Abstract
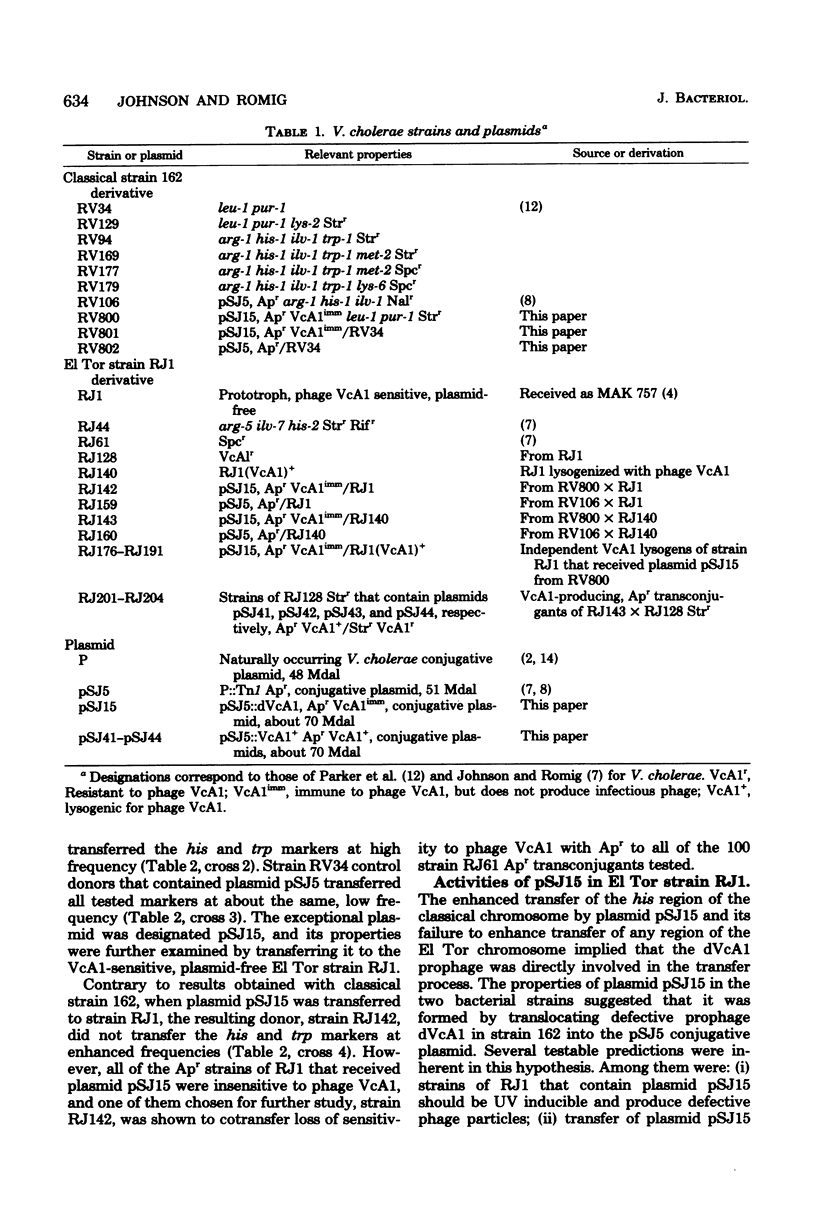
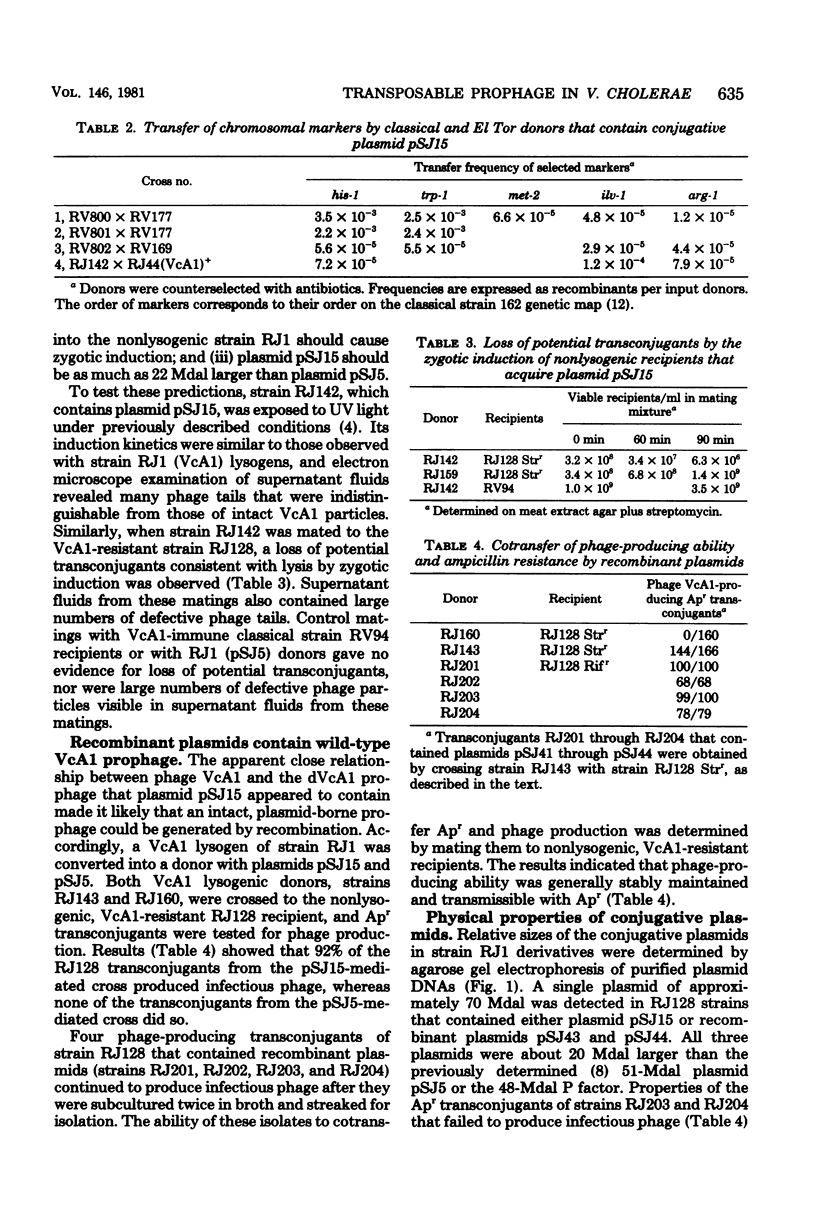
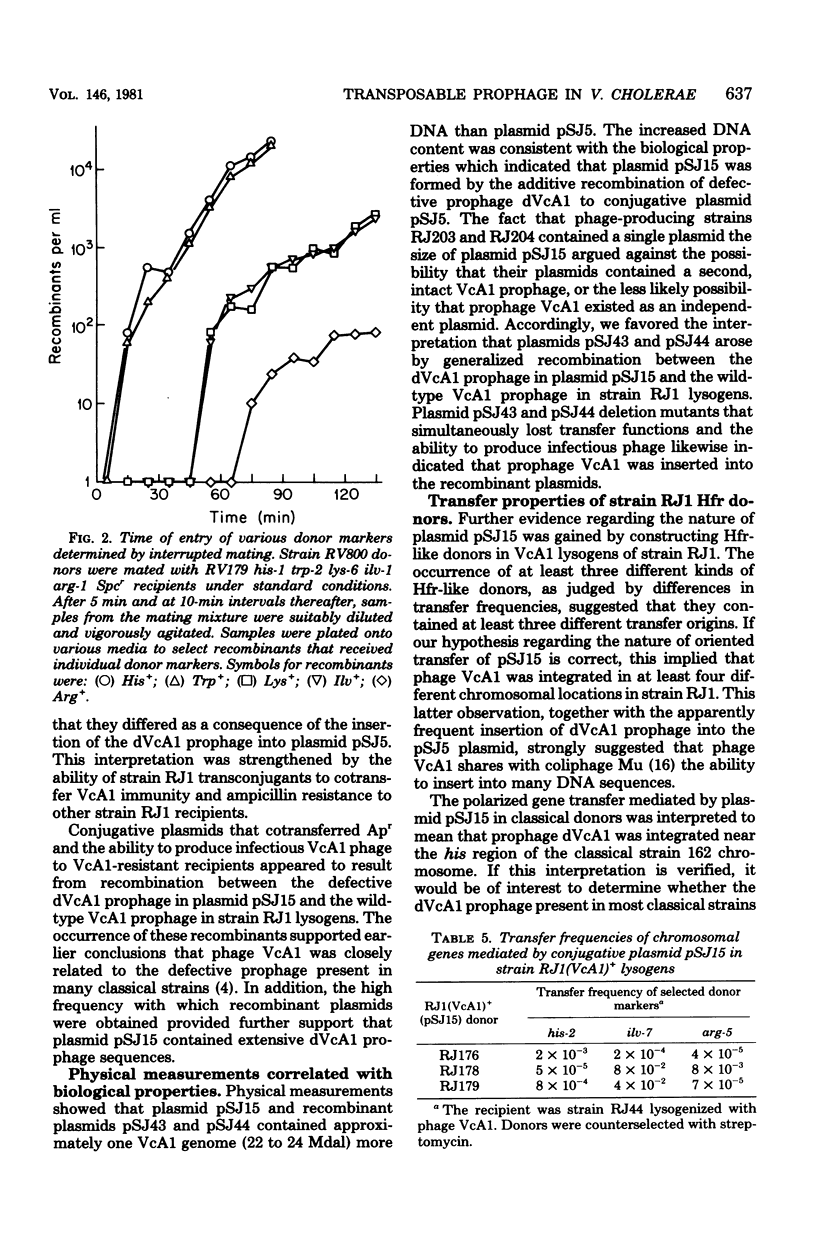
Evidence is presented that defective prophage dVcA1 in Vibrio cholerae strain 162 was transposed to the hybrid P::Tn1 plasmid pSJ5. Properties of the resulting conjugative plasmid, pSJ15, indicated that bacteriophage VcA1, like coliphage Mu, can insert at many sites. By analogy with other Hfr-like donors, the high-frequency, polarized chromosomal transfer mediated by plasmid pSJ15 in strain 162 appeared to depend on plasmid integration through the homologous dVcA1 sequences in both replicons. When strain 162(pSJ15) donors were mated to the nonlysogenic El Tor strain RJ1, many potential ampicillin-resistant transconjugants were zygotically induced. However, surviving transconjugants (i) were immune to phage VcA1, (ii) cotransferred immunity and ampicillin resistance to nonlysogenic recipients, and (iii) did not preferentially transfer any chromosomal markers. Recombinant plasmids that transferred wild-type VcA1 prophages were readily isolated from strain RJ1 (VcA1+) lysogens that contained plasmid pSJ15. Physical measurements revealed that plasmid pSJ15 and the recombinant plasmids were about one VcA1 genome (22 to 24 megadaltons) larger than the 51-megadalton pSJ5 plasmid. Similar Hfr-like donors were constructed by introducing plasmid pSJ15 into different strain RJ1 (VcA1+) lysogens. Transfer properties of these donors indicated that the VcA1 prophage was integrated at several sites in the strain RJ1 chromosome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baine W. B., Vasil M. L., Holmes R. K. Genetic mapping of mutations in independently isolated nontoxinogenic mutants of Vibrio cholerae. Infect Immun. 1978 Jul;21(1):194–200. doi: 10.1128/iai.21.1.194-200.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J. C., Romig W. R. Complete and Defective Bacteriophages of Classical Vibrio cholerae: Relationship to the Kappa Type Bacteriophage. J Virol. 1975 May;15(5):1231–1238. doi: 10.1128/jvi.15.5.1231-1238.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J. C., Romig W. R. Genetic basis of toxin production and pathogenesis in Vibrio cholerae: evidence against phage conversion. Infect Immun. 1975 Mar;11(3):445–452. doi: 10.1128/iai.11.3.445-452.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. R., Romig W. R. Transposon-facilitated recombination in Vibrio cholerae. Mol Gen Genet. 1979 Feb 16;170(1):93–101. doi: 10.1007/BF00268584. [DOI] [PubMed] [Google Scholar]
- Johnson S. R., Romig W. R. Vibrio cholerae hybrid sex factor that contains ampicillin transposon Tn1. J Bacteriol. 1979 Jan;137(1):531–536. doi: 10.1128/jb.137.1.531-536.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C., Gauthier D., Tate A., Richardson K., Romig W. R. Expanded linkage map of Vibrio cholerae. Genetics. 1979 Feb;91(2):191–214. doi: 10.1093/genetics/91.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C., Richardson S. H., Romig W. R. Production of Bacteriophage-Associated Materials by Vibrio cholerae: Possible Correlation with Pathogenicity. Infect Immun. 1970 Apr;1(4):417–420. doi: 10.1128/iai.1.4.417-420.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C., Romig W. R. Self-transfer and genetic recombination mediated by P, the sex factor of Vibrio cholerae. J Bacteriol. 1972 Nov;112(2):707–714. doi: 10.1128/jb.112.2.707-714.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Holmes R. K., Finkelstein R. A. Conjugal transfer of a chromosomal gene determining production of enterotoxin in vibrio cholerae. Science. 1975 Mar 7;187(4179):849–850. doi: 10.1126/science.1114331. [DOI] [PubMed] [Google Scholar]
- Weston L., Drexler H., Richardson S. H. Characterization of vibriophage VA-1. J Gen Virol. 1973 Oct;21:155–158. doi: 10.1099/0022-1317-21-1-155. [DOI] [PubMed] [Google Scholar]
- Zeldis J. B., Bukhari A. I., Zipser D. Orientation of prophage Mu. Virology. 1973 Sep;55(1):289–294. doi: 10.1016/s0042-6822(73)81033-5. [DOI] [PubMed] [Google Scholar]




