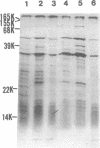
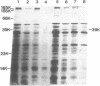
Abstract
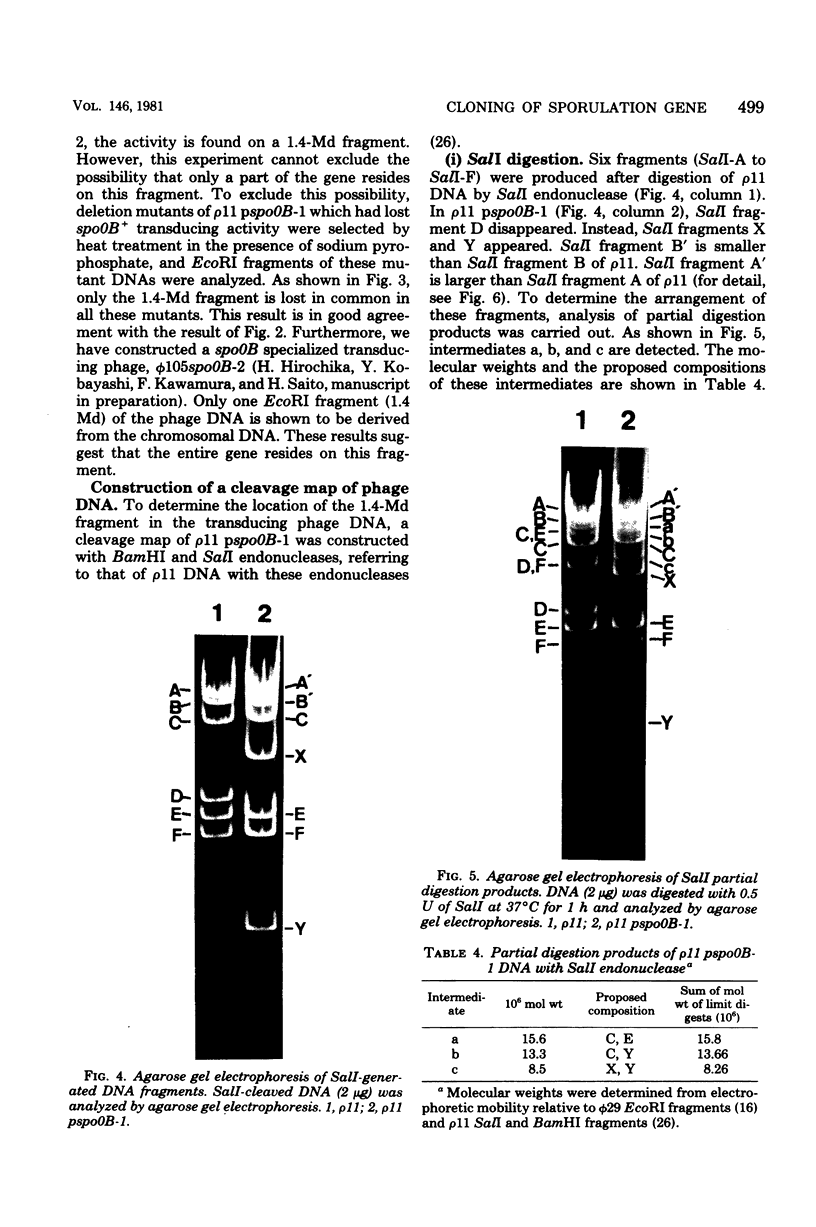
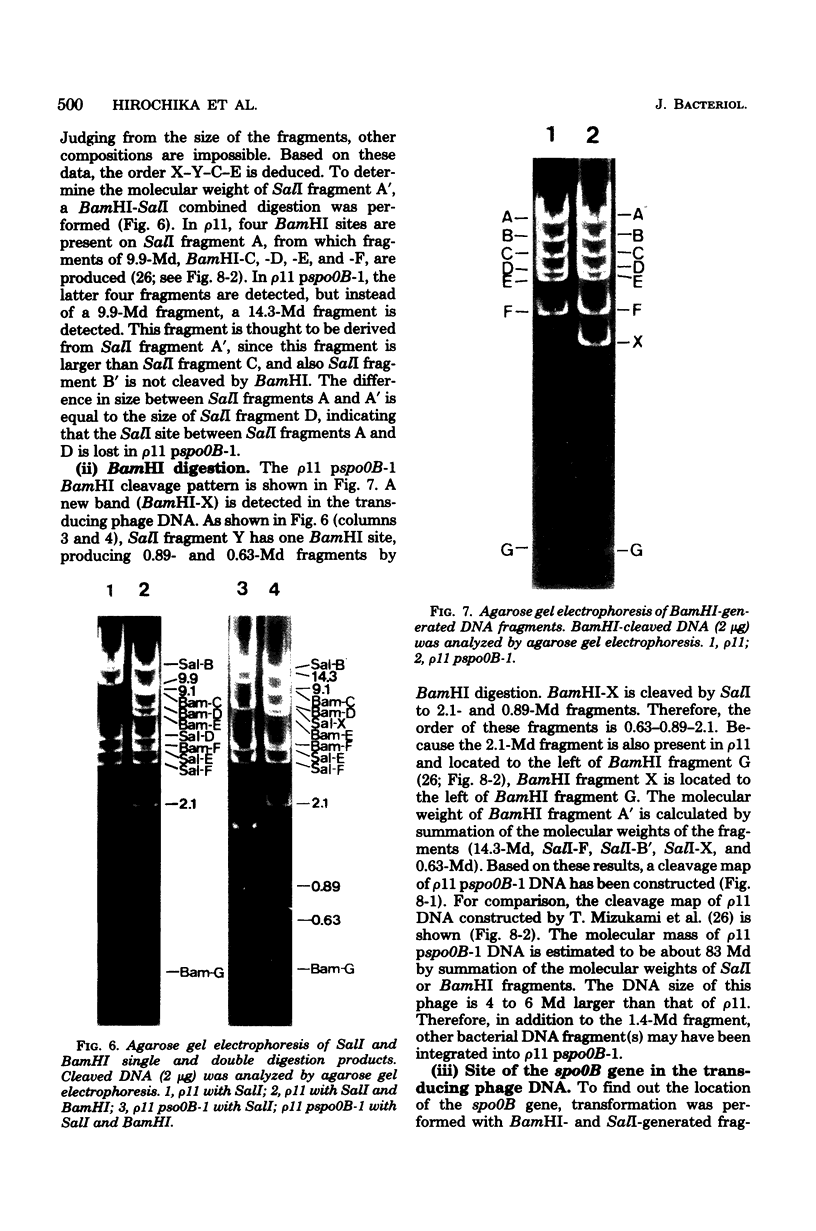
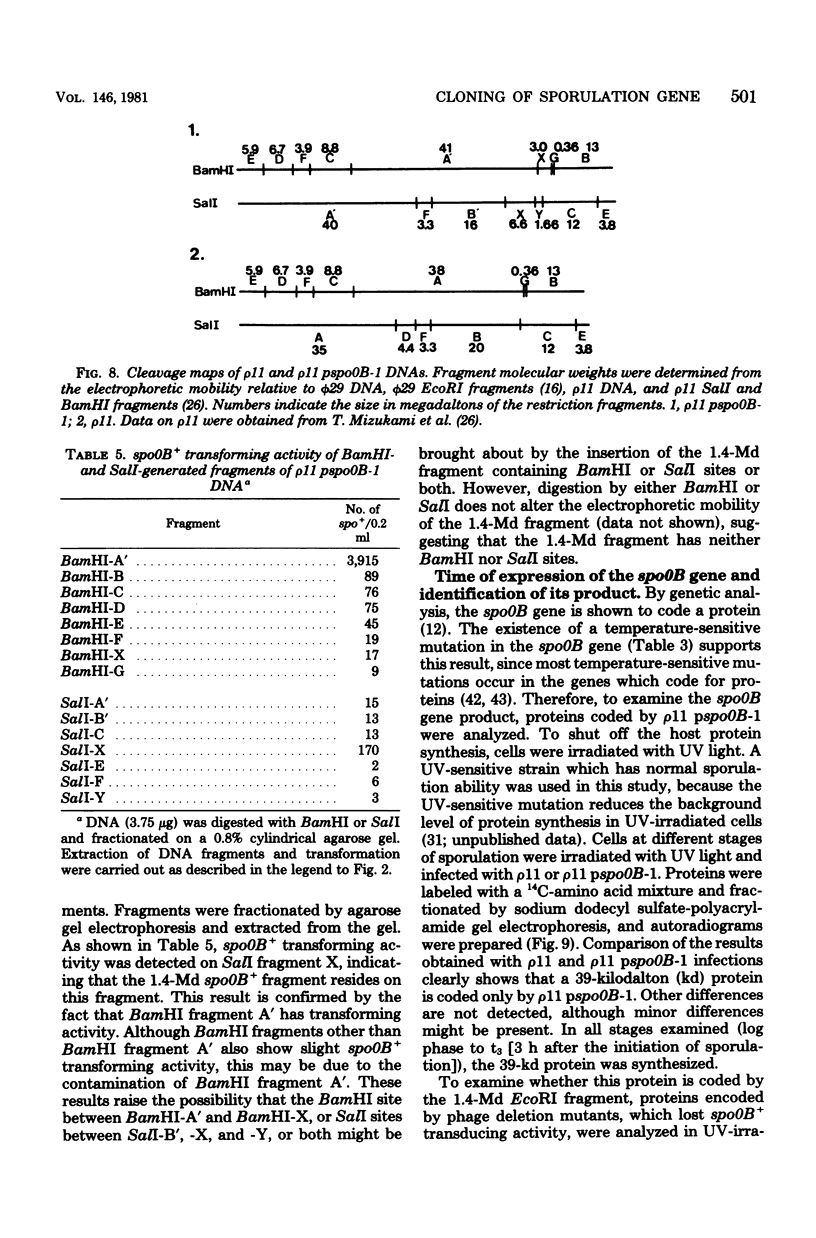
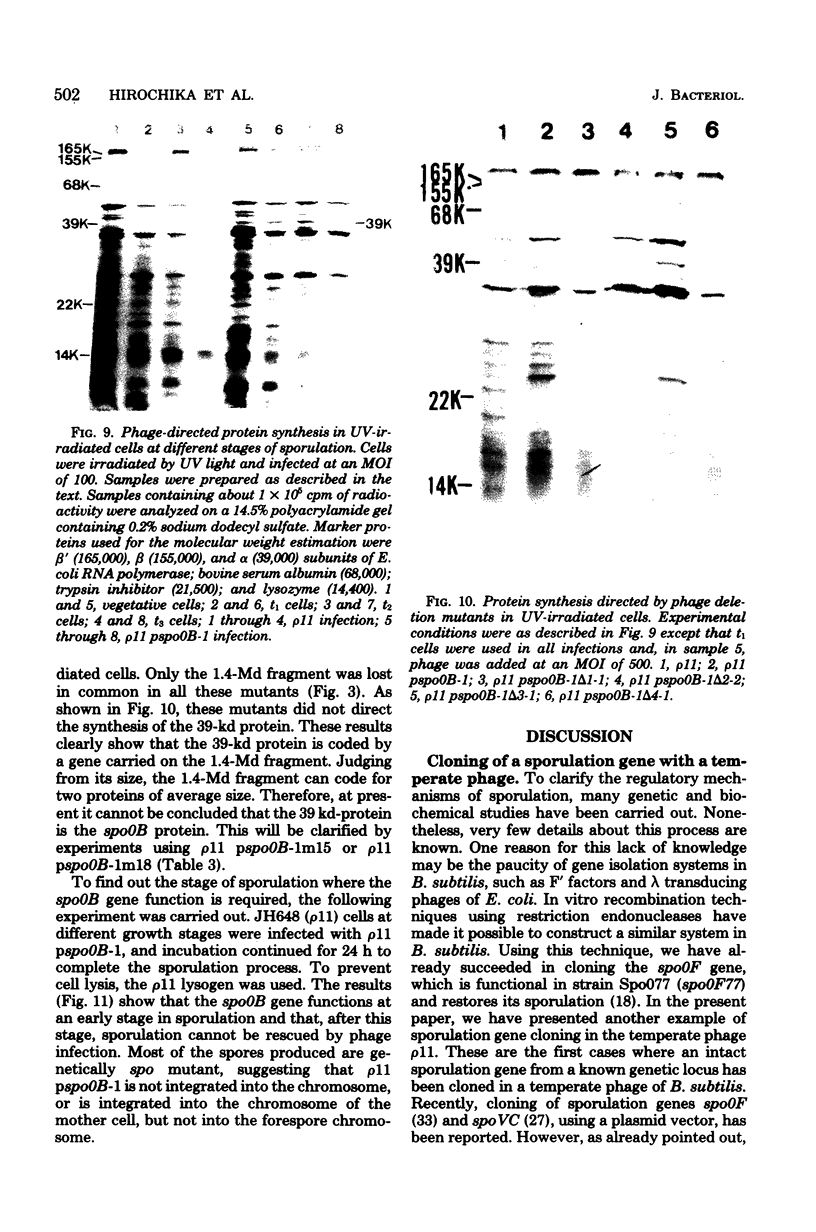
A specialized transducing phage carrying a sporulation gene (spoOB) was constructed from Bacillus subtilis temperate phage rho 11 by in vitro and in vivo recombinations. Transformation experiments showed that the spoOB gene resides on a 1.4-megadalton fragment generated by EcoRI endonuclease treatment of the phage deoxyribonucleic acid (DNA). Mutants of this phage which lost transducing activity were isolated and used for genetic complementation tests and the analysis of protein(s) coded by the 1.4-megadalton fragment. The spoOB locus was shown to be composed of one cistron. Sodium dodecyl sulfate-polyacrylamide gel analysis of proteins synthesized in ultraviolet-irradiated cells infected with these phages showed that the 1.4-megadalton fragment codes at least one protein, of molecular weight 39,000, which is synthesized in both vegetative and sporulating cells. A cleavage map of the phage DNA was constructed by use of restriction endonucleases, EcoRI, BamHI, and SalI, and the site of integration of the 1.4-megadalton fragment was determined. Expression and function of the spoOB gene are discussed.
Full text
PDF


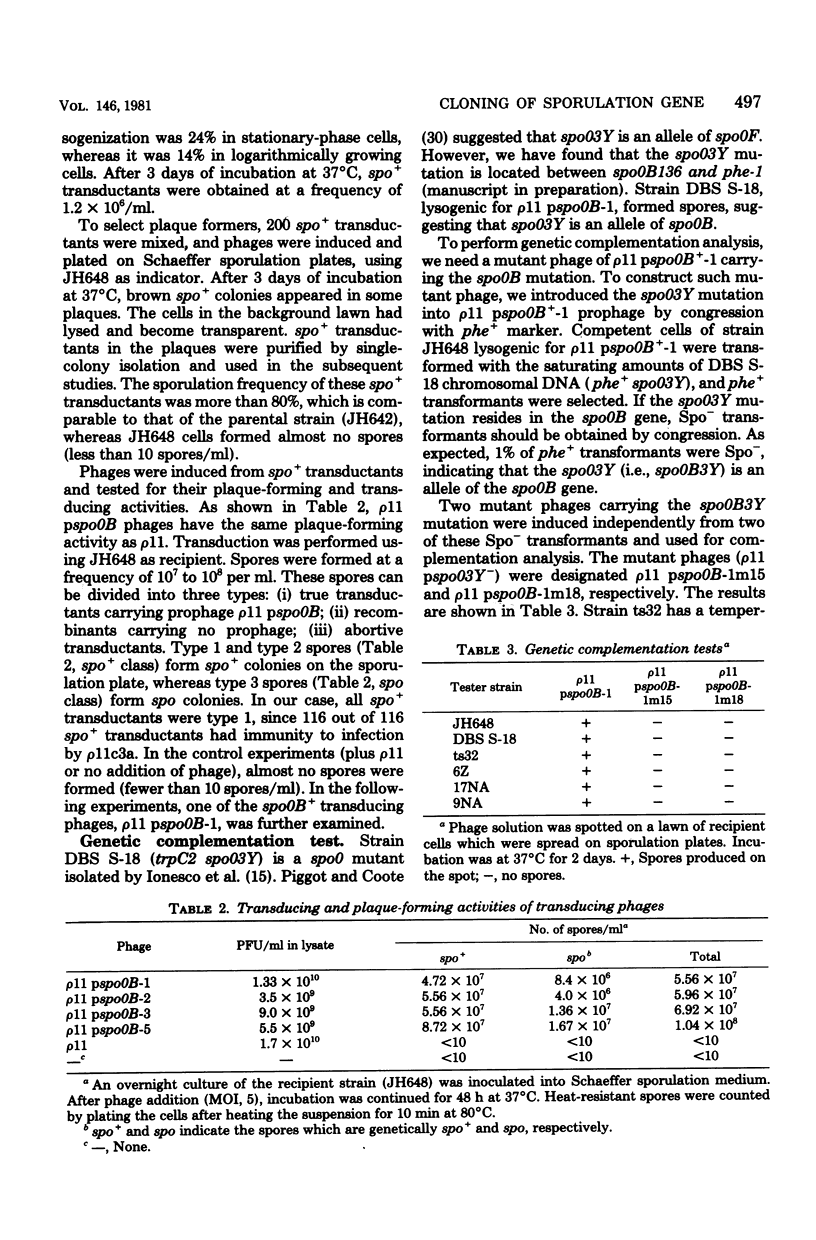
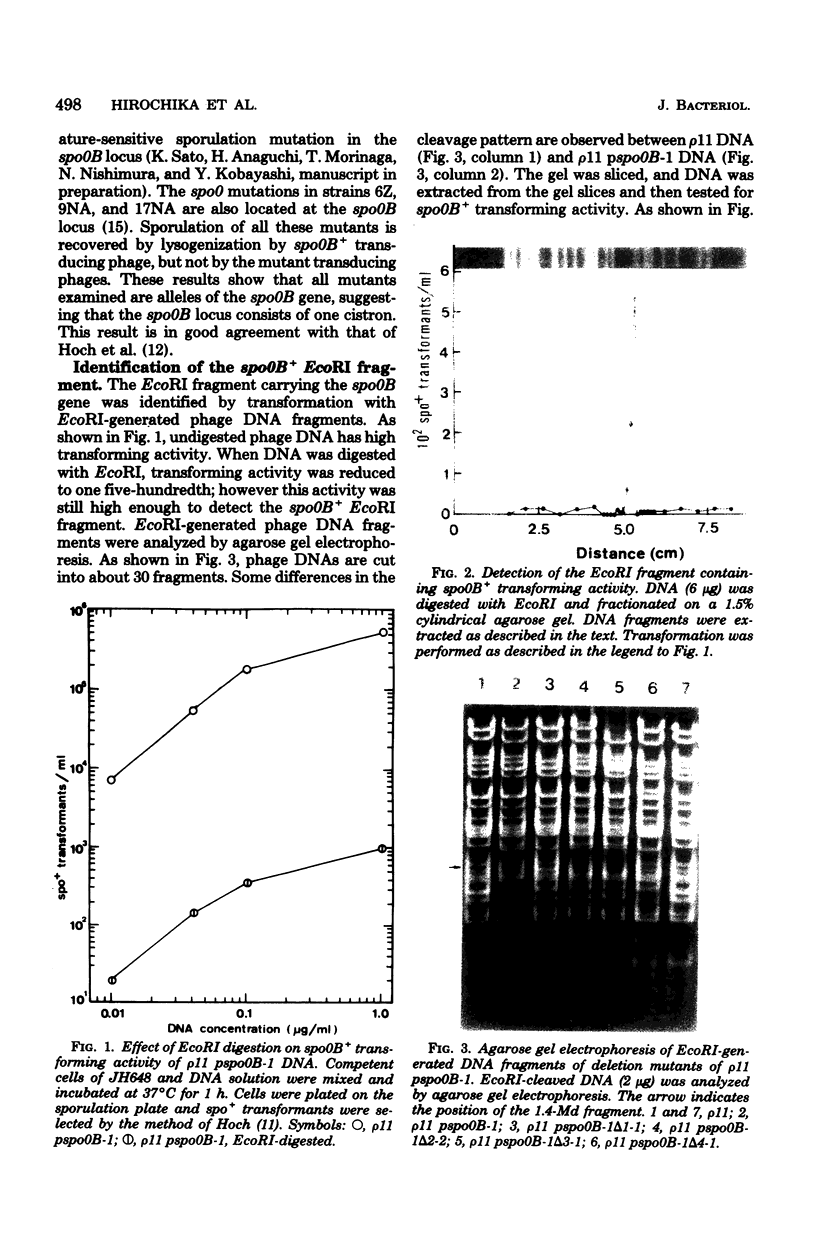


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohin J. P., Bohin A., Schaeffer P. Increased nitrate reductase A activity as a sign of membrane alteration in early blocked asporogenous mutants of Bacillus subtilis. Biochimie. 1976;58(1-2):99–108. doi: 10.1016/s0300-9084(76)80360-4. [DOI] [PubMed] [Google Scholar]
- Brehm S. P., Le Hegarat F., Hoch J. A. Deoxyribonucleic acid-binding proteins in vegetative Bacillus subtilis: alterations caused by stage O sporulation mutations. J Bacteriol. 1975 Nov;124(2):977–984. doi: 10.1128/jb.124.2.977-984.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. H., Orrego J. C., Hutchison K. W., Halvorson H. O. New temperate bacteriophage for Bacillus subtilis, rho 11. J Virol. 1976 Nov;20(2):509–519. doi: 10.1128/jvi.20.2.509-519.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S. D. DNA cloning in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1433–1436. doi: 10.1073/pnas.75.3.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Dubnau D. Construction and properties of chimeric plasmids in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1428–1432. doi: 10.1073/pnas.75.3.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guespin-Michel J. E. Phenotypic reversion in some early blocked sporulation mutants of Bacillus subtilis: isolation and phenotype identification of partial revertants. J Bacteriol. 1971 Oct;108(1):241–247. doi: 10.1128/jb.108.1.241-247.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A. Selection of cells transformed to prototrophy for sporulation markers. J Bacteriol. 1971 Mar;105(3):1200–1201. doi: 10.1128/jb.105.3.1200-1201.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hranueli D., Piggot P. J., Mandelstam J. Statistical estimate of the total number of operons specific for Bacillus subtilis sporulation. J Bacteriol. 1974 Sep;119(3):684–690. doi: 10.1128/jb.119.3.684-690.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi T., Babasaki K., Kurahashi K. Genetic evidence for possible interaction between a ribonucleic acid polymerase subunit and the spo0C gene product of Bacillus subtilis. J Bacteriol. 1979 Aug;139(2):327–332. doi: 10.1128/jb.139.2.327-332.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionesco H., Michel J., Cami B., Schaeffer P. Symposium on bacterial spores: II. Genetics of sporulation in Bacillus subtilis Marburg. J Appl Bacteriol. 1970 Mar;33(1):13–24. doi: 10.1111/j.1365-2672.1970.tb05230.x. [DOI] [PubMed] [Google Scholar]
- Ito J., Kawamura F., Yanofsky S. Analysis of phi 29 and phi 15 genomes by bacterial restriction endonucleases, EcoR1 and Hpal. Virology. 1976 Mar;70(1):37–51. doi: 10.1016/0042-6822(76)90234-8. [DOI] [PubMed] [Google Scholar]
- Kawamura F., Ito J. Bacteriophage gene expression in sporulating cells of Bacillus subtilis 168. Virology. 1974 Dec;62(2):414–425. doi: 10.1016/0042-6822(74)90403-6. [DOI] [PubMed] [Google Scholar]
- Kawamura F., Saito H., Ikeda Y. A method for construction of specialized transducing phage rho 11 of Bacillus subtilis. Gene. 1979 Feb;5(2):87–91. doi: 10.1016/0378-1119(79)90095-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Linn T., Losick R. The program of protein synthesis during sporulation in Bacillus subtilis. Cell. 1976 May;8(1):103–114. doi: 10.1016/0092-8674(76)90191-4. [DOI] [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Mizukami T., Kawamura F., Takahashi H., Saito H. A physical map of the genome of the Bacillus subtilis temperate phage rho 11. Gene. 1980 Oct;11(1-2):157–162. doi: 10.1016/0378-1119(80)90095-5. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Losick R., Sonenshein A. L. Identification of a sporulation locus in cloned Bacillus subtilis deoxyribonucleic acid. J Bacteriol. 1980 Apr;142(1):331–334. doi: 10.1128/jb.142.1.331-334.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Mangan J., Huang W. M., Subbaiah T. V., Marmur J. Properties of the defective phage of Bacillus subtilis. J Mol Biol. 1968 Jun 28;34(3):413–428. doi: 10.1016/0022-2836(68)90169-1. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S., Huskey R. J. Deletion mutants of bacteriophage lambda. I. Isolation and initial characterization. J Mol Biol. 1971 Mar 14;56(2):369–384. doi: 10.1016/0022-2836(71)90471-2. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhaese H. J., Groscurth R. Apparent dependence of sporulation on synthesis of highly phosphorylated nucleotides in Bacillus subtilis. Proc Natl Acad Sci U S A. 1979 Feb;76(2):842–846. doi: 10.1073/pnas.76.2.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhaese H. J., Hoch J. A., Groscurth R. Studies on the control of development: isolation of Bacillus subtilis mutants blocked early in sporulation and defective in synthesis of highly phosphorylated nucleotides. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1125–1129. doi: 10.1073/pnas.74.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Sakaguchi K. Construction of a recombinant plasmid composed of B. subtilis leucine genes and a B. subtilis (natto) plasmid: its use as cloning vehicle in B. subtilis 168. Mol Gen Genet. 1978 Oct 24;165(3):269–276. doi: 10.1007/BF00332526. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Bott K. F. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1968 Apr;95(4):1439–1449. doi: 10.1128/jb.95.4.1439-1449.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann-Liebold B., Jauregui-Adell J., Wittmann H. G. Die primäre Proteinstruktur Temperatur-sensitiver Mutanten des Tabakmosaikvirus. II. Chemisch induzierte Mutanten. Z Naturforsch B. 1965 Dec;20(12):1235–1249. [PubMed] [Google Scholar]
- Wittmann H. G., Wittmann-Liebold B., Jauregui-Adell J. Die primäre Proteinstruktur Temperatur-sensitiver Mutanten des Tabakmosaikvirus I. Spontanmutanten. Z Naturforsch B. 1965 Dec;20(12):1224–1234. [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]