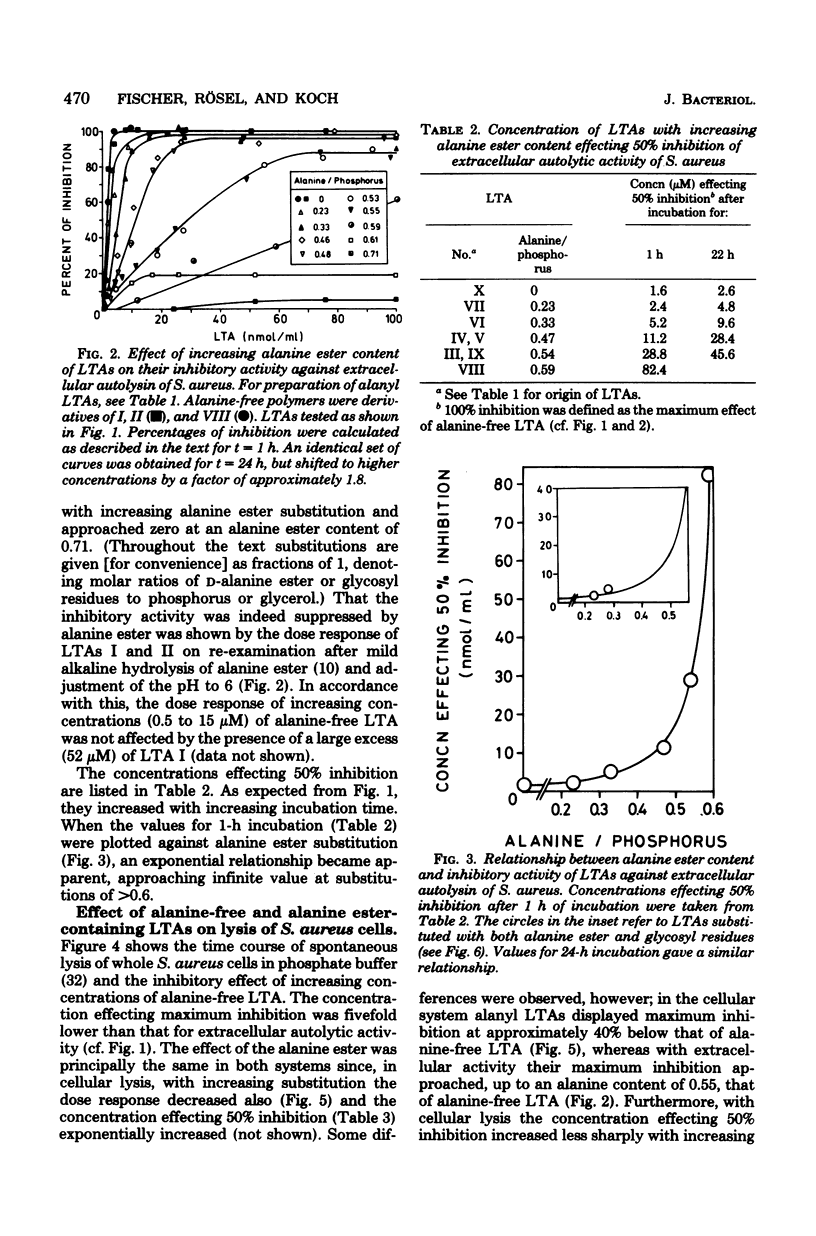
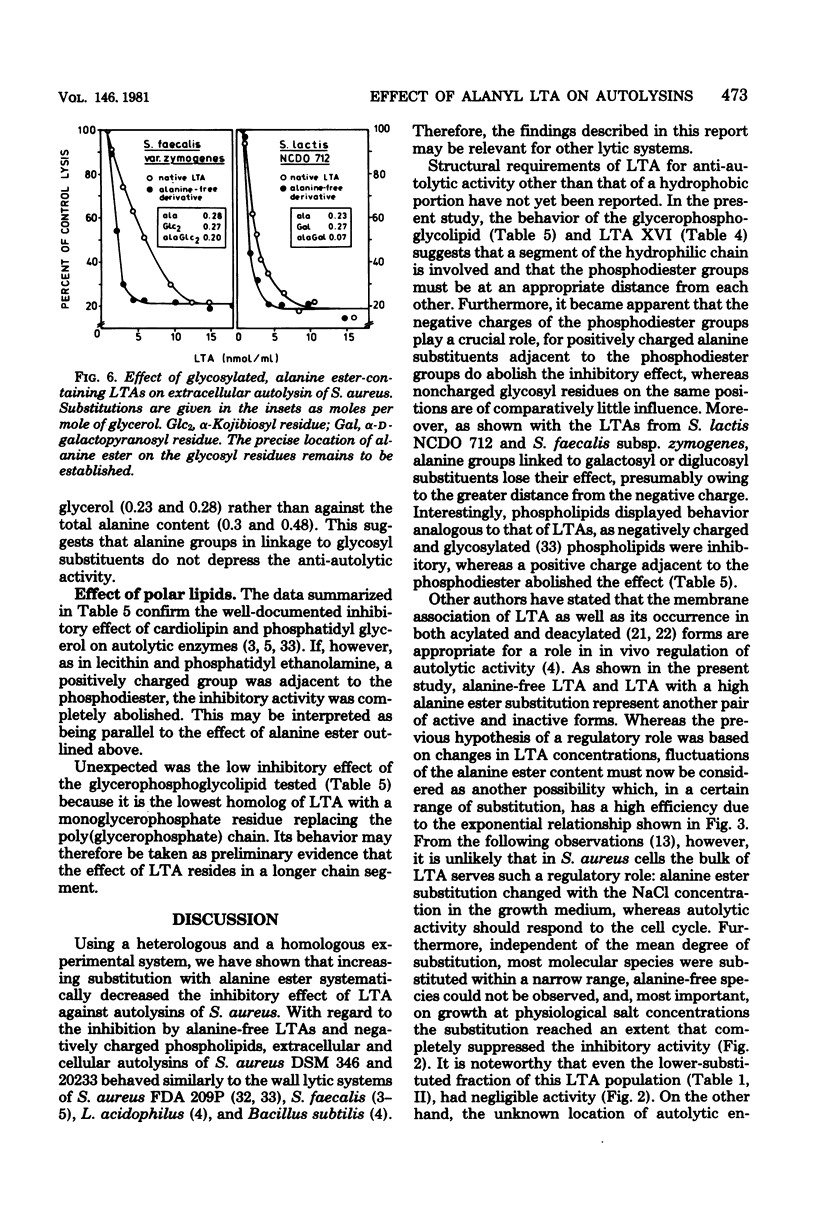
Native substitution with the D-alanine ester of lipoteichoic acids (LTAs) affects their immunological properties, the capacity to bind divalent cations, and LTA carrier activity. In this study we tested the influence of the D-alanine ester on anti-autolytic activity, using extracellular autolysin from Staphylococcus aureus and nine LTAs with alanine/phosphorus molar ratios of between 0.23 and 0.71. The inhibitory activity, highest with alanine-free LTA, exponentially decreased with increasing alanine content, approaching zero at substitutions of greater than 0.6. Correspondingly, dipolar ionic phospholipids were not inhibitory, in contrast to negatively charged ones. Glycosylation of LTA up to an extent of 0.5 did not depress inhibitory activity, and even at a degree of 0.8 the effect was comparatively small. On comparison of LTAs from various sources, differences in lipid structures and chain lengths were without effect. The inhibitory activity drastically decreased when the glycolipid carried a single glycerophosphate residue or the hydrophilic chain had the unusual structure [6 leads to Gal(alpha 1--6)Gal(alpha 1--3)Gro-(2 comes from 1 alpha Gal)-P]n, in which digalactosyl moieties connect the alpha-galactosylated glycerophosphate units. Principally, the same results were obtained with the more complex system of autolysis of S. aureus cells. We hypothesize that the anti-autolytic activity of LTA resides in a sequence of glycerophosphate units and that the negative charges of appropriately spaced phosphodiester groups play a crucial role. The alanine ester effect is discussed with respect to the putative in vivo regulation of autolysins by LTA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald A. R., Baddiley J. The teichoic acids. Adv Carbohydr Chem Biochem. 1966;21:323–375. doi: 10.1016/s0096-5332(08)60320-3. [DOI] [PubMed] [Google Scholar]
- Childs W. C., 3rd, Neuhaus F. C. Biosynthesis of D-alanyl-lipoteichoic acid: characterization of ester-linked D-alanine in the in vitro-synthesized product. J Bacteriol. 1980 Jul;143(1):293–301. doi: 10.1128/jb.143.1.293-301.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Daneo-Moore L., Wicken A. J., Shockman G. D. Effect of lipoteichoic acid and lipids on lysis of intact cells of Streptococcus faecalis. J Bacteriol. 1976 Sep;127(3):1582–1584. doi: 10.1128/jb.127.3.1582-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Holtje J. V., Wicken A. J., Tomasz A., Daneo-Moore L., Shockman G. D. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1128–1135. doi: 10.1016/0006-291x(75)90791-3. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler F., Glaser L. The synthesis of polyribitol phosphate. I. Purification of polyribitol phosphate polymerase and lipoteichoic acid carrier. J Biol Chem. 1974 May 10;249(9):2684–2689. [PubMed] [Google Scholar]
- Fiedler F., Glaser L. The synthesis of polyribitol phosphate. II. On the mechanism of polyribitol phosphate polymerase. J Biol Chem. 1974 May 10;249(9):2690–2695. [PubMed] [Google Scholar]
- Fischer W., Ishizuka I., Landgraf H. R., Herrmann J. Glycerophosphoryl diglucosyl diglyceride, a new phosphoglycolipid from Streptococci. Biochim Biophys Acta. 1973 Mar 8;296(3):527–545. doi: 10.1016/0005-2760(73)90113-6. [DOI] [PubMed] [Google Scholar]
- Fischer W., Koch H. U., Rösel P., Fiedler F. Alanine ester-containing native lipoteichoic acids do not act as lipoteichoic acid carrier. Isolation, structural and functional characterization. J Biol Chem. 1980 May 25;255(10):4557–4562. [PubMed] [Google Scholar]
- Fischer W., Koch H. U., Rösel P., Fiedler F., Schmuck L. Structural requirements of lipoteichoic acid carrier for recognition by the poly(ribitol phosphate) polymerase from Staphylococcus aureus H. A study of various lipoteichoic acids, derivatives, and related compounds. J Biol Chem. 1980 May 25;255(10):4550–4556. [PubMed] [Google Scholar]
- Fischer W., Nakano M., Laine R. A., Bohrer W. On the relationship between glycerophosphoglycolipids and lipoteichoic acids in Gram-positive bacteria. I. The occurrence of phosphoglycolipids. Biochim Biophys Acta. 1978 Mar 30;528(3):288–297. doi: 10.1016/0005-2760(78)90018-8. [DOI] [PubMed] [Google Scholar]
- Fischer W., Rösel P. The alanine ester substitution of lipoteichoic acid (LTA) in Staphylococcus aureus. FEBS Lett. 1980 Oct 6;119(2):224–226. doi: 10.1016/0014-5793(80)80257-2. [DOI] [PubMed] [Google Scholar]
- Fischer W. The polar lipids of group B Streptococci. II. Composition and positional distribution of fatty acids. Biochim Biophys Acta. 1977 Apr 26;487(1):89–104. doi: 10.1016/0005-2760(77)90046-7. [DOI] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Release of lipoteichoic acid from Streptococcus sanguis: stimulation of release during penicillin treatment. J Bacteriol. 1979 Mar;137(3):1180–1184. doi: 10.1128/jb.137.3.1180-1184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques N. A., Hardy L., Knox K. W., Wicken A. J. Effect of growth conditions on the formation of extracellular lipoteichoic acid by Streptococcus mutans BHT. Infect Immun. 1979 Jul;25(1):75–84. doi: 10.1128/iai.25.1.75-84.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemasa Y., Yoshioka T., Hayashi H. Alteration of the phospholipid composition of Staphylococcus aureus cultured in medium containing NaCl. Biochim Biophys Acta. 1972 Nov 30;280(3):444–450. [PubMed] [Google Scholar]
- Kessler R. E., Shockman G. D. Enzymatic deacylation of lipoteichoic acid by protoplasts of Streptococcus faecium (Streptococcus faecalis ATCC 9790). J Bacteriol. 1979 Mar;137(3):1176–1179. doi: 10.1128/jb.137.3.1176-1179.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Shockman G. D. Precursor-product relationship of intracellular and extracellular lipoteichoic acids of Streptococcus faecium. J Bacteriol. 1979 Feb;137(2):869–877. doi: 10.1128/jb.137.2.869-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., van de Rijn I., McCarty M. Characterization and localization of the enzymatic deacylation of lipoteichoic acid in group A streptococci. J Exp Med. 1979 Dec 1;150(6):1498–1509. doi: 10.1084/jem.150.6.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H. U., Fischer W. Acyldiglucosyldiacylglycerol-containing lipoteichoic acid with a poly(3-O-galabiosyl-2-O-galactosyl-sn-glycero-1-phosphate) chain from Streptococcus lactis Kiel 42172. Biochemistry. 1978 Nov 28;17(24):5275–5281. doi: 10.1021/bi00617a030. [DOI] [PubMed] [Google Scholar]
- Lambert P. A., Hancock I. C., Baddiley J. Occurrence and function of membrane teichoic acids. Biochim Biophys Acta. 1977 May 31;472(1):1–12. doi: 10.1016/0304-4157(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J., Talamo B. The chemical characterization and enzymatic synthesis of mannolipids in Micrococcus lysodeikticus. J Biol Chem. 1966 Jun 10;241(11):2707–2719. [PubMed] [Google Scholar]
- MCCARTY M. THE ROLE OF D-ALANINE IN THE SEROLOGICAL SPECIFICITY OF GROUP A STREPTOCOCCAL GLYCEROL TEICHOIC ACID. Proc Natl Acad Sci U S A. 1964 Aug;52:259–265. doi: 10.1073/pnas.52.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Wicken A. J., Hewett M. J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975 Aug;12(2):378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Fischer W. The glycolipids of Lactobacillus casei DSM 20021. Hoppe Seylers Z Physiol Chem. 1977 Nov;358(11):1439–1453. doi: 10.1515/bchm2.1977.358.2.1439. [DOI] [PubMed] [Google Scholar]
- Nakano M., Fischer W. Trihexosyldiacylglycerol and acyltrihexosyldiacylglycerol as lipid anchors of the lipoteichoic acid of Lactobacillus casei DSM 20021. Hoppe Seylers Z Physiol Chem. 1978 Jan;359(1):1–11. doi: 10.1515/bchm.1978.359.1.1. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. Lytic enzymes of Staphylococcus aureus 524. Biochim Biophys Acta. 1959 Feb;31(2):564–565. doi: 10.1016/0006-3002(59)90041-1. [DOI] [PubMed] [Google Scholar]
- Suginaka H., Shimatani M., Ogawa M., Kotani S. Prevention of penicillin-induced lysis of Staphylococcus aureus by cellular lipoteichoic acid. J Antibiot (Tokyo) 1979 Jan;32(1):73–77. doi: 10.7164/antibiotics.32.73. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]


