Abstract
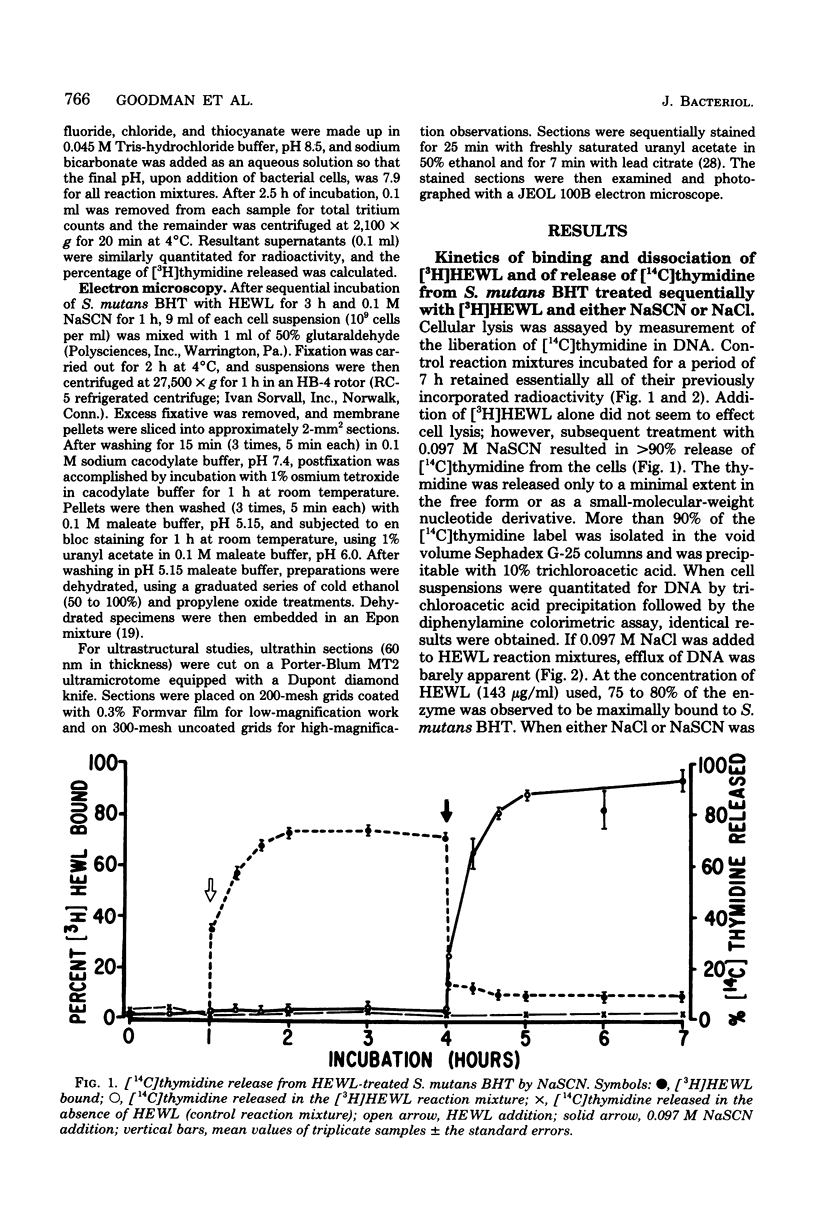
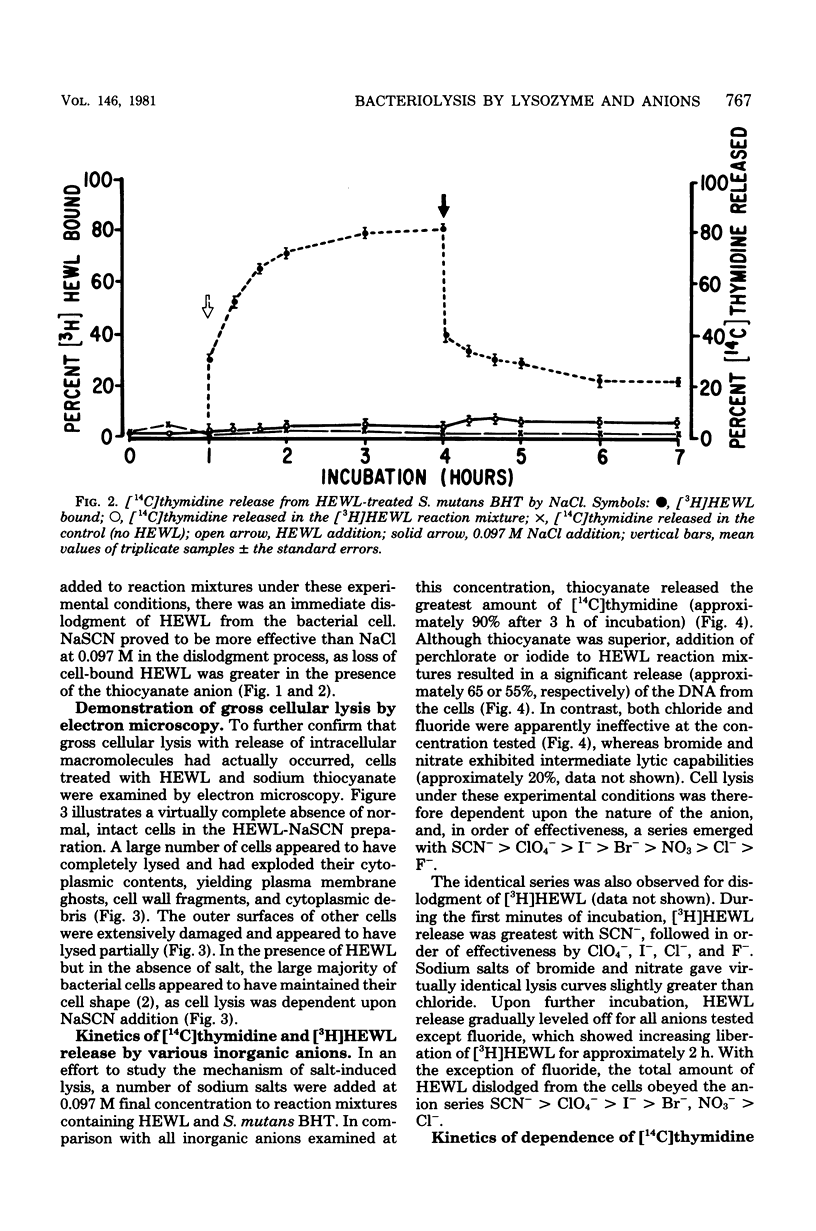
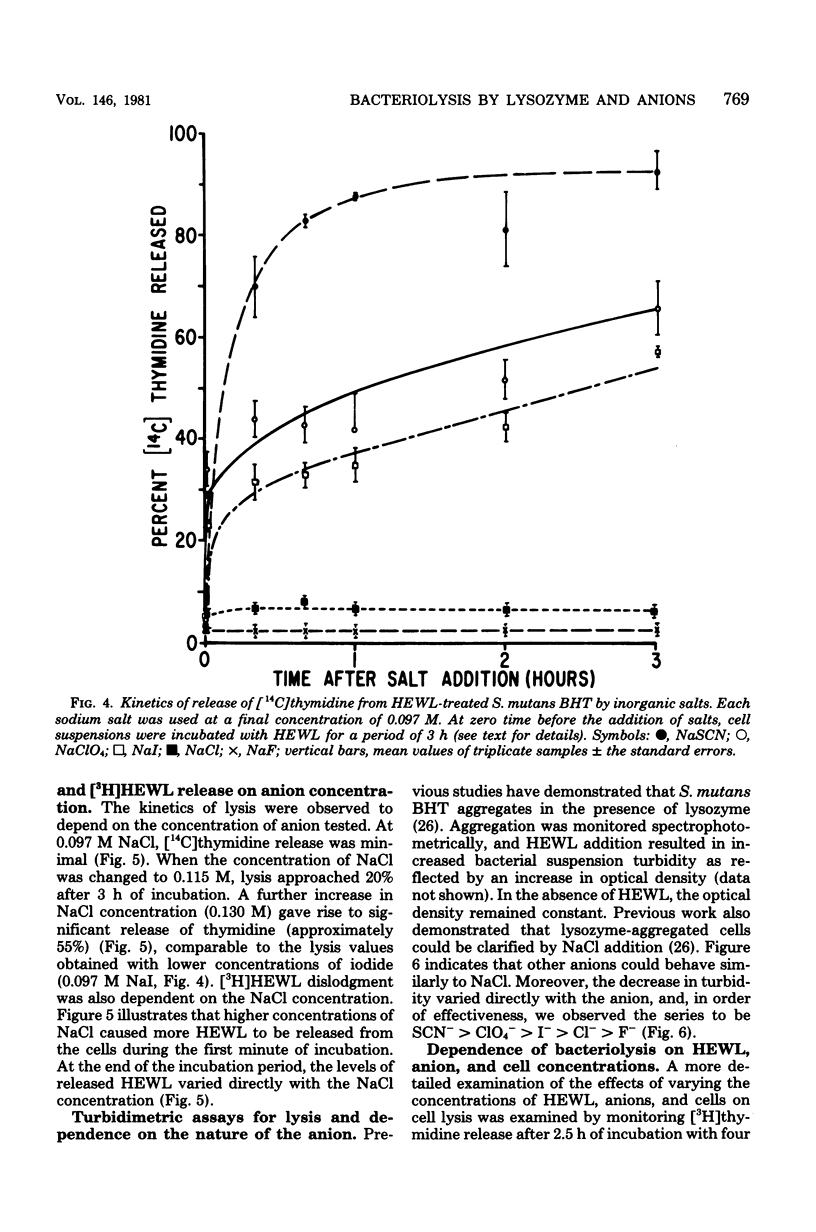
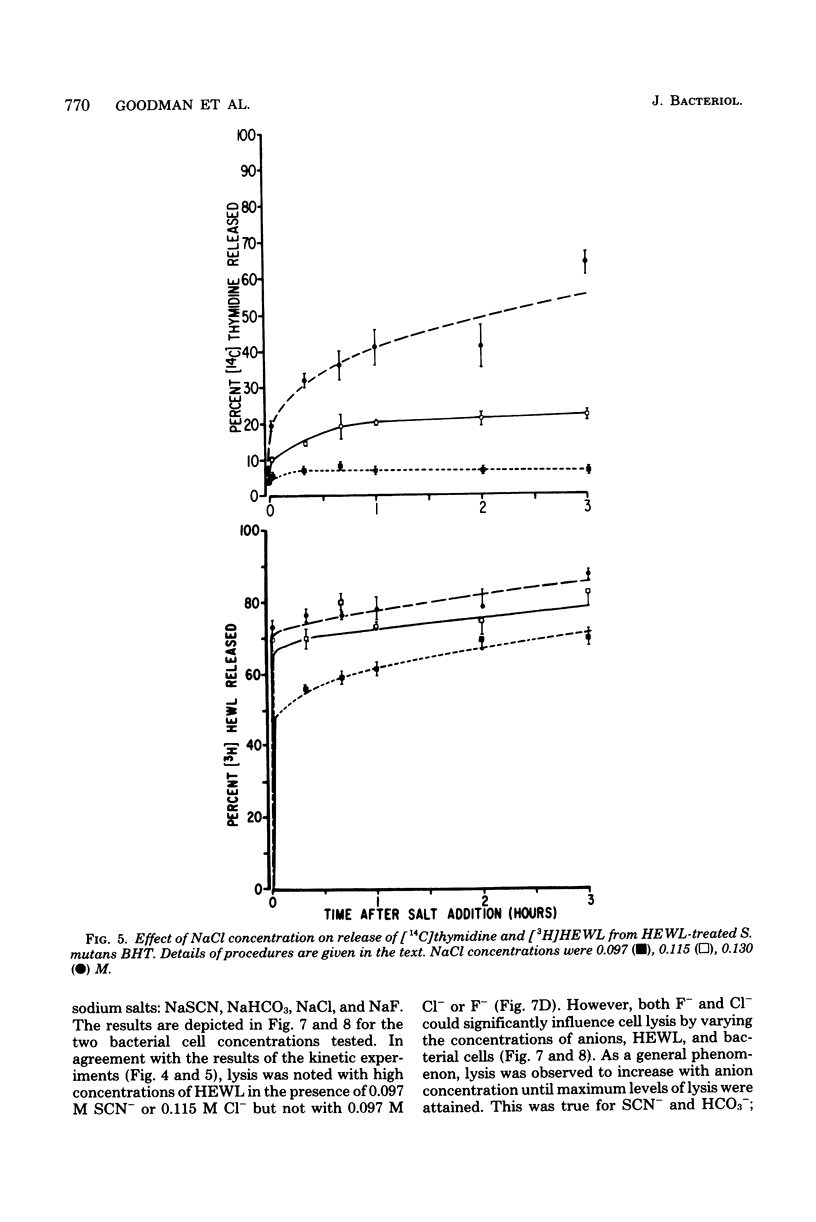
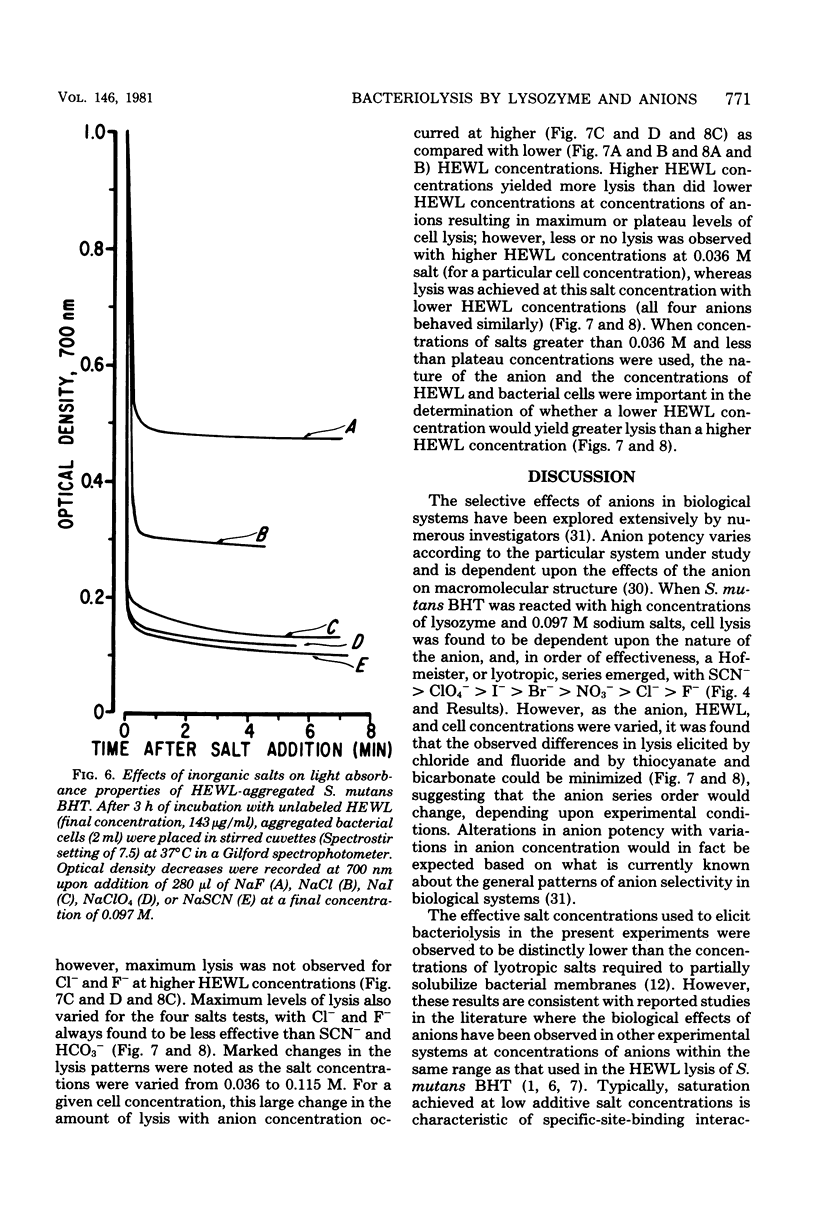
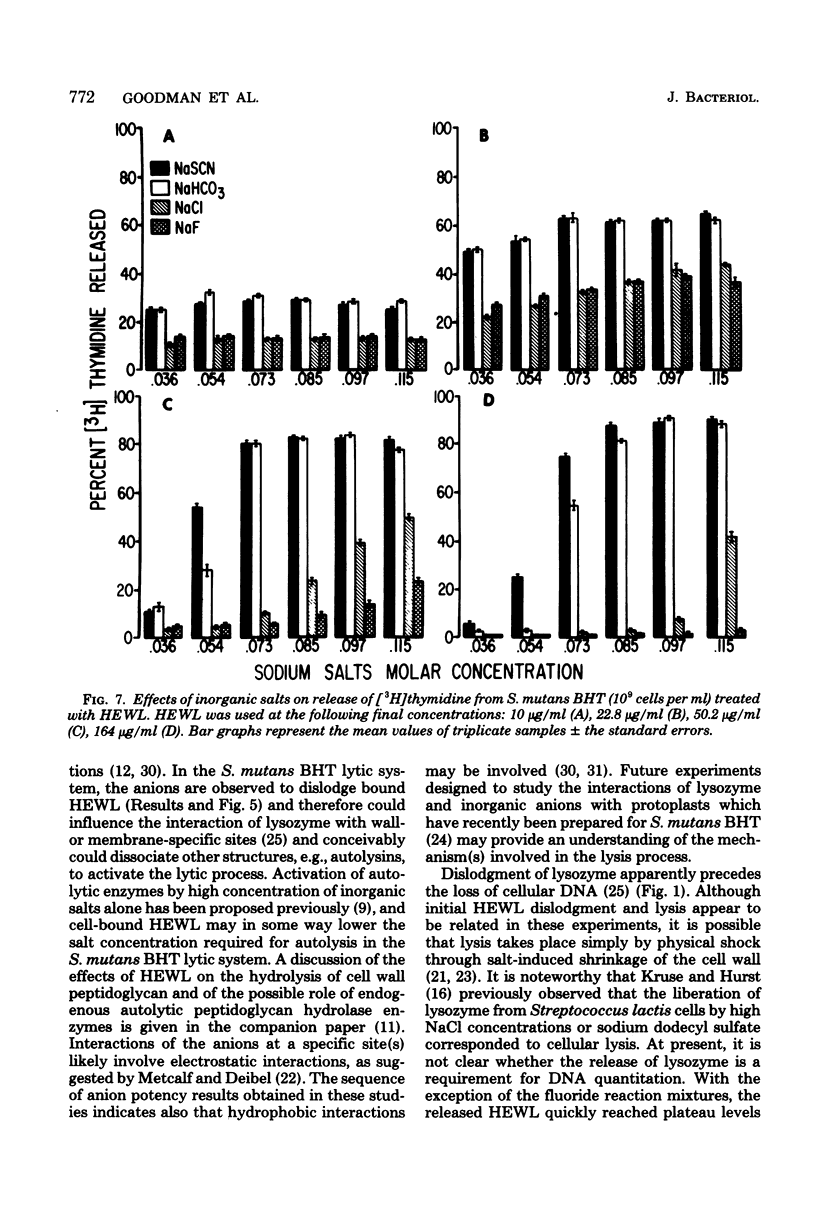
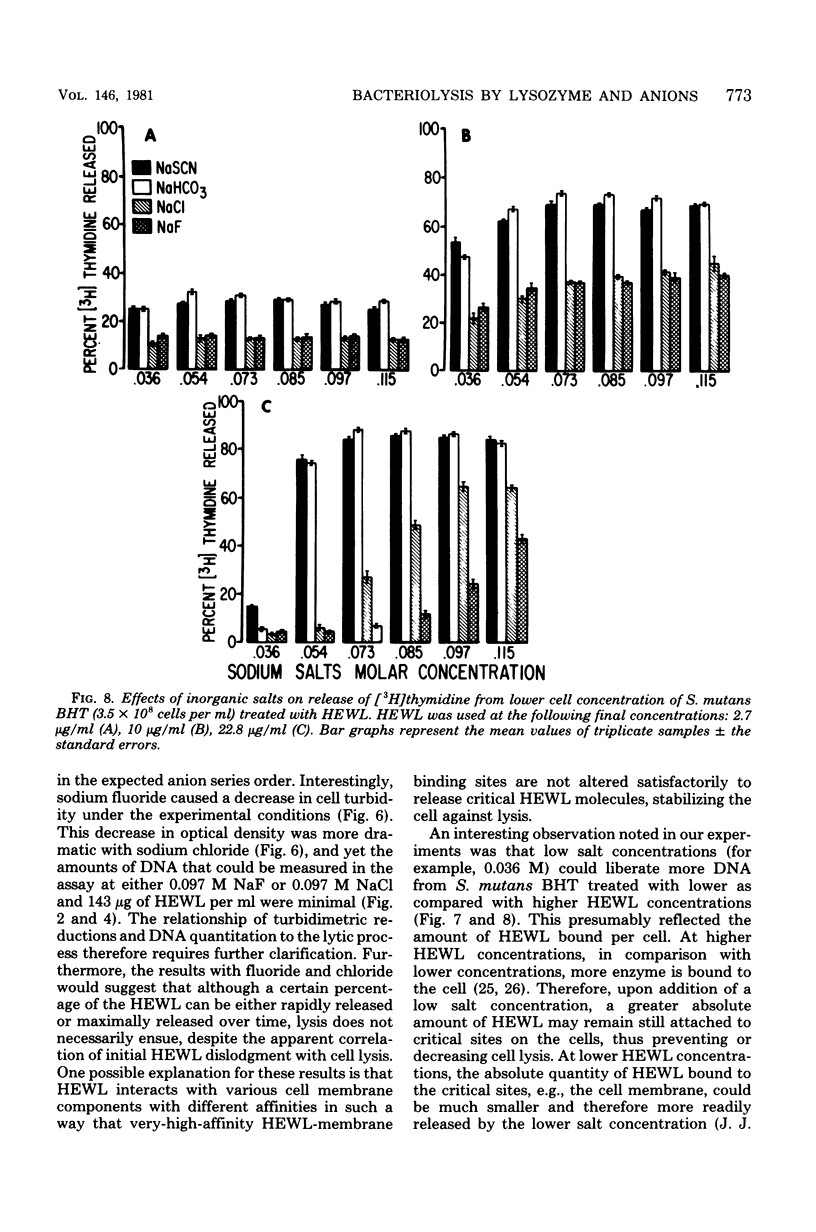
Streptococcus mutans BHT was grown in a synthetic medium containing radioactive thymidine to monitor deoxyribonucleic acid release. Kinetic experiments demonstrated that although lysozyme alone could not liberate deoxyribonucleic acid, cellular deoxyribonucleic acid was liberated from lysozyme-treated cells by addition of low concentrations of inorganic sodium salts. When the salts were tested for their ability to dislodge cell-bound tritiated lysozyme, the extent of the initial release of enzyme by individual anions correlated with the anion potency for deoxyribonucleic acid liberation (SCN- greater than ClO4- greater than I- greater than Br- greater than NO3- greater than Cl- greater than F-), although the total amount of lysozyme dislodged did not correspond directly with cell lysis. Differences in the effectiveness of anions (SCN-, HCO3-, Cl- and F-) in potentiating cell lysis could be enhanced or minimized by varying the lysozyme, anion, and bacterial cell concentrations. As the anion concentration was increased for each enzyme concentration and cell concentration, the lysis increased, in some cases markedly, until maximum levels of released deoxyribonucleic acid were attained. The maximum levels of lysis of SCN- and HCO3- were similar and were greater than those for Cl- and F-. In addition, the maximum levels were observed to increase for each of the anions as the concentration of lysozyme increased.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviram I. The interaction of chaotropic anions with proteins at acid pH. Eur J Biochem. 1973 Dec 17;40(2):631–636. doi: 10.1111/j.1432-1033.1973.tb03235.x. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Giuffrida A. Method for the lysis of Gram-positive, asporogenous bacteria with lysozyme. Appl Environ Microbiol. 1980 Jan;39(1):153–158. doi: 10.1128/aem.39.1.153-158.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman S. E., van de Rijn I., Bleiweis A. S. Lysis of grouped and ungrouped streptococci by lysozyme. Infect Immun. 1970 Nov;2(5):563–569. doi: 10.1128/iai.2.5.563-569.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIDOVICH I. Inhibition of acetoacetic decarboxylase by anions. The Hofmeister lyotropic series. J Biol Chem. 1963 Feb;238:592–598. [PubMed] [Google Scholar]
- Forman H. J., Kennedy J. Effects of chaotropic agents versus detergents on dihydroorotate dehydrogenase. J Biol Chem. 1977 May 25;252(10):3379–3381. [PubMed] [Google Scholar]
- Friedberg I., Avigad G. High lysozyme concentration and lysis of Micrococcus lysodeikticus. Biochim Biophys Acta. 1966 Oct 31;127(2):532–535. doi: 10.1016/0304-4165(66)90409-0. [DOI] [PubMed] [Google Scholar]
- Gilpin R. W., Chatterjee A. N., Young F. E. Autolysis of microbial cells: salt activation of autolytic enzymes in a mutant of Staphylococcus aureus. J Bacteriol. 1972 Jul;111(1):272–283. doi: 10.1128/jb.111.1.272-283.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Lopez R., Tomasz A. Suppression of lytic effect of beta lactams on Escherichia coli and other bacteria. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3293–3297. doi: 10.1073/pnas.73.9.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H., Pollock J. J., Iacono V. J., Wong W., Shockman G. D. Peptidoglycan loss during hen egg white lysozyme-inorganic salt lysis of Streptococcus mutans. J Bacteriol. 1981 May;146(2):755–763. doi: 10.1128/jb.146.2.755-763.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Solubilization of particulate proteins and nonelectrolytes by chaotropic agents. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1129–1136. doi: 10.1073/pnas.62.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono V. J., MacKay B. J., DiRienzo S., Pollock J. J. Selective antibacterial properties of lysozyme for oral microorganisms. Infect Immun. 1980 Aug;29(2):623–632. doi: 10.1128/iai.29.2.623-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto Y., Watanabe T., Tsunemitsu A., Fukui Z., Moriyama T. Lysis of streptococci by lysozyme from human parotid saliva and sodium lauryl sulfate. J Dent Res. 1971 Nov-Dec;50(6):1688–1688. doi: 10.1177/00220345710500066201. [DOI] [PubMed] [Google Scholar]
- Kruse H., Hurst A. Preparation of spheroplasts from Streptococcus lactis. Can J Microbiol. 1972 Jun;18(6):825–831. doi: 10.1139/m72-128. [DOI] [PubMed] [Google Scholar]
- Kuramitsu S., Ikeda K., Hamaguchi K., Miwa S., Nakashima K. Binding of N-acetyl-chitotriose to human lysozyme. J Biochem. 1975 Aug;78(2):327–333. doi: 10.1093/oxfordjournals.jbchem.a130911. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez R., Ronda-Lain C., Tapia A., Waks S. B., Tomasz A. Suppression of the lytic and bactericidal effects of cell wallinhibitory antibiotics. Antimicrob Agents Chemother. 1976 Oct;10(4):697–706. doi: 10.1128/aac.10.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel I. D. Dental caries. Am Sci. 1979 Nov-Dec;67(6):680–688. [PubMed] [Google Scholar]
- Marquis R. E. Salt-induced contraction of bacterial cell walls. J Bacteriol. 1968 Mar;95(3):775–781. doi: 10.1128/jb.95.3.775-781.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf R. H., Deibel R. H. Differential lytic response of enterococci associated with addition order of lysozyme and anions. J Bacteriol. 1969 Sep;99(3):674–680. doi: 10.1128/jb.99.3.674-680.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou L. T., Marquis R. E. Electromechanical interactions in cell walls of gram-positive cocci. J Bacteriol. 1970 Jan;101(1):92–101. doi: 10.1128/jb.101.1.92-101.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESTIDGE L. S., PARDEE A. B. Induction of bacterial lysis by penicillin. J Bacteriol. 1957 Jul;74(1):48–59. doi: 10.1128/jb.74.1.48-59.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks L. C., Shockman G. D., Higgins M. L. Growth of Streptococcus mutans protoplasts is not inhibited by penicillin. J Bacteriol. 1980 Sep;143(3):1491–1497. doi: 10.1128/jb.143.3.1491-1497.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]