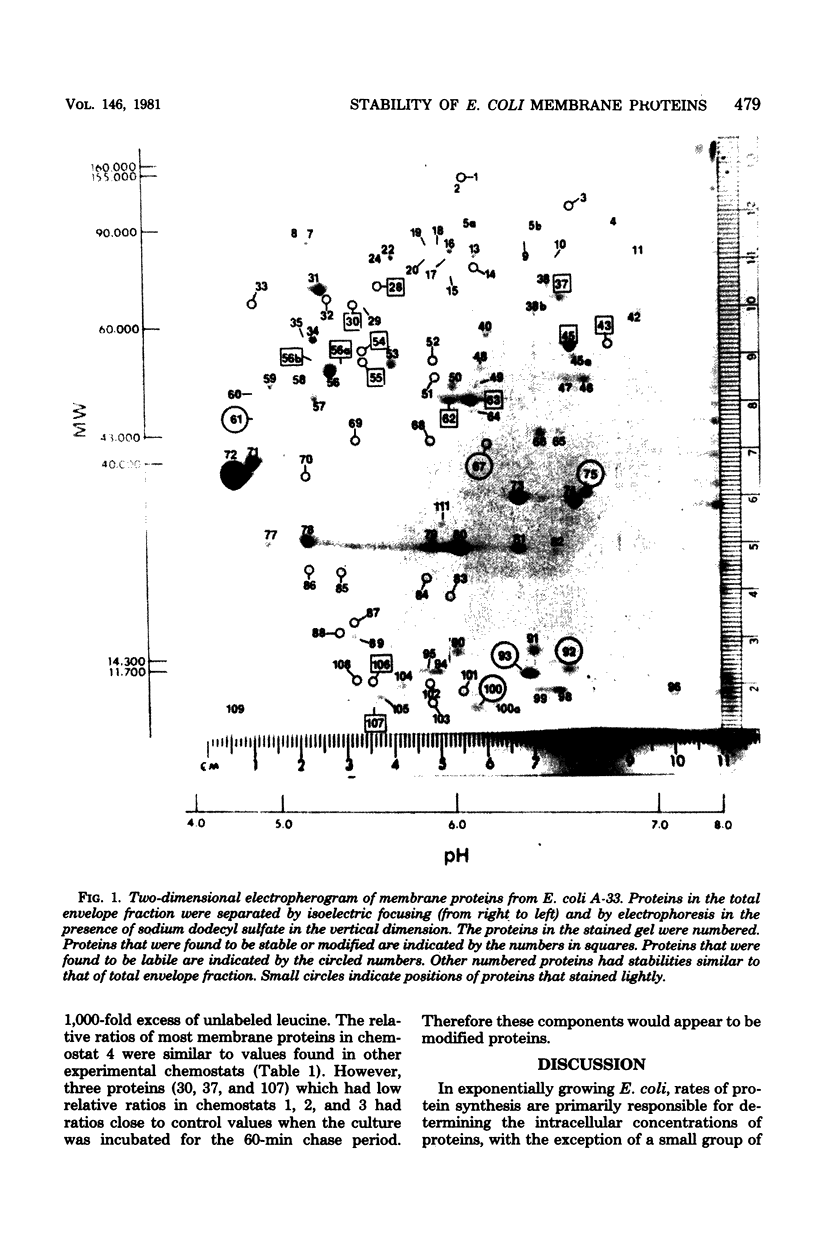
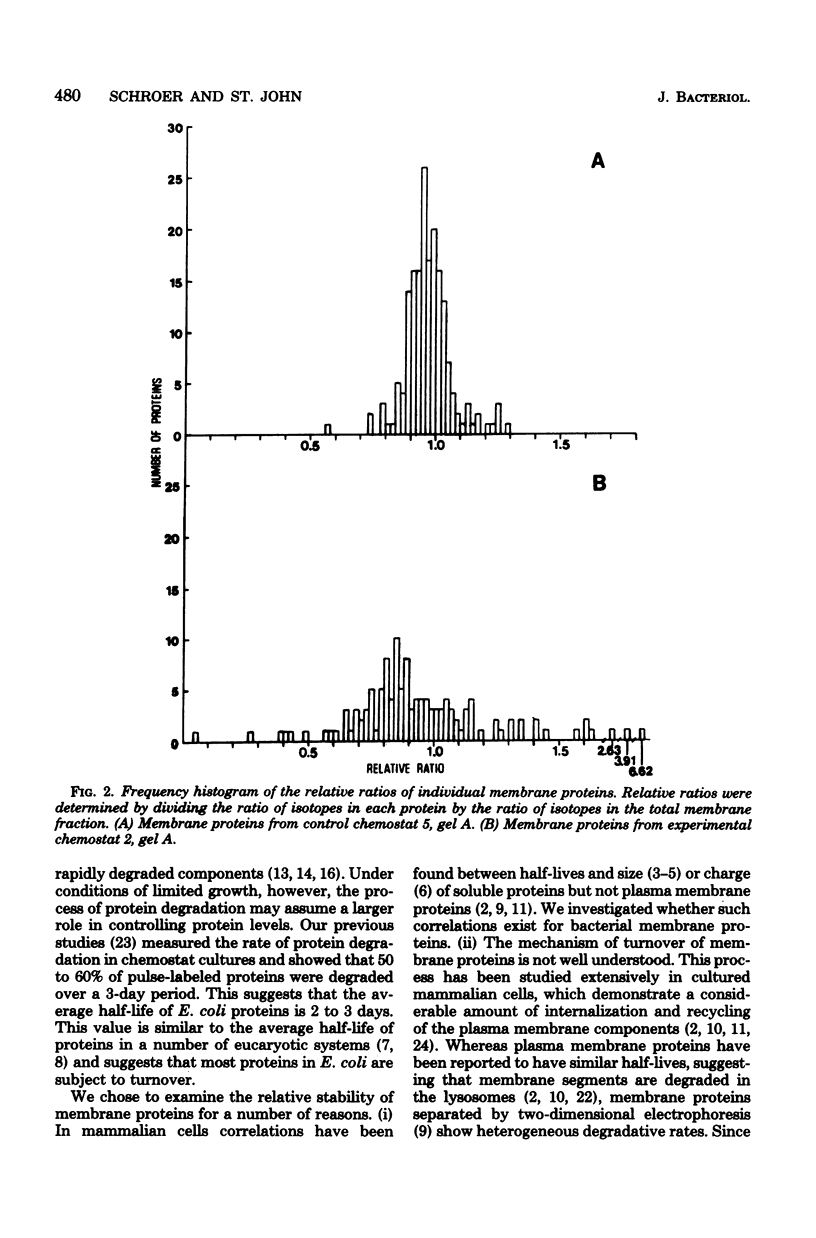
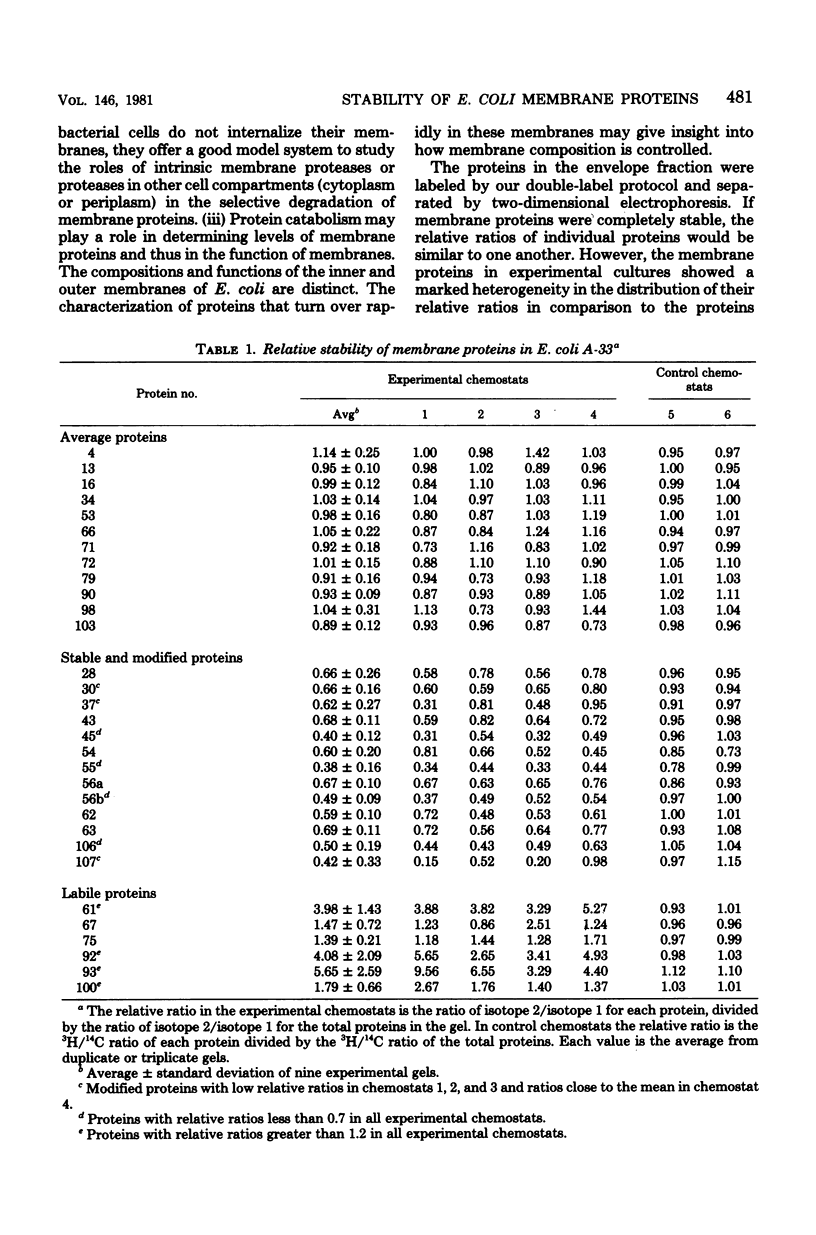
Abstract
The relative stability of membrane proteins in Escherichia coli was investigated to determine whether these proteins are degraded at heterogeneous rates and, if so, whether the degradative rates are correlated with the sizes or charges of the proteins. Cells growing in a glucose-limited chemostat with a generation time of 15 h were labeled with [14C]leucine. After allowing 24 h for turnover of 14C-labeled proteins, the cells were labeled for 15 min with [3H]leucine. By this protocol, the rapidly degraded proteins have a high ratio of 3H to 14C, whereas the stable proteins have a lower ratio. The total cell envelope fraction was collected by differential centrifugation, and the proteins were separated by two-dimensional polyacrylamide gel electrophoresis. The relative ratio for each protein was determined by dividing its 3H/14C ratio by the 3H/14C ratio of the total membrane fraction. Although most of the 125 membrane proteins had relative ratios close to the average for the total membrane fraction, 19 varied significantly from this value. These differences were also observed when the order of addition of [14C]leucine and [3H]leucine was reversed. In control cultures labeled simultaneously with both isotopes, the relative ratios of these 19 proteins were similar to that of the total membrane fraction. Thirteen of these proteins had low relative ratios, which suggested that they were more stable than the average protein. An experiment in which the normal labeling procedure was followed by a 60-min chase period in the presence of excess unlabeled leucine suggested that the low relative ratios of 3 of these 13 proteins may be due to a slow post-translational modification step. Six membrane proteins had high relative ratios, which indicated that they were degraded rapidly. In contrast to the relationships found for soluble proteins in mammalian cells, there were no strong correlations between the degradative rates and either the isoelectric points or the molecular weights of membrane proteins in E. coli.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Baumann H., Doyle D. Turnover of plasma membrane glycoproteins and glycolipids of hepatoma tissue culture cells. J Biol Chem. 1978 Jun 25;253(12):4408–4418. [PubMed] [Google Scholar]
- Dehlinger P. J., Schimke R. T. Size distribution of membrane proteins of rat liver and their relative rates of degradation. J Biol Chem. 1971 Apr 25;246(8):2574–2583. [PubMed] [Google Scholar]
- Dice J. F., Dehlinger P. J., Schimke R. T. Studies on the correlation between size and relative degradation rate of soluble proteins. J Biol Chem. 1973 Jun 25;248(12):4220–4228. [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. A statistical analysis of the relationship between degradative rates and molecular weights of proteins. Arch Biochem Biophys. 1975 Sep;170(1):213–219. doi: 10.1016/0003-9861(75)90112-5. [DOI] [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. Relationship between in vivo degradative rates and isoelectric points of proteins. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3893–3897. doi: 10.1073/pnas.72.10.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Horst M. N., Roberts R. M. Analysis of polypeptide turnover rates in Chinese hamster ovary cell plasma membranes using two-dimensional electrophoresis. J Biol Chem. 1979 Jun 25;254(12):5000–5007. [PubMed] [Google Scholar]
- Kaplan G., Unkeless J. C., Cohn Z. A. Insertion and turnover of macrophage plasma membrane proteins. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3824–3828. doi: 10.1073/pnas.76.8.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Moskowitz M. Studies on the turnover of plasma membranes in cultured mammalian cells. II. Demonstration of heterogeneous rates of turnover for plasma membrane proteins and glycoproteins. Biochim Biophys Acta. 1975 May 6;389(2):306–313. doi: 10.1016/0005-2736(75)90323-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larrabee K. L., Phillips J. O., Williams G. J., Larrabee A. R. The relative rates of protein synthesis and degradation in a growing culture of Escherichia coli. J Biol Chem. 1980 May 10;255(9):4125–4130. [PubMed] [Google Scholar]
- Mosteller R. D., Goldstein R. V., Nishimoto K. R. Metabolism of individual proteins in exponentially growing Escherichia coli. J Biol Chem. 1980 Mar 25;255(6):2524–2532. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Regulation of intracellular proteolysis in Escherichia coli. J Bacteriol. 1973 Jul;115(1):107–116. doi: 10.1128/jb.115.1.107-116.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- Roberts R. M., Yuan B. O. Turnover of plasma membrane polypeptides in nonproliferating cultures of Chinese hamster ovary cells and human skin fibroblasts. Arch Biochem Biophys. 1975 Nov;171(1):234–244. doi: 10.1016/0003-9861(75)90028-4. [DOI] [PubMed] [Google Scholar]
- St John A. C., Jakubas K., Beim D. Degradation of proteins in steady-state cultures of Escherichia coli. Biochim Biophys Acta. 1979 Sep 3;586(3):537–544. doi: 10.1016/0304-4165(79)90044-8. [DOI] [PubMed] [Google Scholar]
- Tulkens P., Schneider Y. J., Trouet A. The fate of the plasma membrane during endocytosis. Biochem Soc Trans. 1977;5(6):1809–1815. doi: 10.1042/bst0051809. [DOI] [PubMed] [Google Scholar]