Abstract
A plasmid, termed pAC25, specifying biodegradation of 3-chlorobenzoate in a strain of Pseudomonas putida has been characterized. During growth of the plasmid-harboring cells with 3-chlorobenzoate, there was an accumulation of 3-chlorocatechol and beta-chloromuconic acid as intermediates and release of more than 80% of the chlorine in the form of inorganic chloride. The plasmid had a mean molecular mass of 68 x 10(6) daltons and was transmissible to a number of Pseudomonas species such as P. aeruginosa, P. putida strain PpG1, and P. putida strain PRS1. Transfer of pAC25 to various catechol-negative mutants of P. putida strain PRS1 showed that the chromosomally coded pyrocatechase was not complemented by the plasmid-specified pyrocatechase, which appeared to be specific for the chlorinated catechols. In contrast to benzoate, which was metabolized by the ortho pathway through beta-ketoadipate as an intermediate, the plasmid specified ortho cleavage of the chlorocatechols through maleylacetate as an intermediate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollag J. M. Microbial transformation of pesticides. Adv Appl Microbiol. 1974;18(0):75–130. doi: 10.1016/s0065-2164(08)70570-7. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M. Genetic basis of the biodegradation of salicylate in Pseudomonas. J Bacteriol. 1972 Nov;112(2):815–823. doi: 10.1128/jb.112.2.815-823.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Olson P. E., Frick T. D. Catabolism of 5-chlorosalicylate by a Bacillus isolated from the Mississippi River. Appl Environ Microbiol. 1979 Sep;38(3):379–384. doi: 10.1128/aem.38.3.379-384.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
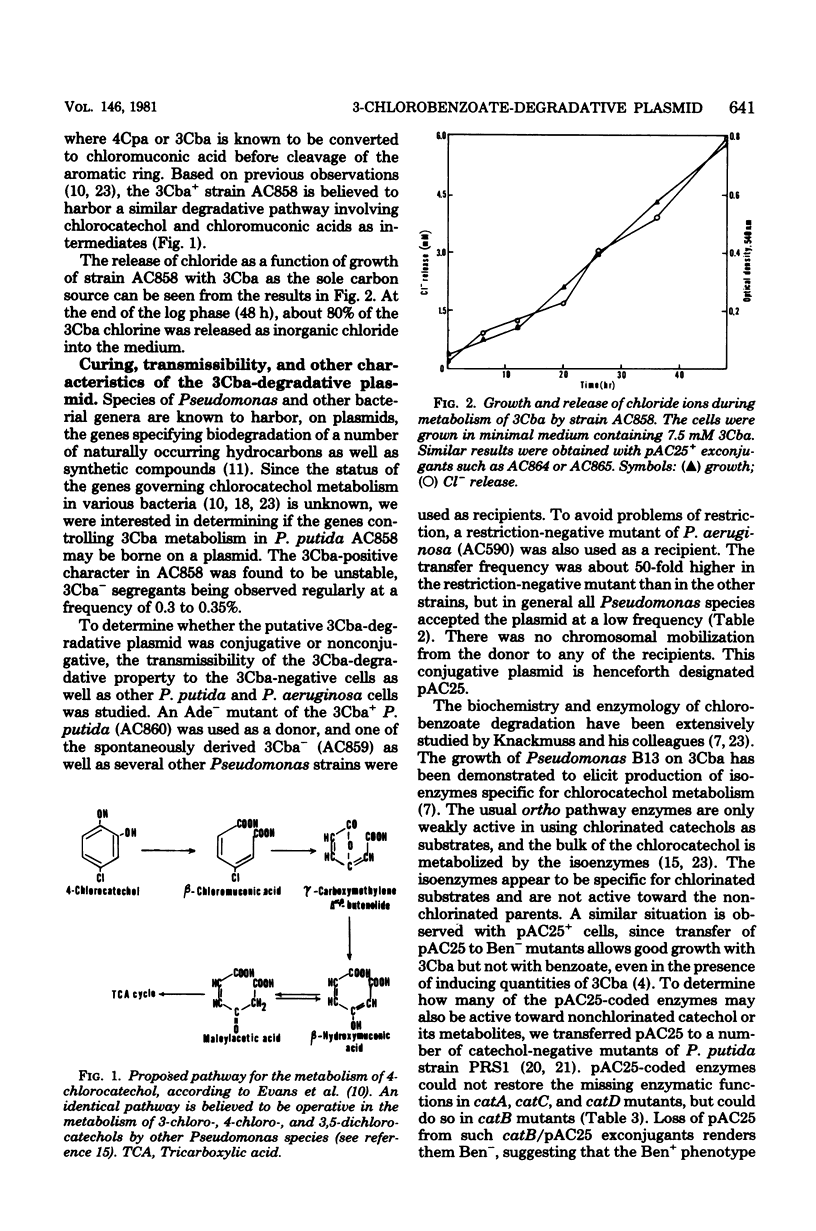
- Dorn E., Hellwig M., Reineke W., Knackmuss H. J. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch Microbiol. 1974;99(1):61–70. doi: 10.1007/BF00696222. [DOI] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J. 1978 Jul 15;174(1):73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C. Oxidation of phenol and benzoic acid by some soil bacteria. Biochem J. 1947;41(3):373–382. doi: 10.1042/bj0410373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C., Smith B. S., Moss P., Fernley H. N. Bacterial metabolism of 4-chlorophenoxyacetate. Biochem J. 1971 May;122(4):509–517. doi: 10.1042/bj1220509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friello D. A., Mylroie J. R., Gibson D. T., Rogers J. E., Chakrabarty A. M. XYL, a nonconjugative xylene-degradative plasmid in Pseudomonas Pxy. J Bacteriol. 1976 Sep;127(3):1217–1224. doi: 10.1128/jb.127.3.1217-1224.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J., Reineke W., Knackmuss H. J. Metabolism of 3-chloro-, 4-chloro-, and 3,5-dichlorobenzoate by a pseudomonad. Appl Environ Microbiol. 1979 Mar;37(3):421–428. doi: 10.1128/aem.37.3.421-428.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R. S. Cometabolism of the herbicide 2,3,6-trichlorobenzoate. J Agric Food Chem. 1971 Mar-Apr;19(2):291–293. doi: 10.1021/jf60174a020. [DOI] [PubMed] [Google Scholar]
- Negoro S., Shinagawa H., Nakata A., Kinoshita S., Hatozaki T., Okada H. Plasmid control of 6-aminohexanoic acid cyclic dimer degradation enzymes of Flavobacterium sp. KI72. J Bacteriol. 1980 Jul;143(1):238–245. doi: 10.1128/jb.143.1.238-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Parke D. Evolution of catabolic pathways. Biochem Soc Trans. 1976;4(3):468–472. doi: 10.1042/bst0040468. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. II. Enzymes of the protocatechuate pathway. J Biol Chem. 1966 Aug 25;241(16):3787–3794. [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Hybrid pathway for chlorobenzoate metabolism in Pseudomonas sp. B13 derivatives. J Bacteriol. 1980 May;142(2):467–473. doi: 10.1128/jb.142.2.467-473.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M., Hegeman G. D. Metabolism of benzoic acid by bacteria. Accumulation of (-)-3,5-cyclohexadiene-1,2-diol-1-carboxylic acid by mutant strain of Alcaligenes eutrophus. Biochemistry. 1971 Jun 22;10(13):2530–2536. doi: 10.1021/bi00789a017. [DOI] [PubMed] [Google Scholar]
- Rothera A. C. Note on the sodium nitro-prusside reaction for acetone. J Physiol. 1908 Dec 15;37(5-6):491–494. doi: 10.1113/jphysiol.1908.sp001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Conversion of chlorinated muconic acids into maleoylacetic acid. Biochem J. 1980 Oct 15;192(1):339–347. doi: 10.1042/bj1920339. [DOI] [PMC free article] [PubMed] [Google Scholar]



