Abstract
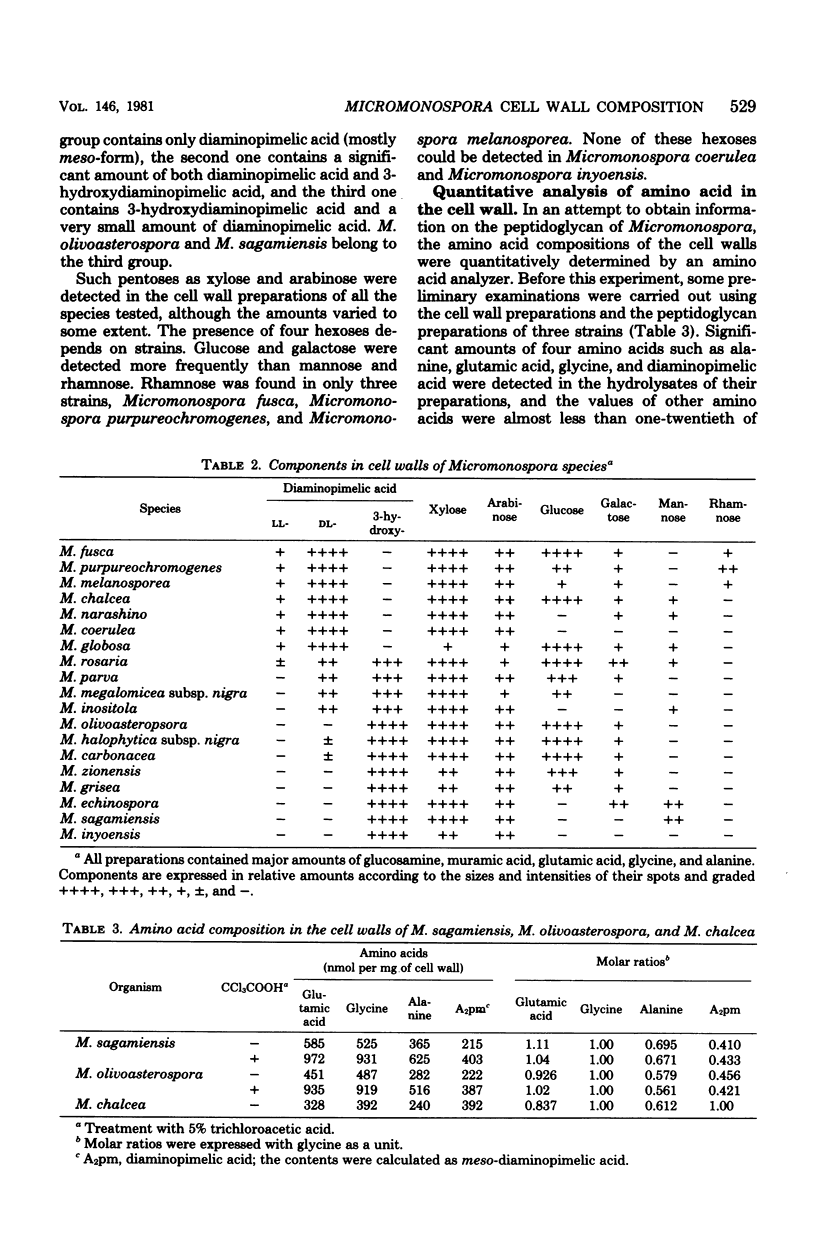
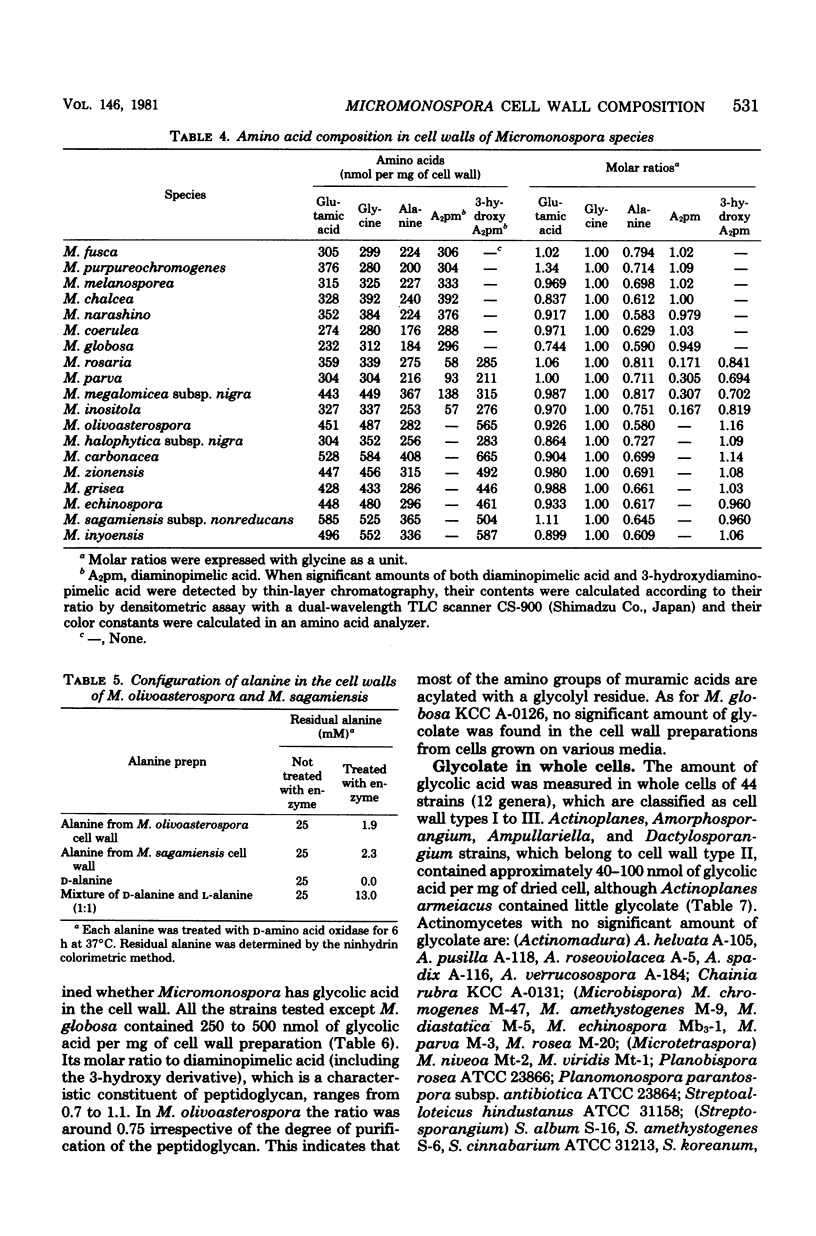
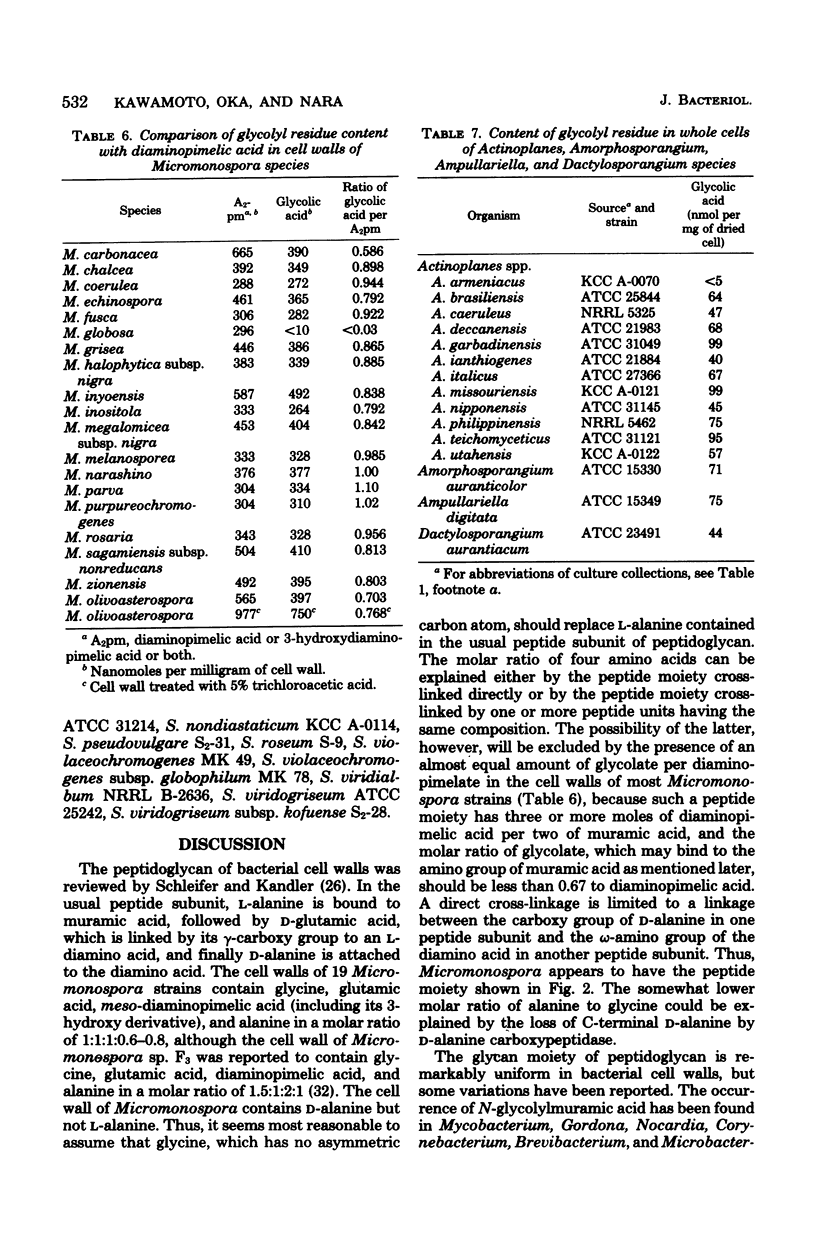
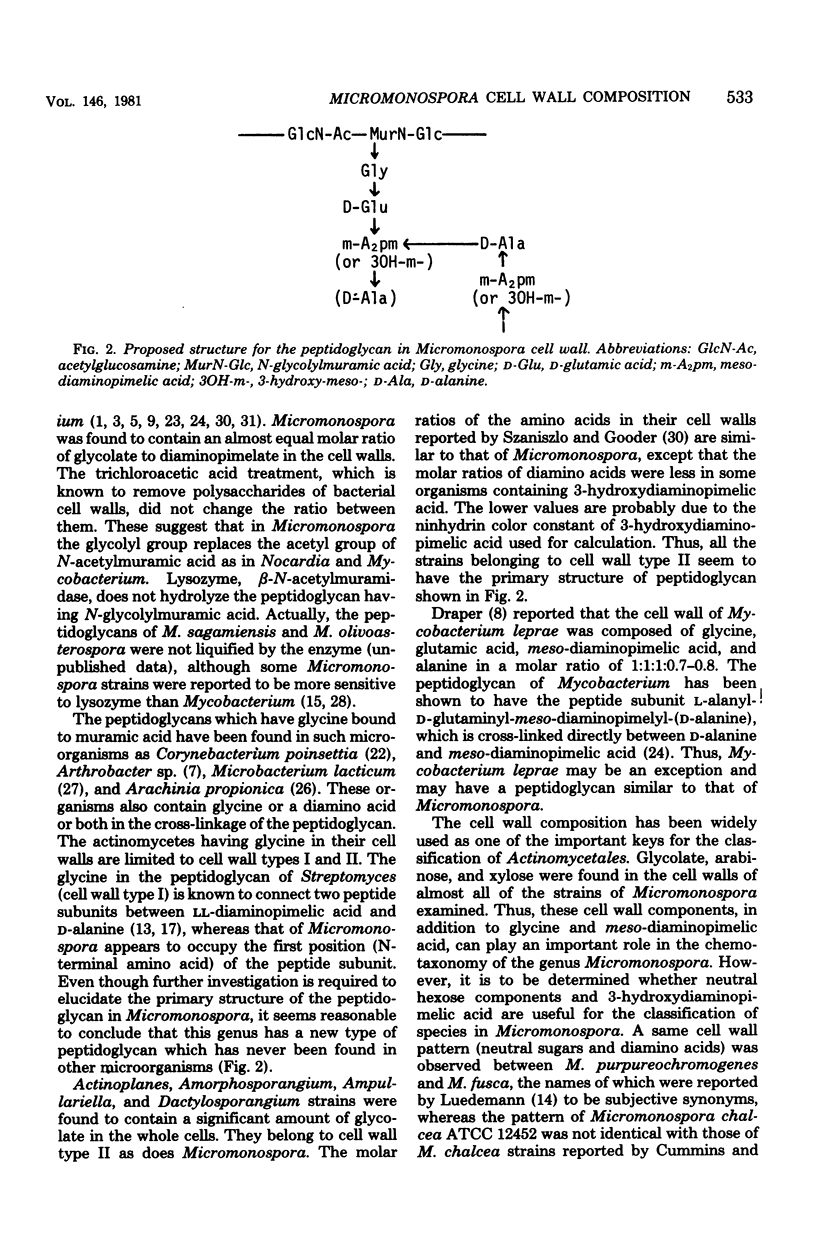
Cell walls of 19 Micromonospora species were analyzed for their components. All the cell walls had xylose and arabinose, but the presence of glucose, galactose, mannose, or rhamnose depended on the strain. Amino acids present in the walls consisted of glycine, glutamic acid, diaminopimelic acid, and alanine, in a molar ratio of approximately 1:1:1:0.6--0.8. 3-Hydroxydiaminopimelic acid, together with meso-diaminopimelic acid, was found in many species and was isolated from Micromonospora olivoasterospora to compare the color constant in an amino acid analyzer with that of meso-diaminopimelic acid. The cell walls of Micromonospora sagamiensis and M. olivoasterospora contained only D-alanine and not L-alanine. All species tested except Micromonospora globosa contained glycolate in an almost equimolar ratio to diaminopimelic acid in their cell walls. Among 45 strains of 12 genera examined, Actinoplanes, Ampullariella, Amorphosporangium, and Dactylosporangium species had a significant amount of glycolate in the whole cells. Based on these results, the primary structure of the peptidoglycan of Micromonospora is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Petit J. F., Wietzerbin-Falszpan J., Sinay P., Thomas D. W., Lederer E. L'acide N-glycolyl-muramique, constituant des parois de Mycobacterium smegmatis: Identification par spectrometrie de masse. FEBS Lett. 1969 Jul;4(2):87–92. doi: 10.1016/0014-5793(69)80203-6. [DOI] [PubMed] [Google Scholar]
- Azuma I., Thomas D. W., Adam A., Ghuysen J. M., Bonaly R., Petit J. F., Lederer E. Occurrence of N-glycolylmuramic acid in bacterial cell walls. A preliminary survey. Biochim Biophys Acta. 1970 Jun;208(3):444–451. doi: 10.1016/0304-4165(70)90217-5. [DOI] [PubMed] [Google Scholar]
- BECKER B., LECHEVALIER M. P., LECHEVALIER H. A. CHEMICAL COMPOSITION OF CELL-WALL PREPARATIONS FROM STRAINS OF VARIOUS FORM-GENERA OF AEROBIC ACTINOMYCETES. Appl Microbiol. 1965 Mar;13:236–243. doi: 10.1128/am.13.2.236-243.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. Studies on the cell-wall composition and taxonomy of Actinomycetales and related groups. J Gen Microbiol. 1958 Feb;18(1):173–189. doi: 10.1099/00221287-18-1-173. [DOI] [PubMed] [Google Scholar]
- Cziharz B., Schleifer K. H., Kandler O. A new type of peptide subunit in the murein of Arthrobacter strain J39. Biochemistry. 1971 Sep 14;10(19):3574–3578. doi: 10.1021/bi00795a014. [DOI] [PubMed] [Google Scholar]
- Draper P. Cell walls of Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1976 Jan-Jun;44(1-2):95–98. [PubMed] [Google Scholar]
- Guinand M., Vacheron M. J., Michel G. Structure des parois cellulaires des nocardia. I-isolement et composition des parois de Nocardia kirovani. FEBS Lett. 1970 Jan 15;6(1):37–39. doi: 10.1016/0014-5793(70)80036-9. [DOI] [PubMed] [Google Scholar]
- HOARE D. S., WORK E. The stereoisomers of alpha epsilon-diaminopimelic acid. II. Their distribution in the bacterial order Actinomycetales and in certain Eubacteriales. Biochem J. 1957 Mar;65(3):441–447. doi: 10.1042/bj0650441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyh-Bouille M., Bonaly R., Ghuysen J. M., Tinelli R., Tipper D. LL-diaminopimelic acid containing peptidoglycans in walls of Streptomyces sp. and of Clostridium perfringens (type A). Biochemistry. 1970 Jul 21;9(15):2944–2952. doi: 10.1021/bi00817a002. [DOI] [PubMed] [Google Scholar]
- Nara T., Kawamoto I., Okachi R., Takasawa S., Yamamoto M. New antibiotic XK-62-2 (sagamicin). II Taxonomy of the producing organism, fermentative production and characterization of sagamicin. J Antibiot (Tokyo) 1975 Jan;28(1):21–28. doi: 10.7164/antibiotics.28.21. [DOI] [PubMed] [Google Scholar]
- Nara T., Yamamoto M., Kawamoto I., Takayama K., Okachi R., Takasawa S., Sato T., Sato S. Fortimicins A and B, new aminoglycoside antibiotics. I. Producing organism, fermentation and biological properties of fortimicins. J Antibiot (Tokyo) 1977 Jul;30(7):533–540. doi: 10.7164/antibiotics.30.533. [DOI] [PubMed] [Google Scholar]
- Okachi R., Takasawa S., Sato T., Sato S., Yamamoto M., Kawamoto I., Nara T. Fortimicins A and B, new aminoglycoside antibiotics. II. Isolation, physico-chemical and chromatographic properties. J Antibiot (Tokyo) 1977 Jul;30(7):541–551. doi: 10.7164/antibiotics.30.541. [DOI] [PubMed] [Google Scholar]
- Perkins H. R. The configuration of 2,6-diamino-3-hydroxypimelic acid in microbial cell walls. Biochem J. 1969 Dec;115(4):797–805. doi: 10.1042/bj1150797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H. R. The use of photolysis of dinitrophenyl-peptides in structural studies on the cell-wall mucopeptide of Corynebacterium poinsettiae. Biochem J. 1967 Feb;102(2):29C–32C. doi: 10.1042/bj1020029c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. F., Adam A., Wietzerbin-Falszpan J., Lederer E., Ghuysen J. M. Chemical structure of the cell wall of Mycobacterium smegmatis. I. Isolation and partial characterization of the peptidoglycan. Biochem Biophys Res Commun. 1969 May 22;35(4):478–485. doi: 10.1016/0006-291x(69)90371-4. [DOI] [PubMed] [Google Scholar]
- Petit J. F. Structure chimique de la paroi des mycobactéries. Ann Microbiol (Paris) 1978 Jan;129(1):39–48. [PubMed] [Google Scholar]
- SOHLER A., ROMANO A. H., NICKERSON W. J. Biochemistry of the Actinomycetales. III. Cell wall composition and the action of lysozyme upon cells and cell walls of the Actinomycetales. J Bacteriol. 1958 Mar;75(3):283–290. doi: 10.1128/jb.75.3.283-290.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Plapp R., Kandler O. Die Aminosäuresequenz des Mureins von Microbacterium lacticum. Biochim Biophys Acta. 1968 Apr 9;154(3):573–582. [PubMed] [Google Scholar]
- Suput J., Lechevalier M. P., Lechevalier H. A. Chemical composition of variants of aerobic actinomycetes. Appl Microbiol. 1967 Nov;15(6):1356–1361. doi: 10.1128/am.15.6.1356-1361.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaniszlo P. J., Gooder H. Cell wall composition in relation to the taxonomy of some Actinoplanaceae. J Bacteriol. 1967 Dec;94(6):2037–2047. doi: 10.1128/jb.94.6.2037-2047.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINSTEIN M. J., LUEDEMANN G. M., ODEN E. M., WAGMAN G. H., ROSSELET J. P., MARQUEZ J. A., CONIGLIO C. T., CHARNEY W., HERZOG H. L., BLACK J. GENTAMICIN, A NEW ANTIBIOTIC COMPLEX FROM MICROMONOSPORA. J Med Chem. 1963 Jul;6:463–464. doi: 10.1021/jm00340a034. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI T. COMPARISON OF THE CELL-WALL COMPOSITION OF MORPHOLOGICALLY DISTINCT ACTINOMYCETES. J Bacteriol. 1965 Feb;89:444–453. doi: 10.1128/jb.89.2.444-453.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]



