Abstract
Patients with chronic fatigue syndrome (CFS) report substantial symptom worsening after exercise. However, the time course over which this develops has not been explored. Therefore, the objective of this study was to investigate the influence of exercise on subjective symptoms and on cognitive function in CFS patients in natural settings using a computerized ecological momentary assessment method, which allowed us to track the effects of exercise within and across days. Subjects were 9 female patients with CFS and 9 healthy women. A watch-type computer was used to collect real time data on physical and psychological symptoms and cognitive function for one week before and two weeks after a maximal exercise test. For each variable, we investigated temporal changes after exercise using multilevel modeling. Following exercise, physical symptoms did get worse but not until a five-day delay in CFS patients. Despite this, there was no difference in the temporal pattern of changes in psychological symptoms or in cognitive function after exercise between CFS patients and controls. In conclusion, physical symptoms worsened after a several day delay in patients with CFS following exercise while psychological symptoms or cognitive function did not change after exercise.
Keywords: chronic fatigue syndrome, ecological momentary assessment, multilevel modeling
Introduction
Chronic fatigue syndrome (CFS) is an illness whose etiology has been not established and which is characterized by severe fatigue of more than six months duration accompanied by at least four of a list of eight rheumatological, infectious and neuropsychiatric symptoms. One particularly common and disabling symptom is the complaint that even minimal exertion produces a dramatic worsening of the entire symptom complex – with much worse fatigue, less activity, and more associated symptoms. Previously, we used paper and pencil questionnaires to investigate this complaint and have found that self-reported fatigue increased 4 days after physical exertion (1). However, the time course over which symptom worsening develops remains unknown.
Although asking a research volunteer to fill out multiple paper diaries would be one way to determine this time course, recent work has pointed out major problems with this approach (2, 3). Stone et al (2) have reported that subjects often did not complete such diaries at specified times. Such faked compliance obviously jeopardizes the research. Recent advances in computer technology permit a solution to this problem, namely the collection of electronic diary data in real time; this process is known as computerized ecological momentary assessment (cEMA) (4, 5) and obviates problems with recall bias or faked compliance (2, 3). Early studies used palmtop devices; however, these devices are not wearable, leading to other problems such as forgetting to take it with her/him or putting it away in a place where its alarm cannot be heard (6). To solve these problems, we used a wearable watch-type computer device.
Therefore, the aim of this study was to use a wrist-worn (cEMA) device to investigate temporal changes of subjective symptoms in the natural environment after exercise because of the common complaint of symptom worsening in CFS following exertion (7). Because an earlier study showed that exercise resulted in a reduction of physical activity after a delay of five days (8), we hypothesized that subjective symptoms would not worsen immediately after exercise but instead would worsen a few days later. We also assessed cognitive function using a continuous performance test (9-11) because cognitive functioning is often impaired in CFS and may be negatively affected by exercise (12, 13).
Methods
Subjects
The subjects studied were 10 healthy women and 10 female patients with CFS who fulfilled both the original (14) and revised (15) Center for Disease Control working case definitions. To reduce patient heterogeneity, the subjects also met the following criteria: illness duration of less than 6 years and at least 7 symptoms reported as being “substantial,” “severe,” or “very severe” in the month before recruitment. All subjects signed informed consent, reviewed and approved by the UMDNJ New Jersey Medical School’s Institutional Review Board. Data from one healthy and one CFS subject were dropped from analysis because of technical problems limiting data collection.
Procedures
Subjects were asked to wear the watch-type computer device on their wrist to provide real time entries into a computerized EMA method for one week before and for two weeks after a maximal exercise test. For the maximal exercise test, subjects were seated on an electronically braked cycle ergometer (Sensormedics, Loma Linda, California), with the seat and handlebars adjusted for optimal performance, and given a few minutes to habituate to the cycle and various monitoring devices. The exercise test began with a three-minute warm-up pedaling at 20 watts. Subjects were instructed to maintain a pedaling cadence between 60-70 rpm. Following the warm-up period, work intensity increased by 5 watts every 20 seconds until volitional exhaustion or a point when the subject could no longer maintain the prescribed pedal rate. The subjects were verbally encouraged to continue pedaling as long as possible. A maximal effort was determined based on meeting at least two of the following criteria: 1) respiratory exchange ratio ≥ 1.1, 2) change in O2 uptake (VO2) < 200ml with an increase in work, 3) rating of perceived exertion of 17 or greater and 4) achieving 85% of age predicted max heart rate.
Data acquisition device and momentary sampling design
The wrist-mounted computer used in this study is OnHand, a watch-type computer, (52 grams, Matsucom Inc., Aurola, Colorado, U.S.A.) loaded with software custom-made for this project (The custom-made software will be supplied to readers by the first author upon request). Before subjects began to wear the device, they were individually trained on how to use the device with our own manual. It took about one hour for the training in using the device. The software displayed questions about symptoms to the subject on the liquid crystal display (LCD) screen of the device, one item at a time. Participants used the “Navigation Joystick” to scroll through a 21-step visual analog scale (0-100) reflecting symptom severity and pushed the “ENTER” button to record the severity of each symptom at that moment. Time and date were recorded after each diary completion. Prior assessments were not accessible to subjects for review or modification. Once started, an assessment had to be completed without stopping. Pausing more than 60 seconds between pushing buttons generated a beep, an event record, and a skip to the next item.
Data entry occurred at several times throughout the day. Subjects responded to an auditory signal emitted randomly by the computer with an average duration of 4 hours; they also input data when going to bed and awakening. After completing a pre-bedtime assessment, random sampling stopped until an assessment was completed after awakening.
Subjects were able to delay responding for up to 30 minutes if occupied with other demands. Auditory signals prompted 15 minutes later and then 15 minutes after the first reminder, if subjects delayed responding. Subjects were instructed to report how they felt at the moment of recording if they had delayed responding. If subjects did not respond to the signal at 30 minutes after the missed prompt, the assessment was missed.
Measurement
The electronic diary asked subjects about the following physical and psychological symptoms: “fatigue”, “sore throat”, “headache”, “weak muscles”, “achy joints”, “achy muscles”, “nausea”, “short of breath”, “tender glands”, “energetic”, “depressed”, “nervous”, “happy”, “can’t concentrate”, and “calm”. These items were presented on the LCD screen in random order. In addition, “quality of sleep” was asked during the data collection epoch following awakening.
After completing this symptom inventory, subjects were asked to respond to a continuous performance test, which was a one-back memory task (11) in this study, requiring subjects to compare the currently visible single digit with the previous single digit and to move the joystick to the right side (different) or to the left side (same) for three minutes. Each digit was presented on the LCD screen of the watch-type computer for 0.5 s with an inter-stimulus interval of 2 s if subjects did not respond while the digit was on the screen and 1 second if subjects responded while the digit was on the screen. Hit rate and reaction time of correct responses were calculated from each result of the task.
Statistical analysis
To reduce the numbers of variables, we divided subjective symptoms into physical and psychological categories and analyzed the sums of each of these. The physical symptoms consisted of “fatigued”, “sore throat”, “headachy”, “weak muscles”, “achy joints”, “achy muscles”, “nausea”, “short of breath”, “tender glands”, and “energetic” (summed as minus value), and the psychological symptoms consisted of “depressed”, “nervous”, “happy” (summed as minus value), “can’t concentrate”, and “calm” (summed as minus value). Cronbach’s alpha coefficients of the physical symptoms and the psychological symptoms were 0.93 and 0.89 respectively.
Regarding physical and psychological symptoms, hit rate, and reaction time of correct responses in the continuous performance test, we investigated the difference between CFS patients and healthy controls in temporal changes after exercise using multilevel modeling (SAS PROC MIXED procedure (16)) as follows:
Level 1 equation:
Level 2 equations:
where DAY1ij is days after exercise with the baseline period of seven days before exercise numbered as 0, X is the delay for worsening of symptoms after exercise, TIME is the time of the day divided into three categories: 6:00:00-11:59:59, 12:00:00-18:00:00 and 18:00:00-05:59:59, and GROUP is a dichotomous variable representing CFS patients or healthy controls. We explored the delay for worsening with changing X between two and nine. Reports after midnight were included as part of the next day.
Regarding “quality of sleep” recorded once a day when awake, we investigated the difference between CFS patients and healthy controls in temporal changes after exercise using multilevel modeling (SAS PROC MIXED procedure) as follows:
Level 1 equation:
Level 2 equations:
where DAY1 is days after exercise with the baseline period of seven days before exercise numbered as 0, DAY2 is days after the fifth day after exercise with days before the day numbered as 0, and GROUP is a dichotomous variable representing CFS patients or healthy controls. All analyses were estimated using full-information maximum likelihood for inferential purposes. A p value of < .05 was considered significant. All final models were a significantly better fit than their associated unconditional (without predictors) models when assessing improvement in goodness of fit.
Results
Data for one week before and 11 days after exercise were analyzed because data beyond this point was not reliably collected due to battery failure. There was no significant difference in age between the CFS group (44.6±10.6 years) and the control group (40.3±13.5 years). The averages of the rates of compliance with the sampling plan were 98.5% (range = 95.9-100%) in CFS patients and 97.9% (range = 96.2-100%) in healthy controls for subjective symptoms and 93.6% (range = 76.6-100%) in CFS patients and 91.0% (range = 82.3-97.3%) in healthy controls for the continuous performance test. There was no significant difference in either compliance rate between CFS patients and healthy controls.
Physical symptoms after exercise (Table 2, Fig. 2)
Table 2.
Multilevel model estimates for physical symptom score*
| (Total record number = 1542) | Mean (S.E.) effect | p value |
|---|---|---|
| Intercept (γ00) | 12.8 (38.5) | 0.74 |
| GROUP (γ01)† | 323.3 (54.3) | < 0.001 |
| DAY1 (γ10) | -1.9 (2.4) | 0.42 |
| DAY2 (γ20) | 3.0 (3.7) | 0.42 |
| GROUP × DAY2 interaction (γ21)† | 3.8 (1.4) | 0.009 |
| TIME (γ30) | < 0.001 | |
| Morning | -49.5 (6.5) | < 0.001 |
| Afternoon | -50.1 (6.6) | < 0.001 |
| Night | referent | . |
| GROUP × TIME interaction (γ31)† | 0.002 | |
| Morning | 33.9 (9.2) | 0.001 |
| Afternoon | 25.9 (9.1) | 0.008 |
| Night | referent | . |
S.E., standard error; CFS, chronic fatigue syndrome group; CON, healthy control group.
Based on the model: physical symptom score = γ00 + γ01GROUP + γ10DAY2 + γ20DAY3 + γ21DAY3 × GROUP + γ30TIME + γ31TIME × GROUP + residuals.
Coefficients for the chronic fatigue syndrome group.
Figure 2.
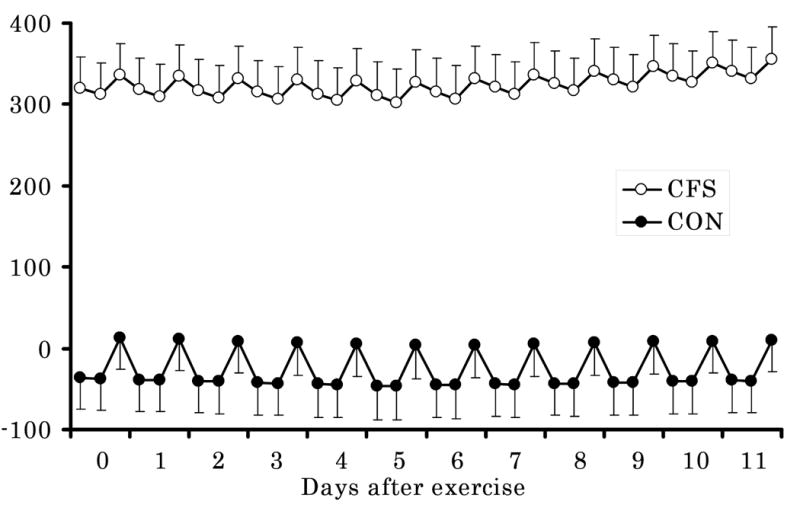
Temporal changes in physical symptom scores after exercise in chronic fatigue syndrome patients and healthy controls estimated by linear mixed models with the baseline period of one week before exercise numbered as “day 0”. There were significant main effects of the group and time of the day and a significant group × time of the day interaction, and a group × day interaction only after the fifth day. Closed squares indicate scores of chronic fatigue syndrome patients and closed circles indicate scores of healthy controls. Error bars shows standard error of means of physical symptom scores. CFS, patients with chronic fatigue syndrome; CON, healthy controls.
There were significant main effects of GROUP (γ01, F(1, 16) = 40.2, p < 0.001) and TIME (γ30, F(2, 32) = 41.1, p < 0.001) and significant GROUP × TIME (γ31, F(2, 32) = 7.80, p = 0.002) and GROUP × DAY2 (γ21, t = 2.63, p = 0.009) interactions only when X (i.e., day after exercise) was 5. No significant interaction was found on other post-exercise days. The final model for physical symptoms is as follows:
Level 1 equation:
Level 2 equations:
These results showed that the CFS group had more physical symptoms, that physical symptoms got worse after the fifth day after exercise (i.e., no change in the first five days after exercise and then progressive increase in symptoms subsequently) only in the CFS group, and that physical symptoms in the morning and in the afternoon were less severe than at night for both groups, while the differences in symptoms between night and the other two periods were smaller for CFS than for controls. This latter result suggests less diurnal variation in symptoms for CFS than for controls.
Psychological symptoms after exercise (Table 3)
Table 3.
Multilevel model estimates for psychological symptom score*
| (Total record number = 1548) | Mean (S.E.) effect | p value |
|---|---|---|
| Intercept (γ00) | -128.0 (16.9) | < 0.001 |
| GROUP (γ01)† | 132.7 (26.1) | < 0.001 |
| TIME (γ30) | 0.004 | |
| Morning | -15.4 (3.7) | < 0.001 |
| Afternoon | -14.9 (3.8) | < 0.001 |
| Night | referent | . |
| GROUP × TIME interaction (γ31)† | 0.009 | |
| Morning | 16.2 (5.2) | 0.004 |
| Afternoon | 12.3 (5.2) | 0.02 |
| Night | referent | . |
S.E., standard error; CFS, chronic fatigue syndrome group; CON, healthy control group.
Based on the model: psychological symptom score = γ00 + γ01GROUP + γ30TIME + γ31TIME × GROUP + residuals.
Coefficients for the chronic fatigue syndrome group.
There were significant main effects of GROUP (γ01, F(1, 16) = 30.1, p < 0.001) and TIME (γ30, F(2, 32) = 6.73, p = 0.004) and a significant interaction of GROUP × TIME (γ31, F(2, 32) = 5.45, p = 0.009). There were no significant interactions of GROUP × DAY1 or GROUP × DAY2, or significant main effects of DAY1 or DAY2. Therefore, the final model is as follows:
Level 1 equation:
Level 2 equations:
These results indicated that the CFS group had more psychological symptoms than controls and that exercise did not affect this relation. Moreover, psychological symptoms in the morning and in the afternoon were less severe than at night for both groups, while the differences in symptoms between night and the other two periods were smaller in the CFS group than in the control group.
Continuous performance test after exercise
Regarding hit rate of the continuous performance test after exercise, there were no significant main effects or interactions.
Regarding reaction time for correct responses in the continuous performance test after exercise, there was only a significant main effect of TIME (γ30, F(2, 32) = 11.6, p < 0.001), which shows that reaction time was significantly better in the afternoon than at night in both groups (Table 4).
Table 4.
Multilevel model estimates for hit rate in continuous performance test*
| (Total record number = 1539) | Mean (S.E.) effect | p value |
|---|---|---|
| Intercept (γ00) | 868.3 (22.9) | < 0.001 |
| TIME (γ30) | < 0.001 | |
| Morning | 1.9 (6.2) | 0.76 |
| Afternoon | -25.5 (6.1) | < 0.001 |
| Night | referent | . |
S.E., standard error; CFS, chronic fatigue syndrome group; CON, healthy control group.
Based on the model: hit rate = γ00 + γ30TIME + residuals.
Quality of sleep after exercise (Table 5)
Table 5.
Multilevel model estimates for sleep quality*
| (Total record number = 305) | Mean (S.E.) effect | p value |
|---|---|---|
| Intercept (γ00) | 75.3 (5.9) | < 0.001 |
| GROUP(γ01)† | -32.1 (8.3.) | 0.001 |
S.E., standard error; CFS, chronic fatigue syndrome group; CON, healthy control group.
Based on the model: sleep quality score = γ00 + γ01GROUP + residuals.
Coefficients for the chronic fatigue syndrome group.
Regarding quality of sleep after exercise, there was only a significant main effect of GROUP (γ01, F(1, 16) = 14.8, p = .001), which shows that CFS patients report worse sleep than controls and that exercise did not affect this relation.
Discussion
CFS patients have an unusual complaint – that even minimal exertion produces a dramatic worsening of their entire symptom complex beginning a day or two later. However, very little work has focused on the scientific validation of this complaint. In our previous work, we showed that activity as monitored by actigraphy diminished but not until 5 days after the period of exertion (8). Importantly, the present follow-up study, focusing on symptoms, showed the same effect. Physical symptoms did not change following exertion until 5 days later. This prolonged delay appears strikingly different from the sort of post-exertional symptom worsening that occurs in cardiopulmonary disease. This delay may distinguish CFS from other fatiguing illnesses. In addition, psychological symptoms did not get worse over time. This dissociation between physical and psychological symptoms is important because it suggests that physical symptom worsening is not associated with altered mood.
We believe that a major reason we were able to capture this delay relates to our use of a cEMA technique. Subjects were asked to provide information about symptoms repeatedly throughout the day and across many days, and responses had to be made at the time of query. Doing this allows for two important differences from paper and pencil diaries: first, time-stamped data assures they represent the patient’s actual response at that particular time, and second, providing repeated input as to symptoms should give more reliable data to assess for change over time.
Finding a prolonged period between the time of exertion and the symptom exacerbation raises questions of mechanism. The cause of this delayed worsening in physical symptoms may attributable to some immunological change in CFS patients (17). In particular, Cannon et al. (18) reported that plasma α2-macroglobulin concentrations in CFS patients were significantly higher than those of control subjects on four of the five days after exercise, and these changes might be associated with the delayed worsening in physical symptoms. In addition, impaired hypothalamo-pituitary-adrenal axis may contribute to the symptoms (19).
An earlier momentary assessment study done on CFS patients doing their usual activities (20) noted that fatigue and arousal were worst early and late in the day for both patients and controls with patients having worse symptoms across the day. Our data were similar to the earlier report in the point that physical symptoms were less severe in the afternoon. In addition, both patients and controls had more psychological symptoms in mornings and afternoons than in nights with the patients having higher scores on these symptoms across the entire day. As was the case for physical symptoms, the CFS group showed less variability of psychological symptoms across the entire day. This diurnal pattern of psychological symptoms was also similar to those in earlier studies (20, 21).
In contrast to increased physical symptoms after exercise, cognitive function did not deteriorate over time although many studies have reported impairment in cognitive function at baseline (22-24) or after exercise (11, 12, 25) for patients with CFS. The reason for this discrepancy may relate to our use of the continuous performance test in this study although one of our previous studies showed that exercise did not alter cognitive function (26). Another possible reason is the time when cognitive tests were performed. In many previous studies, cognitive tests were performed just after exercise while tests were done for a longer period in this study.
There were some limitations in this study. First, the sample size was relatively small and there was some loss of data in the second week after exercise, which might cause difficulty in generalizability of the result in the present study. Therefore, further studies with a larger sample size for a longer period are needed to confirm the results of this study. Second, the subjects in this study were only women. Therefore, it is not possible to apply the results of this study to men with CFS. Third, objective activity was not measured in this study. Therefore, the relationship between subjective symptoms and objective activity remains unknown. Finally, the regression coefficient of the GROUP × DAY2 interaction was not so large compared with the main effect of the GROUP in the model for the physical symptoms. Therefore, the clinical significance should be interpreted with caution.
Nonetheless the results, using an EMA technique, are clear: CFS patients showed worsening of physical symptoms beginning 5 days after performing a standard exercise test to volitional exhaustion; in contrast, exercise did not adversely affect psychological symptoms or cognitive performance. These findings suggest that symptom exacerbation is not a function of altered mood. Documenting symptom worsening itself and the delay preceding it is important in that it provides an outcome measure for therapeutic trials. Moreover, its existence may be useful in moving the diagnosis of CFS from patients’ complaint to objective measures of altered function.
Figure 1.
A picture of the watch-type computer device used in the present study. It is easy to manipulate the device using the joystick to lengthen or shorten the bar like visual analogue scale and pushing the enter-button to record the scale.
Table 1.
Performance during the maximal exercise test
| variables | CFS | Controls | p value |
|---|---|---|---|
| Age | 44.6 (10.6) | 40.3 (13.5) | 0.47 |
| Peak heart rate* (beats/min) | 168.6 (20.2) | 164.4 (21.8) | 0.70 |
| Peak RPE† | 18.3 (1.2) | 17.8 (2.0) | 0.57 |
| Peak VO2* (ml/kg/min) | 21.9 (3.3) | 25.5 (5.7) | 0.16 |
| Peak RER* | 1.13 (0.08) | 1.22 (0.13) | 0.13 |
Values are expressed mean (S.D.).
RPE, rating of perceived exertion; Peak VO2, peak O2 uptake; RER, respiratory exchange ratio.
Sample size of CFS patients was seven because of data missing.
Sample size of CFS patients was eight because of data missing.
Acknowledgments
This study was supported by NIH AI-32247 (BHN). This material is the result of work supported with resources and use of facilities at the VA New Jersey Health Care System (VANJHCS), East Orange, NJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sisto S, LaManca J, Cordero DL, Bergen MT, Drastal S, Boda WL, Tapp WN, Natelson BH. Metabolic and cardiovascular effects of a progressive exercise test in patients with chronic fatigue syndrome. Am J Med. 1996;100:634–640. doi: 10.1016/s0002-9343(96)00041-1. [DOI] [PubMed] [Google Scholar]
- 2.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient non-compliance with paper diaries. Br Med J. 2002;324:1193–1194. doi: 10.1136/bmj.324.7347.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone AA, Broderick JE, Shiffman SS, Schwartz JE. Understanding recall of weekly pain from a momentary assessment perspective: absolute agreement, between- and within-person consistency, and judged change in weekly pain. Pain. 2004;107:61–69. doi: 10.1016/j.pain.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Ann Behav Med. 1994;16:199–202. [Google Scholar]
- 5.Stone AA, Shiffman S. Capturing momentary, self-report data: a proposal for reporting guidelines. Ann Behav Med. 2002;24:236–243. doi: 10.1207/S15324796ABM2403_09. [DOI] [PubMed] [Google Scholar]
- 6.Aaron LA, Mancl L, Turner JA, Sawchuk CN, Klein KM. Reasons for missing interviews in the daily electronic assessment of pain, mood, and stress. Pain. 2004;109:389–393. doi: 10.1016/j.pain.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Paul L, Wood L, Behen WMH, Maclaren WM. Demonstration of delayed recovery from fatiguing exercise in chronic fatigue syndrome. Eur J Neurol. 1999;6:63–69. doi: 10.1046/j.1468-1331.1999.610063.x. [DOI] [PubMed] [Google Scholar]
- 8.Sisto SA, Tapp WN, LaManca JJ, Ling W, Korn LR, Nelson AJ, Natelson BH. Physical activity before and after exercise in women with chronic fatigue syndrome. Q J Med. 1998;91:465–473. doi: 10.1093/qjmed/91.7.465. [DOI] [PubMed] [Google Scholar]
- 9.Rutschmann J, Cornblatt B, Erlenmeyer-Kimling L. Sustained attention in children at risk for schizophrenia. Arch Gen Psychiatry. 1977;34:571–575. doi: 10.1001/archpsyc.1977.01770170081007. [DOI] [PubMed] [Google Scholar]
- 10.Bremer DA. MINI-CPT: a continuous performance test program for the Tandy PC-8 pocket computer. Behav Res Meth Instrum Comp. 1989;21:11–14. [Google Scholar]
- 11.Harvey PO, Le Bastard G, Pochon JB, Levy R, Allilaire JF, Dubois B, Fossati P. Executive functions and updating of the contents of working memory in unipolar depression. J Psychiatr Res. 2004;38:567–576. doi: 10.1016/j.jpsychires.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 12.LaManca JJ, Sisto SA, DeLuca J, Johnson SK, Lange G, Pareja J, Cook S, Natelson BH. Influence of exhaustive treadmill exercise on cognitive functioning in chronic fatigue syndrome. Am J Med. 1998;105:59S–65S. doi: 10.1016/s0002-9343(98)00171-5. [DOI] [PubMed] [Google Scholar]
- 13.Blackwood SK, MacHale SM, Power MJ, Goodwin GM, Lawrie SM. Effects of exercise on cognitive and motor function in chronic fatigue syndrome and depression. J Neurol Neurosurg Psychiatry. 1998;65:541–546. doi: 10.1136/jnnp.65.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes GP, Kaplan JE, Gantz NM, Komaroff AL, Schonberger LB, Straus SE, Jones JF, Dubois RE, Cunningham-Rundles C, Pahwa S. Chronic fatigue syndrome: a working case definition. Ann Intern Med. 1988;108:387–389. doi: 10.7326/0003-4819-108-3-387. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The International Chronic Fatigue Syndrome Study Group. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;21:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 16.Litcher-Kelly L, Stone AA, Broderick JE, Scwartz JE. Association among pain intensity, sensory characteristics, affective qualities, and activity limitations in patients with chronic pain: a momentary, within-person perspective. J Pain. 2004;5:433–439. doi: 10.1016/j.jpain.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Tomoda A, Joudoi T, Raab EM, Matsumoto T, Park TH, Miike T. Cytokine production and modulation: comparison of patients with chronic fatigue syndrome and normal controls. Psychiatry Res. 2005;134:101–104. doi: 10.1016/j.psychres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Cannon JG, Angel JB, Ball RW, Abad LW, Fagioli L, Komaroff AL. Acute phase responses and cytokine secretion in chronic fatigue syndrome. J Clin Immunol. 1999;19:414–421. doi: 10.1023/a:1020558917955. [DOI] [PubMed] [Google Scholar]
- 19.Roberts AD, Wessely S, Chalder T, Papadopoulos A, Cleare AJ. Salivery cortisol response to awakening in chronic fatigue syndrome. Br J Psychiatry. 2004;18:136–141. doi: 10.1192/bjp.184.2.136. [DOI] [PubMed] [Google Scholar]
- 20.Stone AA, Broderick JE, Porter LS, Krupp L. Fatigue and mood in chronic fatigue syndrome patients: results of a momentary assessment protocol examining fatigue and mood levels and diurnal patterns. Ann Behav Med. 1994;16:228–234. [Google Scholar]
- 21.Wood C, Magnello ME, Sharpe MC. Fluctuations in perceived energy and mood among patients with chronic fatigue syndrome. J R Soc Med. 1992;85:195–198. doi: 10.1177/014107689208500405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall PS, Forstot M, Callies A, Peterson PK, Schenck CH. Cognitive slowing and working memory difficulties in chronic fatigue syndrome. Psychosom Med. 1997;59:58–66. doi: 10.1097/00006842-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Vollmer-Conna U, Wakefield D, Lloyd A, Hickie I, Lemon J, Bird KD, Westbrook RF. Cognitive deficits in patients suffering from chronic fatigue syndrome, acute infective illness or depression. Br J Psychiatry. 1997;171:377–381. doi: 10.1192/bjp.171.4.377. [DOI] [PubMed] [Google Scholar]
- 24.Dobbs BM, Dobbs AR, Kiss I. Working memory deficits associated with chronic fatigue syndrome. J Int Neuropsychol Soc. 2001;7:285–293. doi: 10.1017/s1355617701733024. [DOI] [PubMed] [Google Scholar]
- 25.DeLuca J, Christodoulou C, Diamond BJ, Rosenstein ED, Kramer N, Natelson BH. Working memory deficits in chronic fatigue syndrome: differentiating between speed and accuracy of information processing. J Int Neuropsychol Soc. 2004;10:101–109. doi: 10.1017/S1355617704101124. [DOI] [PubMed] [Google Scholar]
- 26.Cook DB, Nagelkirk PR, Peckerman A, Poluri A, Mores J, Natelson BH. Exercise and cognitive performance in Chronic Fatigue Syndrome. Med Sci Sports Exerc. 2005;37:1460–1467. doi: 10.1249/01.mss.0000179921.48404.ef. [DOI] [PubMed] [Google Scholar]


