Abstract
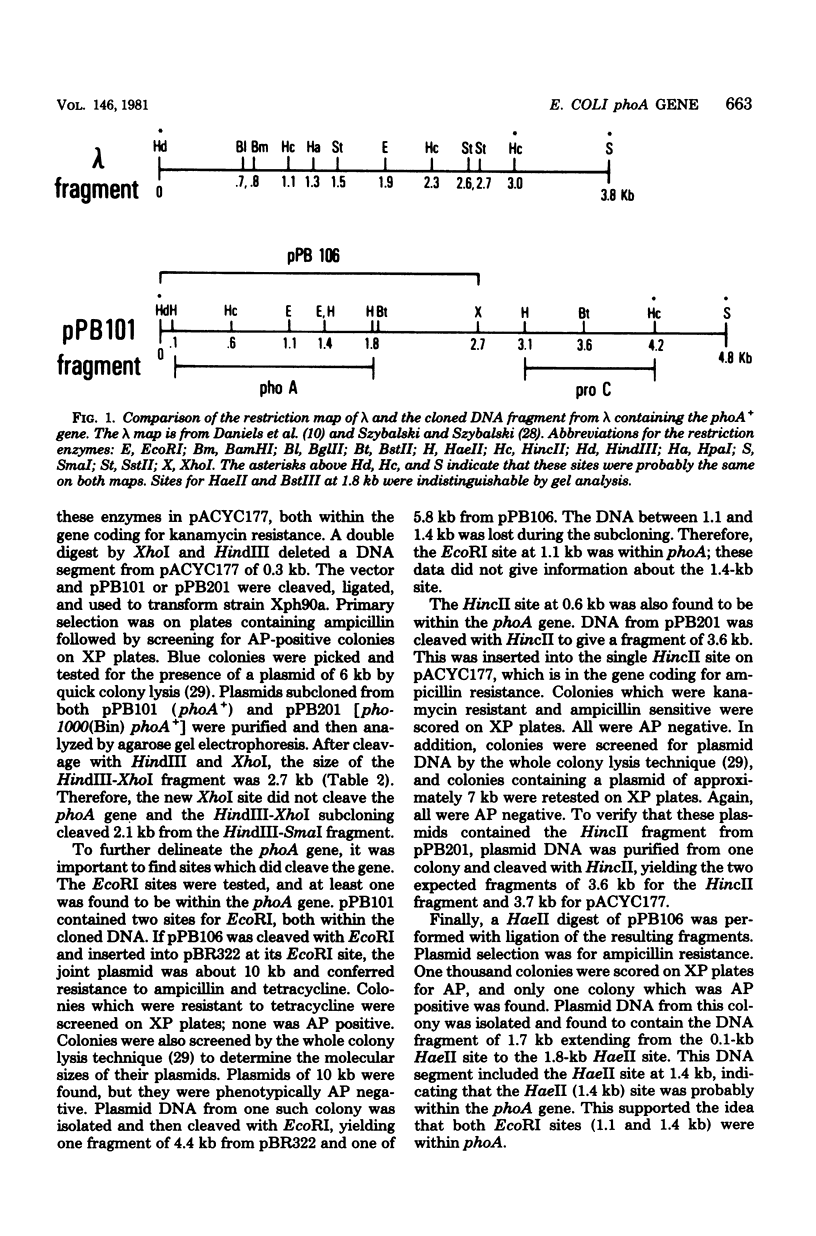
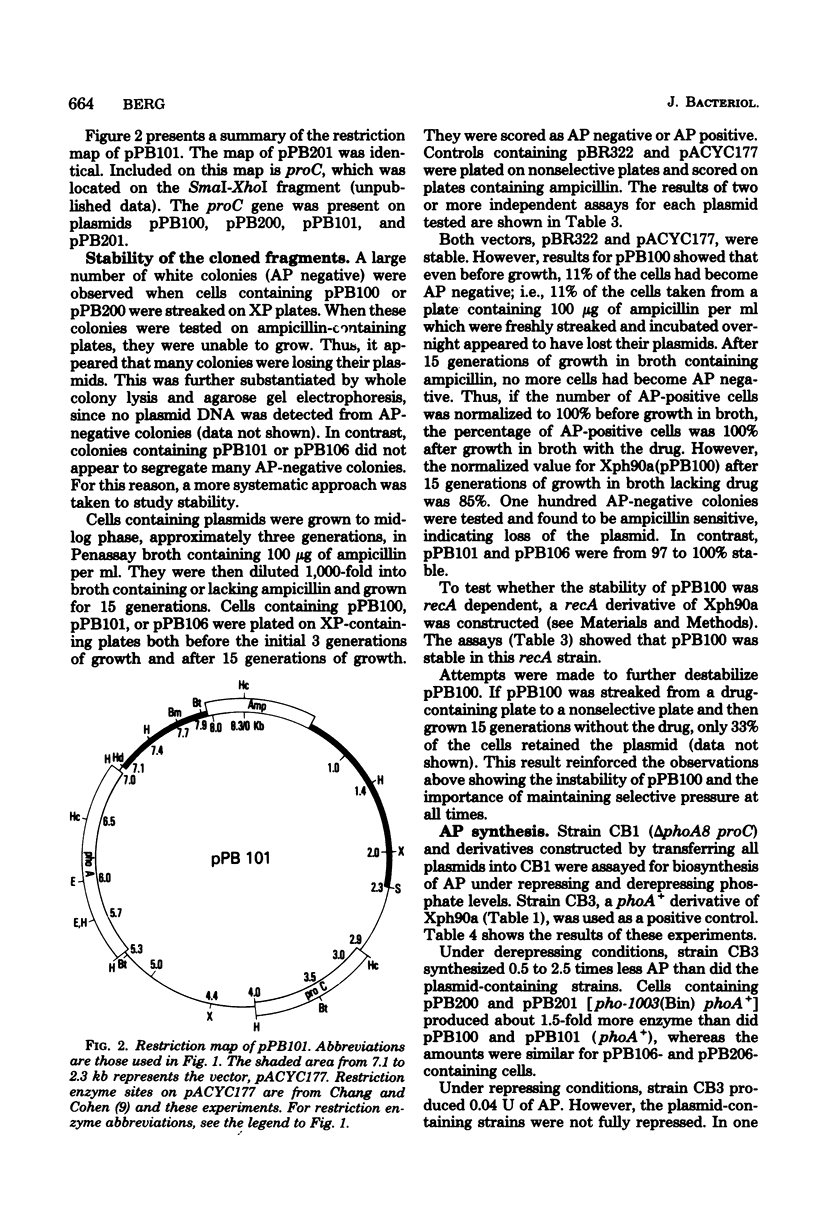
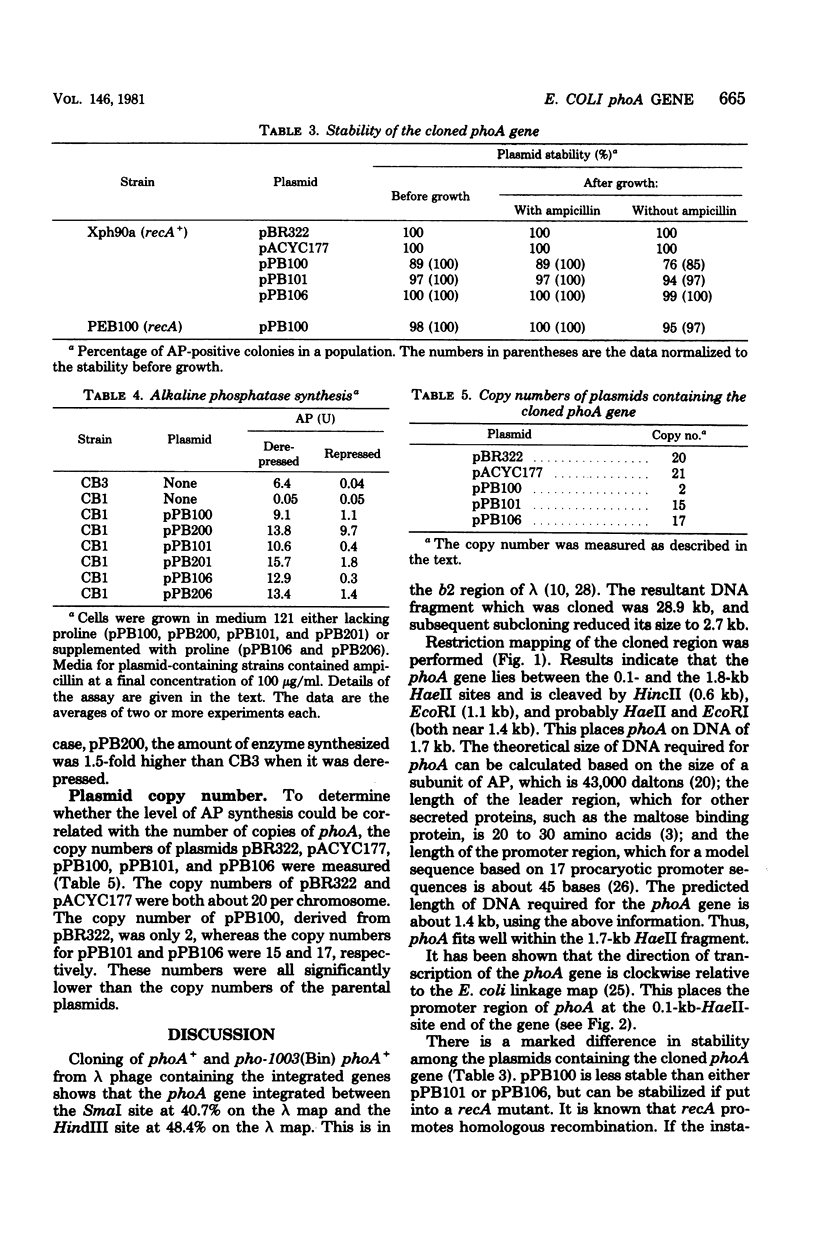
The Escherichia coli structural gene for alkaline phosphatase, phoA, and a promoter-like mutant of phoA, called pho-1003(Bin) phoA+, were cloned by using plasmid vectors. Initially, these genes were cloned on deoxyribonucleic acid fragments of 28.9 kilobases (kb). Subsequently, they were subcloned on fragments and 4.8 and then 2.7 kilobases. A restriction map was developed, and phoA was localized to a 1.7-kb region. The promoter end of the gene was inferred by its proximity to another gene cloned on the same deoxyribonucleic acid fragment, proC. The stability of the largest plasmid (33.3 kb) was found to be recA dependent, although the subcloned plasmids were stable in a recA+ strain. Synthesis of alkaline phosphatase directed by the phoA+ and pho-1003(Bin) phoA+ plasmids in a phoA deletion strain was assayed under repressing and derepressing levels of phosphate. These data were compared with the copy numbers of the plasmids. It was found that synthesis of alkaline phosphatase was tightly regulated, even under derepressing conditions: a copy number of 17 enabled cells to synthesize only about twofold more enzyme than did cells with 1 chromosomal copy of phoA+. Enzyme levels were also compared for cells containing pho-1003(Bin) phoA+ and phoA+.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P., Beckwith J. Escherichia coli mutants accumulating the precursor of a secreted protein in the cytoplasm. Nature. 1979 Feb 15;277(5697):538–541. doi: 10.1038/277538a0. [DOI] [PubMed] [Google Scholar]
- Beckwith J., Rossow P. Analysis of genetic regulatory mechanisms. Annu Rev Genet. 1974;8:1–13. doi: 10.1146/annurev.ge.08.120174.000245. [DOI] [PubMed] [Google Scholar]
- Berg P. E., Gayda R., Avni H., Zehnbauer B., Markovitz A. Cloning of Escherichia coli DNA that controls cell division and capsular polysaccharide synthesis. Proc Natl Acad Sci U S A. 1976 Mar;73(3):697–701. doi: 10.1073/pnas.73.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels D. L., de Wet J. R., Blattner F. R. New map of bacteriophage lambda DNA. J Virol. 1980 Jan;33(1):390–400. doi: 10.1128/jvi.33.1.390-400.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- GAREN A., GAREN S. Genetic evidence on the nature of the repressor for alkaline phosphatase in E. coli. J Mol Biol. 1963 May;6:433–438. doi: 10.1016/s0022-2836(63)80054-6. [DOI] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins S. T., Bennett P. M. Effect of mutations in deoxyribonucleic acid repair pathways on the sensitivity of Escherichia coli K-12 strains to nitrofurantoin. J Bacteriol. 1976 Mar;125(3):1214–1216. doi: 10.1128/jb.125.3.1214-1216.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer K., Pratt C., Torriani A. Genetic analysis of regulatory mutants of alkaline phosphatase of E. coli. Genetics. 1975 Nov;81(3):459–468. doi: 10.1093/genetics/81.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAMY M., HORECKER B. L. The localization of alkaline phosphatase in E. coli K12. Biochem Biophys Res Commun. 1961 Jun 2;5:104–108. doi: 10.1016/0006-291x(61)90020-1. [DOI] [PubMed] [Google Scholar]
- Nagahari K., Tanaka T., Hishinuma F., Kuroda M., Sakaguchi K. Control of tryptophan synthetase amplified by varying the numbers of composite plasmids in Escherichia coli cells. Gene. 1977 Mar;1(2):141–152. doi: 10.1016/0378-1119(77)90025-7. [DOI] [PubMed] [Google Scholar]
- PLOCKE D. J., LEVINTHAL C., VALLEE B. L. Alkaline phosphatase of Escherichia coli: a zinc metalloenzyme. Biochemistry. 1962 May 25;1:373–378. doi: 10.1021/bi00909a001. [DOI] [PubMed] [Google Scholar]
- Pogue-Geile K. L., DasSarma S., King S. R., Jaskunas S. R. Recombination between bacteriophage lambda and plasmid pBR322 in Escherichia coli. J Bacteriol. 1980 Jun;142(3):992–1003. doi: 10.1128/jb.142.3.992-1003.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt C. Kinetics and regulation of cell-free alkaline phosphatase synthesis. J Bacteriol. 1980 Sep;143(3):1265–1274. doi: 10.1128/jb.143.3.1265-1274.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy A., Fowler A., Zabin I., Beckwith J. Use of gene fusions to determine a partial signal sequence of alkaline phosphatase. J Bacteriol. 1979 Sep;139(3):932–939. doi: 10.1128/jb.139.3.932-939.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy A., Michaelis S., Beckwith J. Deletion map of the Escherichia coli structural gene for alkaline phosphatase, phoA. J Bacteriol. 1981 Jan;145(1):288–292. doi: 10.1128/jb.145.1.288-292.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy A., Michaelis S., Beckwith J. Use of gene fusions to determine the orientation of gene phoA on the Escherichia coli chromosome. J Bacteriol. 1981 Jan;145(1):293–298. doi: 10.1128/jb.145.1.293-298.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G. E., Walkinshaw M. D., Arnott S. A computer aided oligonucleotide analysis provides a model sequence for RNA polymerase-promoter recognition in E.coli. Nucleic Acids Res. 1978 Oct;5(10):3759–3773. doi: 10.1093/nar/5.10.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein S., Inouye M. Studies on the role of bacteriophage T7 lysozyme during phage infection. J Mol Biol. 1975 Jul 25;96(1):1–11. doi: 10.1016/0022-2836(75)90178-3. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Thompson R. C., Davis B. D. Extracellular labeling of nascent polypeptides traversing the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2830–2834. doi: 10.1073/pnas.74.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szybalski E. H., Szybalski W. A comprehensive molecular map of bacteriophage lambda. Gene. 1979 Nov;7(3-4):217–270. doi: 10.1016/0378-1119(79)90047-7. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Telford J., Boseley P., Schaffner W., Birnstiel M. Novel screening procedure for recombinant plasmids. Science. 1977 Jan 28;195(4276):391–393. doi: 10.1126/science.318763. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., Sarthy A., Beckwith J. Escherichia coli pleiotropic mutant that reduces amounts of several periplasmic and outer membrane proteins. J Bacteriol. 1979 Oct;140(1):229–239. doi: 10.1128/jb.140.1.229-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Willsky G. R., Bennett R. L., Malamy M. H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973 Feb;113(2):529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble D. D., Taylor D. P., Rownd R. H. Method for obtaining more-accurate covalently closed circular plasmid-to-chromosome ratios from bacterial lysates by dye-buoyant density centrifugation. J Bacteriol. 1977 Apr;130(1):148–153. doi: 10.1128/jb.130.1.148-153.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagil E., Bracha M., Lifshitz Y. The regulatory nature of the phoB gene for alkaline phosphatase synthesis in Escherichia coli. Mol Gen Genet. 1975;137(1):11–16. doi: 10.1007/BF00332537. [DOI] [PubMed] [Google Scholar]



