Abstract
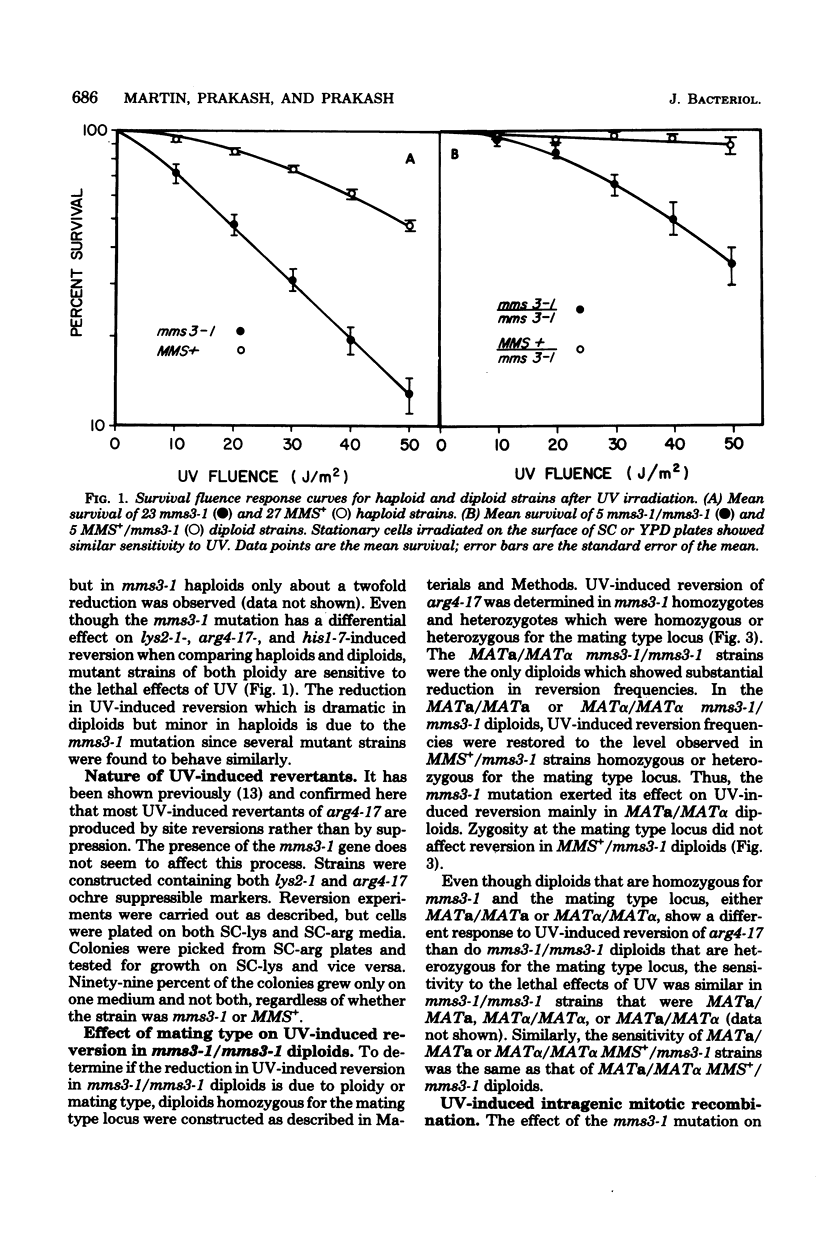
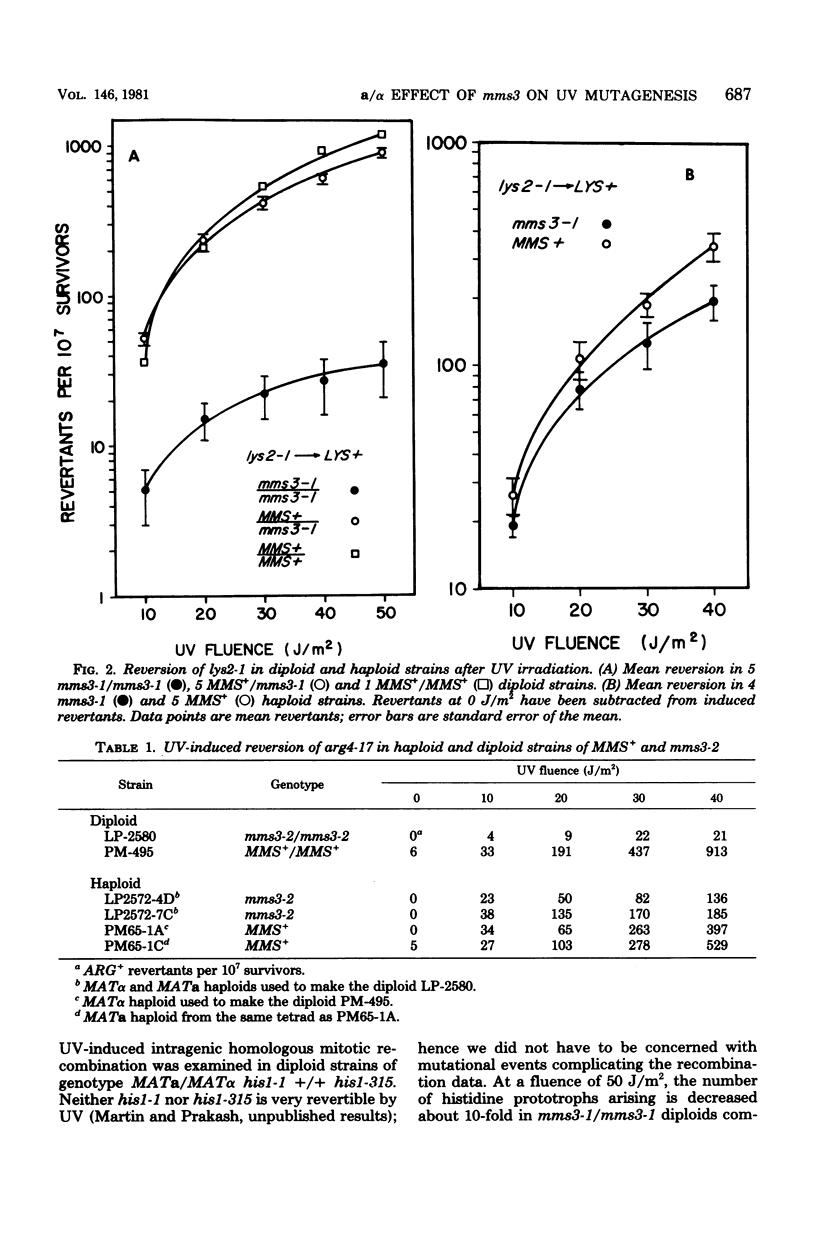
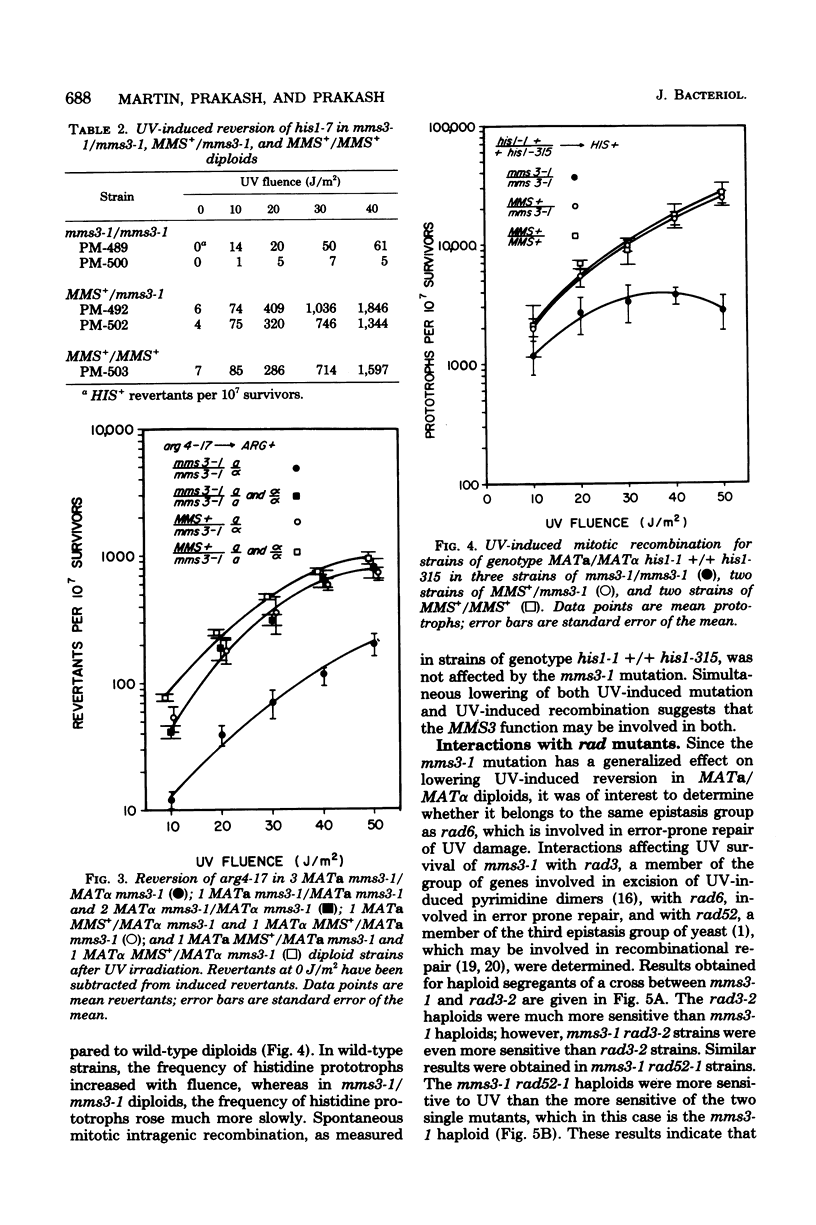
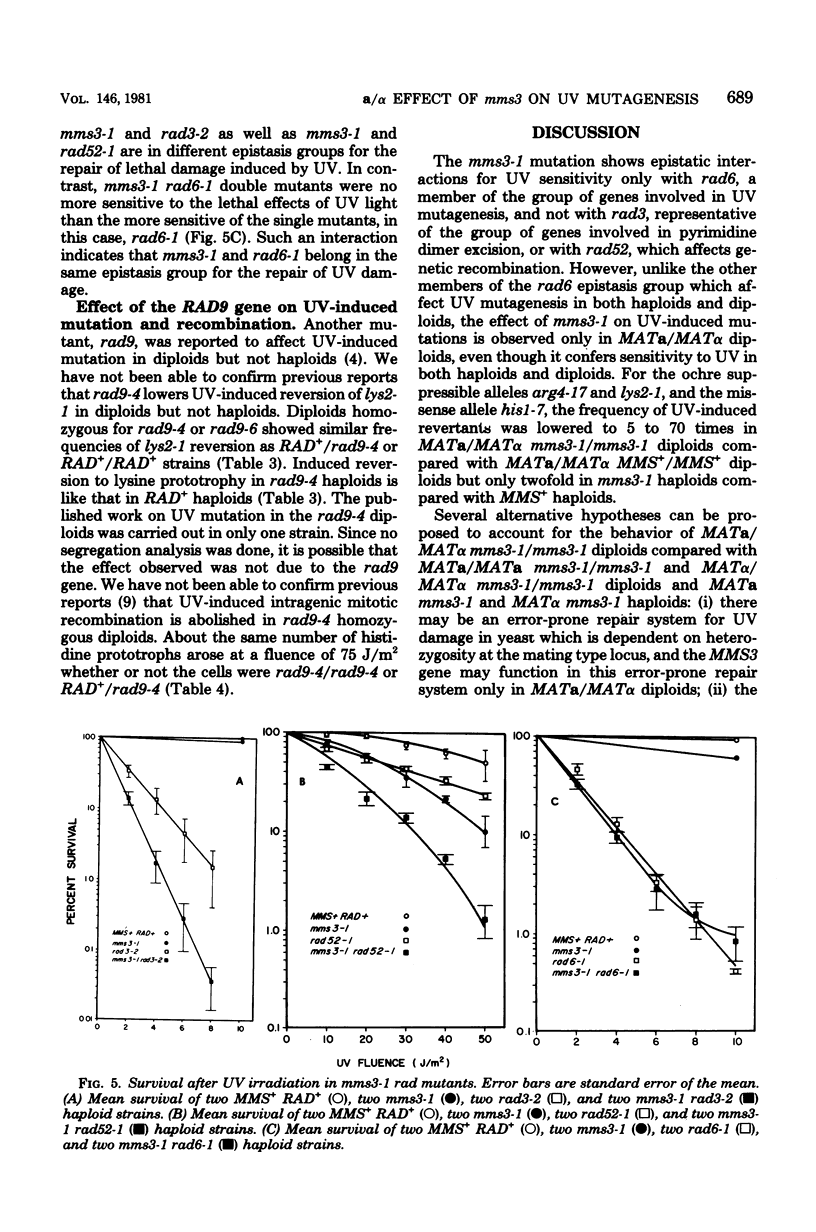
A new gene involved in error-prone repair of ultraviolet (UV) damage has been identified in Saccharomyces cerevisiae by the mms3-1 mutation. UV-induced reversion is reduced in diploids that are homozygous for mms3-1, only if they are also heterozygous (MATa/MAT alpha) at the mating type locus. The mms3-1 mutation has no effect on UV-induced reversion either in haploids or MATa/MATa or MAT alpha/MAT alpha diploids. The mutation confers sensitivity to UV and methyl methane sulfonate in both haploids and diploids. Even though mutation induction by UV is restored to wild-type levels in MATa/MATa mms3-1/mms3-1 or MAT alpha/MAT alpha mms3-1/mms3-1 diploids, such strains still retain sensitivity to the lethal effects of UV. Survival after UV irradiation in mms3-1 rad double mutant combinations indicates that mms3-1 is epistatic to rad6-1 whereas non-epistatic interactions are observed with rad3 and rad52 mutants. When present in the homozygous state in MATa/MAT alpha his1-1/his1-315 heteroallelic diploids, mms3-1 was found to lower UV-induced mitotic recombination.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox B. S., Parry J. M. The isolation, genetics and survival characteristics of ultraviolet light-sensitive mutants in yeast. Mutat Res. 1968 Jul-Aug;6(1):37–55. doi: 10.1016/0027-5107(68)90101-2. [DOI] [PubMed] [Google Scholar]
- Cox B., Game J. Repair systems in Saccharomyces. Mutat Res. 1974 Aug;26(4):257–264. doi: 10.1016/s0027-5107(74)80023-0. [DOI] [PubMed] [Google Scholar]
- Deschamps J., Wiame J. M. Mating-type effect on cis mutations leading to constitutivity of ornithine transaminase in diploid cells of Saccharomyces cerevisiae. Genetics. 1979 Jul;92(3):749–758. doi: 10.1093/genetics/92.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt F., Kowalskí S., Laskowski W. The effects of three rad genes on UV induced mutation rates in haploid and diploid Saccharomyces cells. Mol Gen Genet. 1975;136(3):261–272. doi: 10.1007/BF00334021. [DOI] [PubMed] [Google Scholar]
- Errede B., Cardillo T. S., Sherman F., Dubois E., Deschamps J., Wiame J. M. Mating signals control expression of mutations resulting from insertion of a transposable repetitive element adjacent to diverse yeast genes. Cell. 1980 Nov;22(2 Pt 2):427–436. doi: 10.1016/0092-8674(80)90353-0. [DOI] [PubMed] [Google Scholar]
- Game J. C., Mortimer R. K. A genetic study of x-ray sensitive mutants in yeast. Mutat Res. 1974 Sep;24(3):281–292. doi: 10.1016/0027-5107(74)90176-6. [DOI] [PubMed] [Google Scholar]
- Grant P., Sánchez L., Jiménez A. Cryptopleurine resistance: genetic locus for a 40S ribosomal component in Saccharomyces cerevisiae. J Bacteriol. 1974 Dec;120(3):1308–1314. doi: 10.1128/jb.120.3.1308-1314.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne D. C. Identification of nonsense codons in yeast. J Mol Biol. 1969 Jul 14;43(1):71–75. doi: 10.1016/0022-2836(69)90079-5. [DOI] [PubMed] [Google Scholar]
- Kowalski S., Laskowski W. The effect of three rad genes on survival, inter- and intragenic mitotic recombination in Saccharomyces. I. UV irradiation without photoreactivation or liquid-holding post-treatment. Mol Gen Genet. 1975;136(1):75–86. doi: 10.1007/BF00275450. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Christensen R. B. Ultraviolet-induced reversion of cyc1 alleles in radiation-sensitive strains of yeast. III. rev3 mutant strains. Genetics. 1979 Jun;92(2):397–408. doi: 10.1093/genetics/92.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. W., Stewart J. W., Sherman F., Christensen R. Specificity and frequency of ultraviolet-induced reversion of an iso-1-cytochrome c ochre mutant in radiation-sensitive strains of yeast. J Mol Biol. 1974 May 5;85(1):137–162. doi: 10.1016/0022-2836(74)90134-x. [DOI] [PubMed] [Google Scholar]
- Lemoine Y., Dubois E., Wiame J. M. The regulation of urea amidolyase of Saccharomyces cerevisiae: mating type influence on a constitutivity mutation acting in cis. Mol Gen Genet. 1978 Nov 9;166(3):251–258. [PubMed] [Google Scholar]
- Lemontt J. F. Induction of forward mutations in mutationally defective yeast. Mol Gen Genet. 1972;119(1):27–42. doi: 10.1007/BF00270441. [DOI] [PubMed] [Google Scholar]
- Lemontt J. F. Mutants of yeast defective in mutation induced by ultraviolet light. Genetics. 1971 May;68(1):21–33. doi: 10.1093/genetics/68.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L. Lack of chemically induced mutation in repair-deficient mutants of yeast. Genetics. 1974 Dec;78(4):1101–1118. doi: 10.1093/genetics/78.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L., Prakash S. Isolation and characterization of MMS-sensitive mutants of Saccharomyces cerevisiae. Genetics. 1977 May;86(1):33–55. doi: 10.1093/genetics/86.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L., Prakash S. Three additional genes involved in pyrimidine dimer removal in Saccharomyces cerevisiae: RAD7, RAD14 and MMS19. Mol Gen Genet. 1979 Nov;176(3):351–359. doi: 10.1007/BF00333097. [DOI] [PubMed] [Google Scholar]
- Prakash L. Repair of pyrimidine dimers in radiation-sensitive mutants rad3, rad4, rad6 and rad9 of Saccharomyces cerevisiae. Mutat Res. 1977 Oct;45(1):13–20. doi: 10.1016/0027-5107(77)90038-0. [DOI] [PubMed] [Google Scholar]
- Prakash S., Prakash L., Burke W., Montelone B. A. Effects of the RAD52 Gene on Recombination in SACCHAROMYCES CEREVISIAE. Genetics. 1980 Jan;94(1):31–50. doi: 10.1093/genetics/94.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J., Sherman F. Dependence on mating type for the overproduction of iso-2-cytochrome c in the yeast mutant CYC7-H2. Genetics. 1980 Apr;94(4):891–898. doi: 10.1093/genetics/94.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogerson L., McLaughlin C., Wakatama E. Modification of ribosomes in cryptopleurine-resistant mutants of yeast. J Bacteriol. 1973 Nov;116(2):818–822. doi: 10.1128/jb.116.2.818-822.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



