Abstract
We analyzed the ability of two rat coronavirus (RCoV) strains, sialodacryoadenitis virus (SDAV) and Parker's RCoV (RCoV-P), to infect rat alveolar type I cells and induce chemokine expression. Primary rat alveolar type II cells were transdifferentiated into the type I cell phenotype. Type I cells were productively infected with SDAV and RCoV-P, and both live virus and UV-inactivated virus induced mRNA and protein expression of three CXC chemokines: CINC-2, CINC-3, and LIX, which are neutrophil chemoattractants. Dual immunolabeling of type I cells for viral antigen and CXC chemokines showed that chemokines were expressed primarily by uninfected cells. Virus-induced chemokine expression was reduced by the IL-1 receptor antagonist, suggesting that IL-1 produced by infected cells induces uninfected cells to express chemokines. Primary cultures of alveolar epithelial cells are an important model for the early events in viral infection that lead to pulmonary inflammation.
Keywords: Alveolar epithelium, Chemokine, Sialodacryoadenitis virus, Parker rat coronavirus, CINC-2, CINC-3, LIX, Neutrophil chemoattractant, IL-1 receptor antagonist
Introduction
The large surface area of the alveolar epithelium makes it an important interface between respiratory pathogens and the host. Alveolar type I cells, which make up 95% of this surface area, are terminally differentiated and function in gas exchange and fluid homeostasis (Williams, 2003). Alveolar type II cells are dividing cells that transdifferentiate in vivo into the type I cell phenotype during the repair of damaged alveolar epithelium. Type II cells also produce surfactant proteins and lipids, which keep the alveoli expanded and function in innate defense of the lung (Evans et al., 1975, Mason, 2006). Epithelial cells that line the respiratory tract initiate pulmonary inflammation in response to pollutants, allergens, or infectious agents (Martin et al., 1997). Bronchial epithelial cells and alveolar type II cells express and secrete proinflammatory cytokines and chemokines in response to infection with respiratory viruses including respiratory syncytial virus, influenza A virus, and the SARS-associated coronavirus (SARS-CoV) (Chan et al., 2005, Yen et al., 2006, Zhang et al., 2001). The role of alveolar type I cells in immune responses to viral infection is largely unknown.
Coronaviruses are an important cause of respiratory infections of animals, humans, and birds. Five coronaviruses are known to cause respiratory disease in humans. Human coronaviruses (HCoVs) 229E and OC43 are etiologic agents of the common cold and can cause more serious lower respiratory tract disease in elderly or immunocompromised patients (Birch et al., 2005, Pene et al., 2003). Infection with SARS-CoV was associated with an epidemic of severe acute respiratory syndrome in 2002–2003 that had a case fatality rate of ∼ 10% (Ksiazek et al., 2003, Tsui et al., 2003). HCoV-NL63 and HCoV-HKU1 are recently identified coronaviruses that are associated with both upper and lower respiratory tract diseases in children and adults (Fouchier et al., 2004, Kuypers et al., 2007, Pyrc et al., 2007, Van der Hoek et al., 2004, Woo et al., 2005). Coronaviruses also cause respiratory diseases in rats, dogs, cows, pigs, and poultry (Weiss and Navas-Martin, 2005).
Several strains of rat coronavirus (RCoV) have been isolated from the lungs of rats with clinical or subclinical infections (Compton et al., 1999, Kojima and Okaniwa, 1991, Parker et al., 1970). The two prototype strains of RCoV have different tissue tropisms and disease associations in rats. Sialodacryoadenitis virus (SDAV) causes disease primarily in the upper respiratory tract, salivary and lacrimal glands, and eyes (Bhatt et al., 1972, Jacoby et al., 1975). SDAV has also been isolated from the lower respiratory tract and can cause mild interstitial pneumonia in young rats (Wojcinski and Percy, 1986). In contrast, Parker's RCoV (RCoV-P) has only been isolated from the respiratory tract and causes fatal pneumonia in suckling rats (Bhatt and Jacoby, 1977, Parker et al., 1970). In adult rats inoculated intranasally with RCoV-P, virus replicates in the upper and lower respiratory tracts. Neutrophils are observed in the nasal cavity, trachea, and alveoli on days 2–5, followed by infiltration of mononuclear cells in the lung on day 5 after infection. RCoV-P infection of adult rats causes interstitial pneumonia and focal edema in the alveoli, which resolves by day 8 after infection (Bhatt and Jacoby, 1977). As with RCoV-P, upon intranasal inoculation of adult rats with SDAV, neutrophils are the primary infiltrating cell type in the respiratory epithelium early in infection (Jacoby et al., 1975, Wojcinski and Percy, 1986). RCoV is an important model in which to study the pathogenesis of pulmonary infection caused by a coronavirus in its natural host. Here we examine the role of alveolar type I cells in the innate immune response to rat coronavirus infection.
Primary cultures of rat alveolar epithelial cells are a well-characterized model of differentiated lung epithelium, which have not been studied previously with respect to viral infection (Wang et al., 2006). Alveolar type II cells isolated from rat lung can be cultured in vitro to either maintain the type II cell phenotype or transdifferentiate into the type I cell phenotype (Dobbs et al., 1985, Dobbs et al., 1988, Mason et al., 2002, Nishina et al., 2005). Because alveolar type I cells are difficult to isolate at a high yield and purity, transdifferentiated type II cells are used as a model to study the characteristics and functions of type I cells in vitro (Borok et al., 1998a, Dobbs et al., 1985, Williams, 2003). After 3–5 days in culture on plastic in the absence of rat serum and keratinocyte growth factor (KGF), rat alveolar type II cells change in morphology from a cuboidal to a flattened shape, and have decreased expression of surfactant proteins and lamellar bodies and increased expression of the type I cell markers, T1α, aquaporin V, and caveolin-1 (Borok et al., 1998a, Borok et al., 1995, Borok et al., 1998b, Danto et al., 1995, Manzer et al., 2006, Mason et al., 2002).
In this study, cultures of rat alveolar type I cells were evaluated for susceptibility to RCoV infection, and for expression and secretion of proinflammatory cytokines and chemokines in response to RCoV inoculation. To our knowledge, this is the first study to evaluate the in vitro response of rat alveolar epithelial cells to infection with a rat virus. Two strains of RCoV, RCoV-P and SDAV, caused productive infection of alveolar type I cells. Infection of type I cell cultures with these coronaviruses increased expression and secretion of CXC chemokines, CINC-2, CINC-3, and LIX, which are important neutrophil chemoattractants. Secretion of these chemokines was also induced by UV-inactivated viruses, showing that viral replication was not required for the induction of chemokine expression. Dual immunolabeling of viral antigen and CXC chemokines showed that expression of CXC chemokines was primarily from uninfected cells in these cultures. Virus-induced chemokine expression was reduced by IL-1 receptor antagonist, but not soluble TNF receptor, indicating that IL-1 produced by infected cells may induce chemokine expression from the uninfected type I cells. These data suggest that alveolar type I cells can secrete chemokines in response to viral infection, which may lead to infiltration of neutrophils into the lung.
Results
Rat coronavirus strains RCoV-P and SDAV infect primary cultures of rat alveolar type I cells
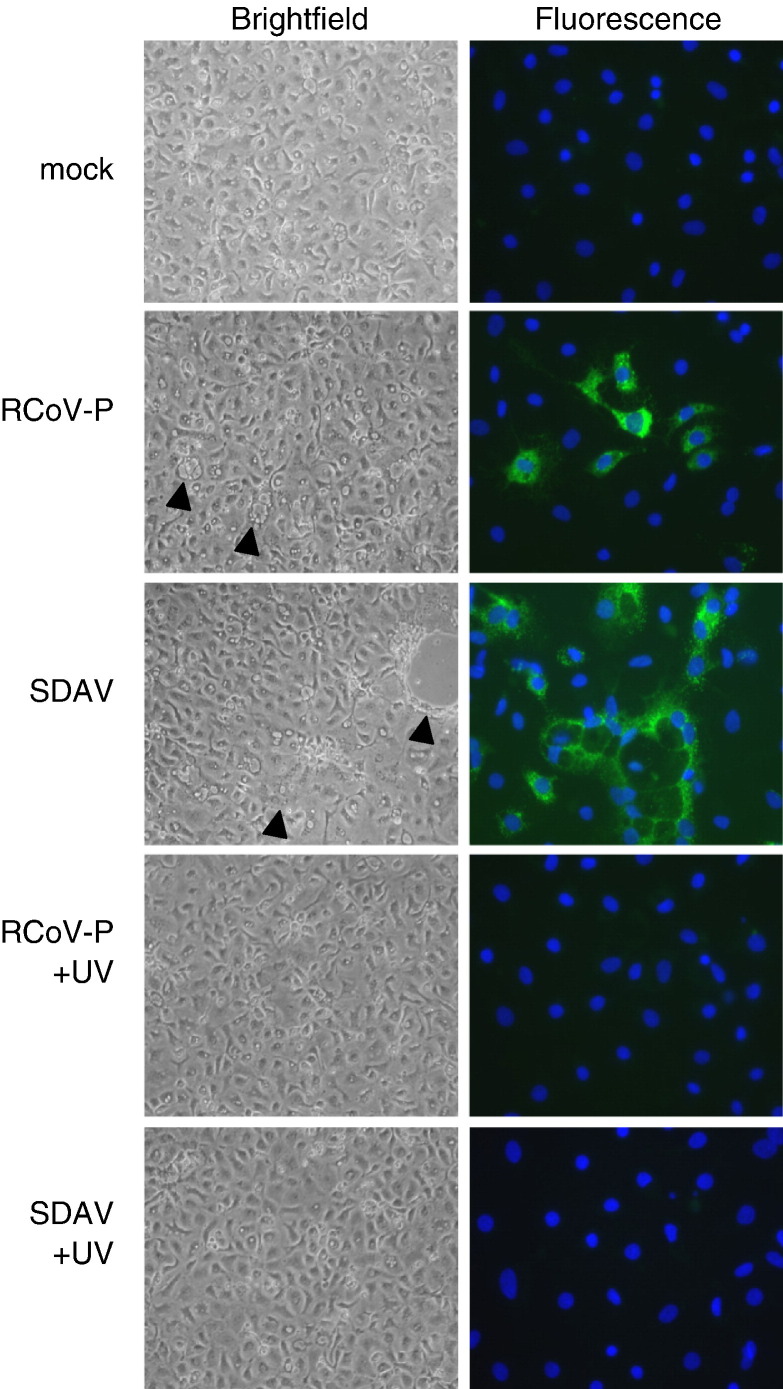
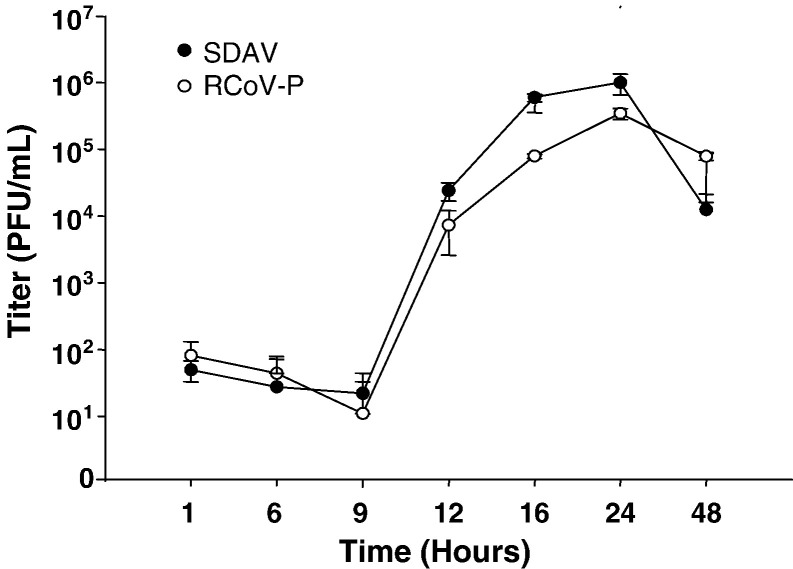
Type I cells infected with RCoV-P showed mild cytopathic effects including cell rounding and vacuolation at 24 h after inoculation (Fig. 1 ). Type I cells infected with SDAV had more pronounced cytopathic effects including syncytia formation and cell detachment. No cytopathic effects were observed in mock-inoculated type I cell cultures. At 24 h after virus inoculation, viral nucleocapsid protein was detected in type I cells inoculated with RCoV-P or SDAV, but not in mock-inoculated cells. Culture media from type I cells inoculated with RCoV-P or SDAV were collected at various times after inoculation and titrated by plaque assay. RCoV-P and SDAV replicated with similar kinetics in alveolar type I cells (Fig. 2 ). Infectious virus was first detected in the supernatant medium by 12 h after inoculation, and reached a maximum titer of 105–106 PFU/ml at 24 h.
Fig. 1.
Cytopathic effects and viral antigen expression in alveolar type I cells infected with RCoV-P or SDAV. Live cells were photographed at 10× magnification 24 h after inoculation with RCoV-P, SDAV, mock inoculation, or exposure to UV-inactivated RCoV-P or SDAV. Arrowheads point to cytopathic effects in the virus-inoculated cells. To visualize viral antigen, cells were fixed 24 h after inoculation with virus and immunolabeled for viral nucleocapsid antigen using a FITC-conjugated secondary antibody. Nuclei were stained with DAPI. Representative fields from 6 experiments are shown.
Fig. 2.
Growth curves of RCoV-P and SDAV replication in alveolar type I cells. Type I cells were inoculated with RCoV-P or SDAV at an MOI of 2. Media were harvested at the indicated times after inoculation and titrated by plaque assay on L2P-41.a cells. Data shown are averages from duplicate experiments.
Infection of alveolar type I cells by RCoV-P or SDAV induces expression of pro-inflammatory chemokines
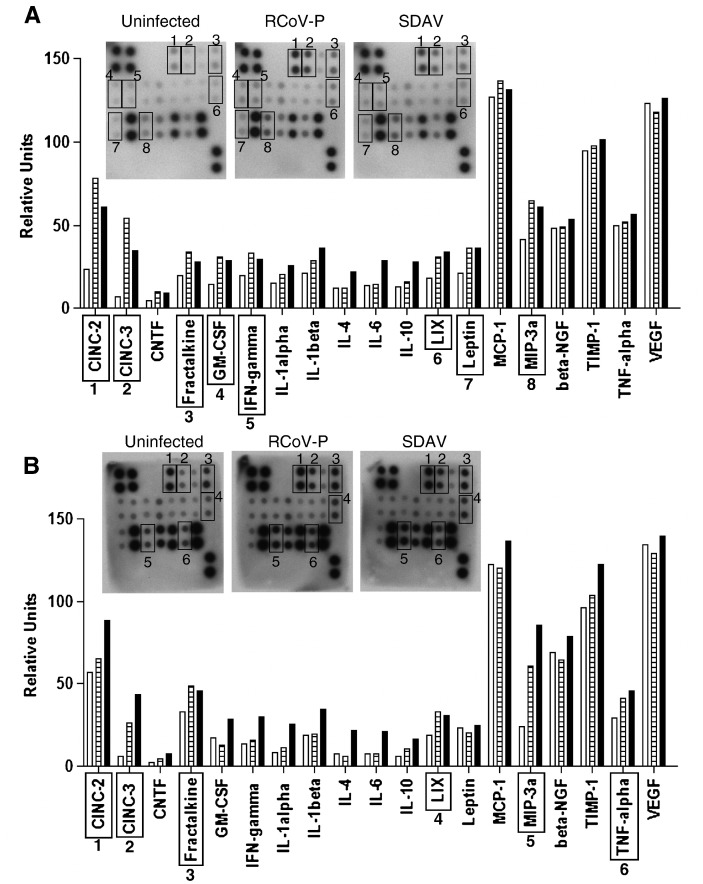
Culture media from type I cells collected 6 h or 24 h after inoculation with sucrose density gradient purified RCoV-P or SDAV were assayed for the presence of 19 rat cytokine and chemokine proteins by protein macroarray analysis (Fig. 3 ). At 6 h after inoculation, both RCoV-P- and SDAV-inoculated type I cells showed increased secretion of the chemokines CINC-2, CINC-3 (CXCL-2/3, MIP-2), fractalkine (CX3CL-1), GM-CSF, IFN-γ, LIX (CXCL-5), Leptin, and MIP-3α (CCL20), compared to mock-inoculated cells. By 24 h after inoculation, CINC 2, CINC-3, fractalkine, LIX, MIP-3α, and TNF-α were increased in cultures that were inoculated with each of these viruses. Infection of type I cells with SDAV increased the expression of several other cytokines, including IL-1α, IL-1β, IL-4, IL-6, and IL-10, compared to mock or RCoV-P inoculated cultures.
Fig. 3.
Protein macroarray analysis of cytokine and chemokine secretion from alveolar type I cell cultures infected with RCoV-P or SDAV. Type I cells were inoculated with RCoV-P, SDAV, or mock inoculated. Media were harvested at (A) 6 h or (B) 24 h after inoculation and incubated with RayBio membrane arrays as directed by the manufacturer. Representative membranes from duplicate experiments are shown. Densitometry data were normalized to internal positive controls on each membrane and graphed as relative units. Open bars = Mock, hatched bars = RCoV-P, black bars = SDAV. Scans of autoradiographs for each membrane are inset with relevant spots boxed with numbers corresponding to cytokines on the graph.
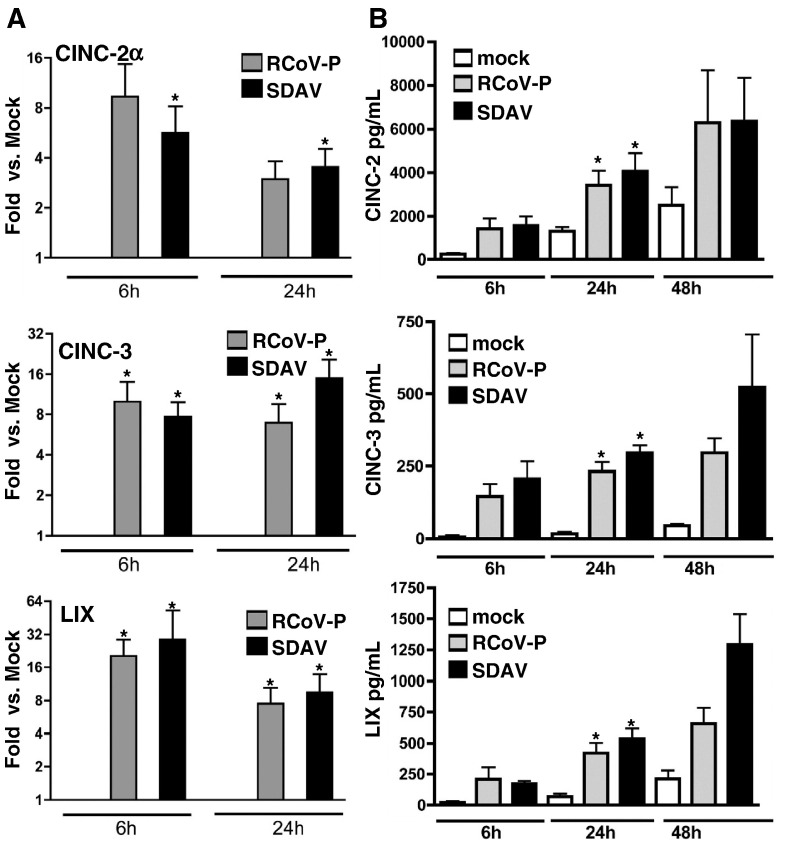
Because CINC-2, CINC-3, and LIX are closely related CXC chemokines that are expressed by rat alveolar epithelial cells in response to LPS or cytokines (Jeyaseelan et al., 2005, Manzer et al., 2006, Nishina et al., 2005, Vanderbilt et al., 2003), we further quantified their expression and secretion in RCoV-infected type I cells. The expression of CXC chemokines, CINC-2, CINC-3, and LIX, was confirmed by quantitative RT-PCR and ELISA (Fig. 4 ). Infection of type I cells with RCoV-P or SDAV induced expression of mRNA encoding CINC-2α, CINC-3, and LIX at 6 h and 24 h after inoculation (Fig. 4A). Infection with RCoV-P or SDAV also induced secretion of CINC-2, CINC-3, and LIX proteins by 6 h after inoculation (Fig. 4B). Secretion of the CXC proteins increased further at 24 h and 48 h after inoculation. The ELISA for CINC-2 did not differentiate between CINC-2α and CINC-2β, whereas the probe used in RT-PCR was specific for CINC-2α. Rat alveolar type I cells do not express CINC-2β constitutively or in response to cytokines or ozone (Nishina et al., 2005, Wang et al., 2006).
Fig. 4.
CXC chemokines have increased levels of mRNA and protein in alveolar type I cells infected with RCoV-P or SDAV. Type I cells were inoculated with RCoV-P, SDAV, or mock-inoculated. Total cellular RNA was extracted at 6 h and 24 h after inoculation. (A) mRNA expression for CINC-2α, CINC-3, and LIX was evaluated by quantitative RT-PCR as described in Materials and methods. Asterisks (∗) indicate a significant difference compared to mock at the same time point, p < 0.05. (B) Media from type I cell cultures were analyzed by ELISA at 6 h, 24 h, and 48 h after inoculation for the expression of CINC-2, CINC-3, and LIX. Asterisks (∗) indicate significant difference from mock-inoculated cells at the same time point, p < 0.01. All data are average values of duplicate samples from 3 to 6 experiments.
UV-inactivated virus also induces expression of CINC-2, CINC-3, and LIX
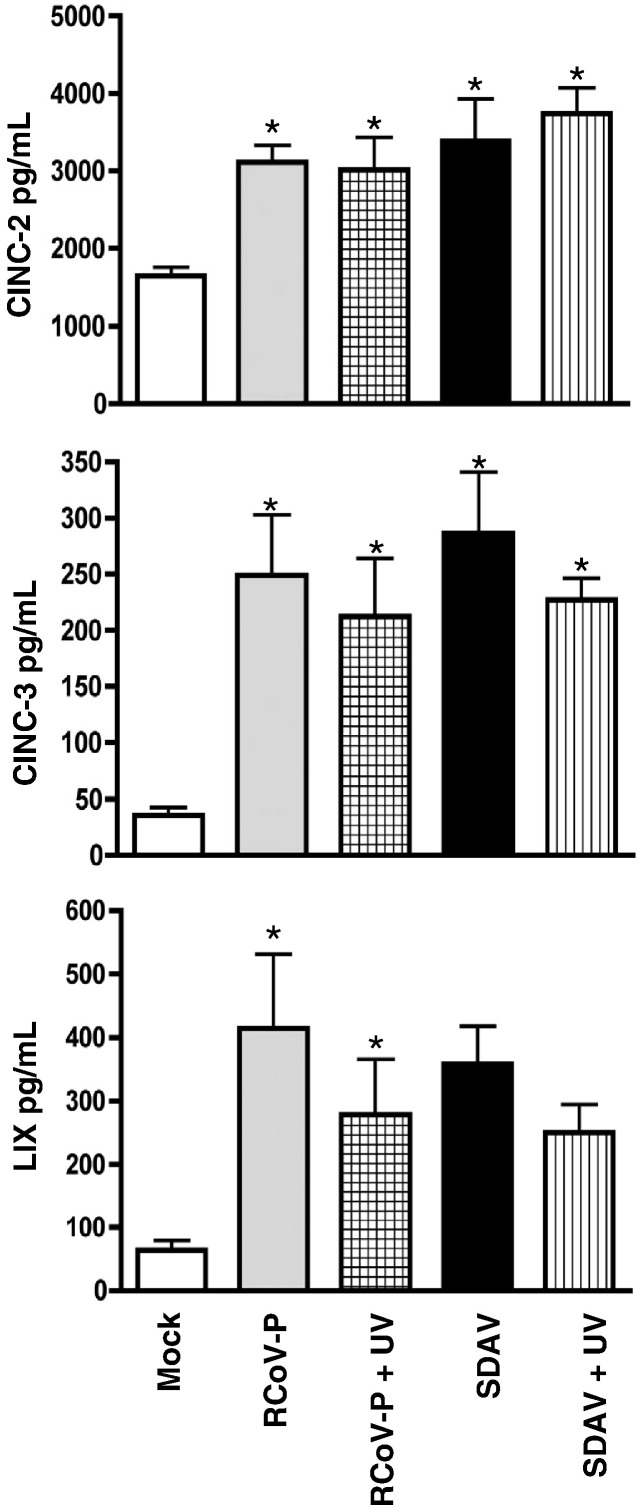
To determine whether viral replication was required to induce CXC chemokine expression, we inactivated the infectivity of RCoV-P and SDAV by UV irradiation. Inactivation of virus infectivity was confirmed by plaque assay of the UV-treated viral inoculum or medium from type I cells 24 h after exposure to UV-treated virus, in comparison to untreated virus. The titers of UV-treated RCoV-P and SDAV were below the limit of detection of our plaque assay (11.1 PFU/ml). Alveolar type I cells exposed to UV-treated virus for 24 h did not express viral nucleocapsid protein (Fig. 1), but nevertheless induced secretion of CINC-2, CINC-3, and LIX compared to mock-inoculated type I cells (Fig. 5 ). There was no difference in the amount of CXC chemokines secreted from type I cells treated with UV-inactivated virus compared to cells treated with infectious virus (p < 0.05).
Fig. 5.
UV-inactivated viruses induce secretion of chemokines from alveolar type I cells. Type I cells were inoculated with RCoV-P or SDAV, mock-inoculated, or treated with UV-inactivated RCoV-P or SDAV (see Fig. 1). Media were harvested 24 h after treatment and chemokine secretion was analyzed by ELISA. Asterisks (∗) indicate significant difference from mock-inoculated cells, p < 0.05. Data are average values of duplicate samples from 3 experiments.
To determine whether the CXC chemokine response to infectious or UV-inactivated RCoV-P or SDAV was specific for the rat coronaviruses, we inoculated rat alveolar type I cells with a related group 2a coronavirus, mouse hepatitis virus (MHV) strain A59 that had been purified by sucrose density gradient centrifugation. No viral antigen was detected by immunolabeling at 24 h after inoculation of type I cells with MHV-A59, and the expression of CXC chemokines CINC-2, CINC-3, and LIX was not increased compared to mock-inoculated cells (data not shown).
Uninfected cells in the type I cultures express CXC chemokines
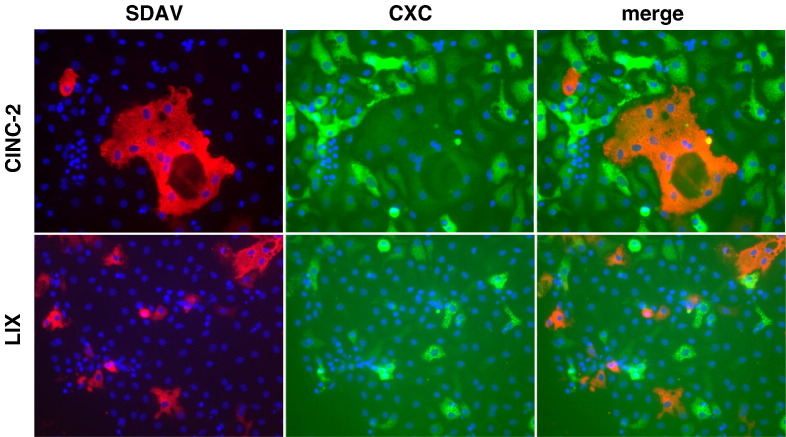
To determine whether the increase in CXC chemokine expression in alveolar type I cell cultures was limited to virus-infected cells, at 16 h after virus inoculation type I cell cultures were treated with brefeldin A for 6 h before dual immunolabeling of viral nucleocapsid protein and CXC chemokines (Fig. 6 ). Brefeldin A inhibits export of secreted proteins from the distal Golgi to the cell surface (Miller et al., 1992). As expected from the ELISA data, cultures of type I cells that had been inoculated with RCoV-P or SDAV had increased immunolabeling with CINC-2, CINC-3, and LIX antibodies compared to mock inoculated cultures. CINC-2 and LIX were much more brightly labeled than CINC-3 (data not shown), corresponding to the concentrations of protein detected by ELISA (Fig. 4B). The majority of cells that were positive for CXC chemokine expression did not express viral nucleocapsid protein. Therefore, the ELISA data showing increased chemokine secretion in RCoV-infected type I cell cultures is mostly from uninfected cells. These data suggest that RCoVs induce CXC chemokine expression in type I cell cultures through an indirect mechanism.
Fig. 6.
Virus infection induces expression of chemokines from uninfected type I cells in the culture. Type I cells were treated with brefeldin A 16 h after inoculation with SDAV and fixed 6 h later. Fixed cells were immunolabeled for expression of viral nucleocapsid protein (red) and CINC-2 or LIX (green). Nuclei were stained blue with DAPI. Representative fields from duplicate experiments are shown.
Signaling through the IL-1 receptor, but not the TNF receptor, promotes SDAV-induced CXC chemokine expression in rat alveolar type I cells
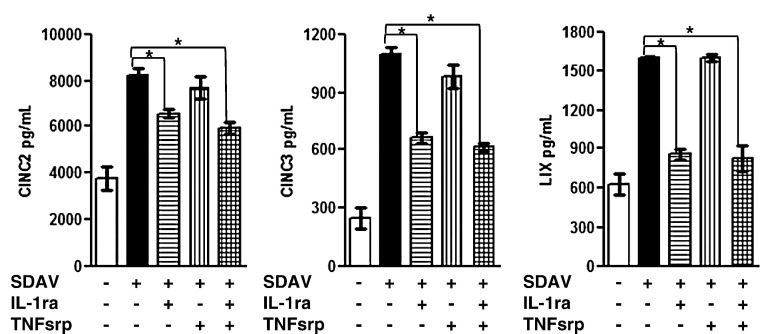
To test the hypothesis that a paracrine factor secreted from infected cells induces CXC chemokine expression in uninfected cells in the culture, we analyzed the ability of antagonists for the IL-1 and TNF receptors to block the CXC chemokine response. The IL-1 receptor antagonist (IL-1ra) binds strongly to the IL-1 type I receptor, thereby preventing signal transduction by IL-1α and IL-1β (Dinarello, 1996). The TNF soluble receptor protein (TNFsrp) binds to both soluble and membrane-bound forms of TNF-α, thereby inhibiting their activity (Dinarello, 2005). Treatment of SDAV-inoculated cells with IL-1ra decreased the level of CINC-2, CINC-3, and LIX secretion when it was added to type I cells prior to inoculation and maintained in the medium throughout infection with SDAV (Fig. 7 ). The presence of TNFsrp had no effect on the chemokine secretion during SDAV infection of type I cells. When both IL-1ra and TNFsrp were present during SDAV infection, the levels of CINC-2, CINC-3, and LIX expression were the same as levels from cells that were treated with IL-1ra alone. These data suggest that IL-1 that is secreted from infected alveolar type I cells induces expression of CXC chemokines in uninfected cells in the culture.
Fig. 7.
There is decreased expression of CINC-2, CINC-3, and LIX in SDAV-infected type I cells that have been treated with IL-1ra. Type I cells were treated with IL-1ra, TNFsrp, or both proteins and inoculated with SDAV. After 1 h, the inoculum was removed, cells were rinsed and refed with medium with or without the indicated receptor antagonists. After 18 h the media were analyzed for CXC chemokines by ELISA. Asterisks (*) indicate a statistically significant difference between SDAV-infected cells without receptor antagonist treatment and SDAV-infected cells with receptor antagonist treatment (p < 0.05). Data are representative of duplicate experiments with triplicate samples.
Discussion
Although type I cells make up 95% of the surface area of the alveolar epithelium, their role in the pathogenesis of respiratory virus infections and in innate immunity is largely unknown. In this study, we evaluated the ability of rat coronaviruses to infect rat alveolar type I cells in vitro. Despite differences in tissue tropism and disease association in vivo, two strains of RCoV were able to infect primary alveolar epithelial cells in vitro. We showed that infection of alveolar type I cells with either RCoV-P or SDAV induced expression of chemokines, CINC-2, CINC-3, LIX, MIP-3α, and fractalkine at both 6 h and 24 h after inoculation. Our protein array analysis also identified cytokines that are increased in SDAV-infected, but not RCoV-P-infected, alveolar type I cell cultures. This differential cytokine response may play a role in the different pathogenesis of RCoV-P and SDAV infections in vivo. Fractalkine, the sole member of the CX3C chemokine family, is predominately a membrane-bound chemokine and functions in the recruitment and adhesion of cytotoxic lymphocytes to endothelial cell surfaces (Stievano et al., 2004). MIP-3α, which is chemotactic for dendritic cells, B cells, and memory T cells, is expressed by human bronchial epithelial cells in response to inflammatory mediators IL-1β and TNF-α (Starner et al., 2003).
Previous studies showed that alveolar type II cells express CXC chemokines both constitutively and in response to treatment with cytokines, LPS, or acid and infection with Pseudomonas aeruginosa (Gonzalez et al., 2005, Jeyaseelan et al., 2005, Mason, 2006, Nishina et al., 2005, Vanderbilt et al., 2003). Our study contributes to the growing body of data that supports a role for alveolar type I cells in chemokine expression (Manzer et al., 2006, Wang et al., 2006). CXC chemokines are important mediators of neutrophil chemotaxis and immunopathology associated with respiratory virus infections (Londhe et al., 2005, Tumpey et al., 2005, Turner, 1990, Yen et al., 2006, Zhang et al., 2001). Therefore, we further quantified the expression of CXC family members CINC-2, CINC-3, and LIX made by rat alveolar type I cells in response to RCoV infection by quantitative RT-PCR and ELISA. Levels of CINC-2, CINC-3, and LIX mRNAs and secreted proteins were increased in RCoV-infected type I cell cultures. CINC-2, CINC-3, and LIX are closely related CXC-ELR+ chemokines that are similar to IL-8/CXCL8 in humans (Shibata et al., 1995, Smith et al., 2002). CINC-2, CINC-3, and LIX specifically bind and signal through CXCR2, a seven transmembrane domain receptor expressed on rat type II alveolar cells, endothelial cells, and neutrophils (Chandrasekar et al., 2003, Dunstan et al., 1996, Shibata et al., 2000, Vanderbilt et al., 2003). Neutrophil chemotaxis and activation are the primary functions of CXCR2 signaling. However, CXCR2 also mediates cellular proliferation and morphogenesis, angiogenesis, and wound healing (Devalaraja et al., 2000). CXC chemokines secreted from infected alveolar epithelial cells could potentially signal through CXCR2 on type II pneumocytes to stimulate type II cell proliferation and trans-differentiation into the type I cell phenotype, resulting in repair of damaged epithelium. Expression of CXCR2 and secretion of CXC chemokines are upregulated in alveolar type II cells by lung injury (Vanderbilt et al., 2003). In addition, CINC-1 signaling through CXCR2 on type II cells promotes alveolar development in newborn rats that are exposed to hyperoxic conditions (Auten et al., 2001). CXC chemokine signaling through CXCR2 on alveolar type II cells and endothelial cells may also promote retraction of these cells, which would promote increased migration of neutrophils and other inflammatory cells into the alveoli (Schraufstatter et al., 2001). Although CINC-2, CINC-3, and LIX signal through the same receptor, they may have some distinct functions during viral infection. For example, although CINC-2 and CINC-3 have similar neutrophil chemotactic activity, CINC-3 induces greater calcium mobilization than CINC-2, resulting in increased neutrophil activation (Shibata et al., 2000). Further studies are needed to elucidate the roles of CINC-2, CINC-3, and LIX in the innate immune response to RCoV infection in vivo.
The production of CXC chemokines by alveolar epithelial cells during viral infection is probably responsible for the recruitment of neutrophils to the infected alveoli, an early event in a variety of respiratory viral infections. Respiratory viruses, including influenza A virus, rhinovirus, and respiratory syncytial virus (RSV), induce expression of CXC chemokines, which results in an influx of neutrophils into the respiratory tract (Abu-Harb et al., 1999, Tumpey et al., 2005, Turner, 1990, Wang et al., 1998, Zhang et al., 2001, Zhu et al., 1997). Infiltration of neutrophils into lung and other tissues occurs soon after infection with coronaviruses including rat coronaviruses, mouse hepatitis virus strains 1 and JHM, and SARS-CoV, and has been implicated in both immunopathology and virus clearance (Bhatt and Jacoby, 1977, De Albuquerque et al., 2006, Haagmans et al., 2004, Iacono et al., 2006, Tsui et al., 2003, Yen et al., 2006, Zhou et al., 2003). After intranasal inoculation of adult rats with RCoV-P, neutrophils are found in the upper respiratory tract on day 2 and in the lung on day 5, corresponding to the presence of viral antigen and histological lesions (Bhatt and Jacoby, 1977). Neutrophils are also the earliest infiltrating cell type in the respiratory epithelium after intranasal inoculation with SDAV (Jacoby et al., 1975, Wojcinski and Percy, 1986). The role of neutrophils in RCoV clearance and immunopathology is not yet known. Primary cultures of alveolar epithelial cells are an important model in which to study the early events in viral infection that lead to pulmonary inflammation.
Like infection with RCoV-P or SDAV, other UV-inactivated viruses also induce expression of CXC chemokines. UV-inactivated murine coronavirus, MHV-4 variant V5A13.1, induces expression of CXC and CC chemokine genes in primary mouse astrocyte cultures and in the CNS of infected mice during acute and chronic phases of CNS disease (Lane et al., 1998). UV-inactivated RSV or soluble viral glycoproteins (F and G proteins) induce expression of IL-8 in A549 cells (Tirado et al., 2005). Our data show that a component of purified virions that was not destroyed by UV light was responsible for inducing chemokine expression. The viral hemagglutinin esterase glycoprotein (HE) was not required for the induction of CXC chemokines because SDAV virions do not contain HE (Gagneten et al., 1996; Miura and Holmes, unpublished observation). Perhaps interaction of the viral spike glycoprotein with as yet unknown cellular receptor(s) triggers chemokine expression, as SARS-CoV spike protein does in A549 cells (Chang et al., 2004). Alternatively, other viral structural proteins may induce expression of chemokines (Law et al., 2007, Tirado et al., 2005). For several RNA viruses, dsRNA replicative intermediates induce chemokine expression (Hewson et al., 2005, Imaizumi et al., 2004, Londhe et al., 2005). However, this is unlikely to be the case for RCoV because viral replication was not required for chemokine expression.
Viral infection can induce chemokine expression either by a direct or indirect mechanism (Melchjorsen et al., 2003). In our RCoV-infected type I cell cultures, dual immunolabeling of viral antigen and CXC chemokines demonstrated that the CXC chemokines were expressed principally in cells that did not express viral nucleocapsid protein. This suggests that RCoV infection of alveolar type I cells can indirectly induce expression of chemokines in uninfected cells. Infected cells may release a soluble factor such as IL-1 or TNF-α, which could stimulate nearby cells to express and secrete CXC chemokines (Manzer et al., 2006, Nishina et al., 2005). To test this hypothesis, we inoculated type I cells with SDAV in the presence of antagonists for the IL-1 and/or TNF receptors, IL-1ra and TNFsrp, respectively. Treatment with IL-1ra, but not TNFsrp, decreased CXC chemokine secretion from SDAV-infected cultures (Fig. 7). Thus, signaling through the IL-1 receptor contributes to the induction of CXC chemokine expression in SDAV-infected type I cell cultures.
IL-1ra blocks signaling of both IL-1α and IL-1β through the IL-1 receptor (Dinarello, 1996). IL-1 is important in the induction of CXC chemokines in lung cells in vitro and in vivo. Both IL-1α and IL-1β induce expression and secretion of CXC chemokines in primary cultures of rat alveolar type I and type II cells (Manzer et al., 2006; Nishina et al., 2005; Unpublished observations). Furthermore, IL-1 receptor knock-out mice have a decreased neutrophil response to influenza infection, likely due to decreased IL-1-dependent expression of CXC chemokines (Schmitz et al., 2005).
A potential limitation of this study is that we isolated type II cells and transdifferentiated them into a type I-like cell phenotype in vitro (Manzer et al., 2006, Wang et al., 2006). While transdifferentiated type I cells express T1α and many other type I cell markers, are morphologically like type I cells, and secrete MIP-2 and MCP-1 in response to LPS and IL-1, they may not express all of the genes expressed in freshly isolated type I cells (Gonzalez et al., 2005, Manzer et al., 2006, Wang et al., 2006). Although we think that it is unlikely, it is possible that type I cells in vivo will respond differently to viral infection.
Animal models to evaluate the mechanisms of viral induction of chemokines in the lung are valuable in studying viral pathogenesis and designing treatments for viral diseases. The expression of chemokines in SARS patients has been correlated with increased disease severity (Tang et al., 2005). SARS pathogenesis has been studied in rodent models: aged mice, SARS-CoV adapted to cause disease in rodents, or transgenic mice that express the SARS receptor, human angiotensin converting enzyme 2 (McCray et al., 2007, Nagata et al., 2007, Roberts et al., 2007, Roberts et al., 2005, Tseng et al., 2007). We are studying rat coronaviruses as a model for respiratory coronavirus infection in the natural host of the virus. The study of other rodent viruses in their natural hosts has advanced the understanding of other respiratory viral pathogens, such as Sendai virus and pneumonia virus of mice as models for human parainfluenza and RSV respectively (Faisca and Desmecht, 2007, Rosenberg et al., 2005). Recently the MHV-1 strain of murine coronavirus was shown to induce severe respiratory disease in mice and was proposed as a model for SARS-CoV (De Albuquerque et al., 2006). Primary cultures of rat alveolar epithelial cells will be a valuable model for dissecting the virus-cell interactions and innate immune responses that are important in the critical early steps in pulmonary infection of a virus in its natural host.
Materials and methods
Isolation and culture of alveolar epithelial cells
Alveolar type II cells were isolated from 6–8-week-old, specific pathogen-free male Sprague–Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) as previously described (Dobbs and Mason, 1979, Manzer et al., 2006). Briefly, type II cells were dissociated from rat lung with porcine pancreatic elastase (Roche Molecular Biochemicals, Indianapolis, IN) and were partially purified on discontinuous Optiprep (Axis-Shield Poc As, Oslo, Norway) density gradients. Cells were plated on plastic tissue culture dishes in DMEM supplemented with 5% FBS, 2 mM glutamine, 2.5 ug/ml amphotericin B, 100 ug/ml streptomycin, and 100 ug/ml penicillin G, and incubated at 37 °C and 10% CO2. Culture of type II cells on plastic in DMEM containing 5% FBS for 3–5 days results in trans-differentiation into the type I cell phenotype, characterized by loss of expression of surfactant proteins, gain of expression of T1α and aquaporin V, and changes in morphology and lectin-binding specificities (Borok et al., 1998a, Borok et al., 1995, Borok et al., 1998b, Danto et al., 1995, Dobbs et al., 1985, Manzer et al., 2006, Wang et al., 2006).
Viruses
RCoV-P and SDAV were obtained from Dr. D. Percy (University of Guelph, Guelph, Ontario) (Percy et al., 1991). Viruses were propagated in a subclone of murine L929 cells, L2P-41.a (Gagneten et al., 1996), purified by sucrose density gradient ultracentrifugation as previously described, and stored in TMS buffer (25 mM Tris, 25 mM maleic acid, 100 mM NaCl, pH 6.5) with 5% glycerol (Sturman et al., 1980). Viral titers were determined by plaque assay on L2P-41.a cells (described below). Purified virus was diluted in DMEM to achieve equivalent doses of RCoV-P and SDAV for inoculation. For experiments with UV-inactivated viruses, the diluted viruses were exposed on ice to UV irradiation in a Stratalinker UV Crosslinker (Stratagene, La Jolla, CA) at a cumulative dose of 120 mJ/cm2. Inactivation of virus was demonstrated by plaque assay of the UV-irradiated virus and medium from type I cells 24 h after inoculation with the UV-irradiated virus, as well as by immunofluorescent detection of viral antigens in type I cells 24 h after inoculation with the UV-irradiated virus, using a cross-reactive mAb to the mouse hepatitis virus nucleocapsid protein (described below). These analyses confirmed that the infectivity of the RCoVs was completely eliminated by this UV treatment. UV-irradiated viral inocula were shown to be free of bacterial contamination by inoculation of blood agar and incubation at 37 °C.
Viral infection of alveolar type I cells
Primary alveolar epithelial cells were cultured as described above for 5 days to obtain a type I cell phenotype, then inoculated with RCoV-P, SDAV, UV-inactivated RCoV-P or SDAV, or mock inoculated. After a 1 h adsorption period, the cells were washed twice with DMEM, refed with DMEM/5% FBS and antibiotics, and incubated at 37 °C and 10% CO2. For mock inoculation of alveolar type I cells, TMS buffer with 5% glycerol was diluted in DMEM at the same ratio as the virus inoculum. Twenty-four hours after inoculation, type I cells on coverslips were fixed in methanol/acetic acid (3:1). Viral nucleocapsid antigen was detected using a monoclonal antibody to mouse hepatitis virus nucleocapsid protein (kindly provided by Dr. J. Leibowitz, Texas A&M University, College Station, TX) that cross-reacts with RCoV nucleocapsid protein, followed by a FITC-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). At intervals after inoculation, media from inoculated cultures were collected for viral titration, cytokine protein arrays and ELISAs. Total cellular RNA was extracted for quantification of chemokine mRNA as described below. For experiments with receptor antagonists, IL-1ra (10 ug/ml), TNFsrp (10 ug/ml), neither antagonist, or both IL-1ra and TNFsrp were added to type I cells 30 min prior to inoculation with SDAV. The concentration of antagonist was maintained in the culture throughout the 18 h infection. IL-1ra and TNFsrp were kindly provided by Dr. Charles Dinarello (University of Colorado Health Sciences Center, Denver, CO).
Plaque assay
Stocks of purified virus or medium from virus infected cultures was serially diluted in DMEM without FBS and used to inoculate triplicate wells of near confluent L2P-41.a cells. After a 1 h adsorption period at 37 °C, the inoculum was removed and the cells were overlaid with MEM, 4% FBS, antibiotics, and 0.5% SeaKem LE agarose (Cambrex, Rockland, ME). Plaques were stained after 72 h incubation at 37 °C, with the agarose overlay medium containing 6% neutral red (Sigma-Aldrich).
Cytokine protein array
Type I cells were inoculated with RCoV-P or SDAV, or were mock-inoculated as described above. Media from type I cells were collected 24 h after inoculation, centrifuged to remove cellular debris, and incubated with membranes spotted with antibodies specific for 19 rat cytokines and chemokines (RayBiotech, Inc., Norcross, GA). The membranes were processed according to the manufacturer's recommendations. The membranes were exposed to X-ray film and the film was scanned for densitometry analysis using Kodak Molecular Imaging Software (Eastman Kodak Company, Rochester, NY). Densitometry data were normalized to internal positive controls on each membrane and graphed as relative units.
Real-Time quantitative RT-PCR
Total RNA was isolated from alveolar epithelial cells using Trizol reagent according to the manufacturer's specifications (Invitrogen Corp., Carlsbad, CA). Reverse transcription and TaqMan PCR (Applied Bioscience) were performed on an ABI Prism 7700 sequence detection system as previously described (Wang et al., 2006), using published primer pairs and fluorogenic probes for CINC-2α (Wang et al., 2006), CINC-3 (Nishina et al., 2005), and LIX (Jeyaseelan et al., 2005). The mRNA expression levels of CINC-2, CINC-3, and LIX were normalized to the level of GAPDH mRNA for each sample, using a commercially available rat GAPDH kit (Applied Bioscience).
Enzyme linked immunosorbent assay (ELISA)
Media from RCoV-inoculated or mock-inoculated alveolar type I cells were harvested 6, 24, and 48 h after inoculation for the measurement of CINC-2, CINC-3, and LIX by ELISA. ELISA assays were developed by ELISAtech (Aurora, CO) using standards and antibodies from R&D Systems (Minneapolis, MN). The CINC-2β antibody (R&D Systems) used in the ELISA shows significant cross-reactivity to CINC-2α, hence the product of this ELISA is referred to as CINC-2. Chemokine levels were quantified by comparison of values with that of a standard curve for each chemokine. The reported values are averages ± standard error of the mean of at least three independent experiments with two replicate wells per experiment. The chemokine concentrations were compared between samples using one-way ANOVA. P values were computed using the Newman–Keuls multiple comparison test.
Dual immunolabeling of viral antigen and CXC chemokines
Type I cells were inoculated with RCoV-P or SDAV, or were mock-inoculated. After 18 h, a protein transport inhibitor that contained brefeldin A (BD GolgiPlug, BD Biosciences) was added to the culture medium as recommended by the manufacturer. Six hours later, the cells were fixed with 2% paraformaldehyde and permeabilized with 0.2% Triton X-100 (Sigma Aldrich). Viral nucleocapsid protein was detected using a monoclonal antibody to MHV nucleocapsid (described above) followed by an Alexafluor 594-conjugated anti-mouse secondary antibody (Invitrogen). CXC chemokines were detected with goat antibodies (R&D Systems) to rat LIX (AF543), CINC-3 (AF525), or CINC-2α (AF516) followed by an Alexafluor 488-conjugated anti-goat secondary antibody (Invitrogen).
Acknowledgments
This work was support by NIH grants AI-059576 and HL-029891. The authors would like to thank Julian Leibowitz, Dean Percy, and Charles Dinarello for reagents; Rizwan Manzer, Dennis Voelker, Emily Travanty, and Samuel Dominguez for helpful discussions; and Karen Edeen, Anna Castano, and Christian Pontillo for expert technical support.
References
- Abu-Harb M., Bell F., Finn A., Rao W.H., Nixon L., Shale D., Everard M.L. IL-8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis. Eur. Respir. J. 1999;14(1):139–143. doi: 10.1034/j.1399-3003.1999.14a23.x. [DOI] [PubMed] [Google Scholar]
- Auten R.L., Jr., Mason S.N., Tanaka D.T., Welty-Wolf K., Whorton M.H. Anti-neutrophil chemokine preserves alveolar development in hyperoxia-exposed newborn rats. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2001;281(2):L336–L344. doi: 10.1152/ajplung.2001.281.2.L336. [DOI] [PubMed] [Google Scholar]
- Bhatt P.N., Jacoby R.O. Experimental infection of adult axenic rats with Parker's rat coronavirus. Arch. Virol. 1977;54(4):345–352. doi: 10.1007/BF01314779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P.N., Percy D.H., Jonas A.M. Characterization of the virus of sialodacryoadenitis of rats: a member of the coronavirus group. J. Infect. Dis. 1972;126(2):123–130. doi: 10.1093/infdis/126.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch C.J., Clothier H.J., Seccull A., Tran T., Catton M.C., Lambert S.B., Druce J.D. Human coronavirus OC43 causes influenza-like illness in residents and staff of aged-care facilities in Melbourne, Australia. Epidemiol. Infect. 2005;133(2):273–277. doi: 10.1017/s0950268804003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borok Z., Hami A., Danto S.I., Zabski S.M., Crandall E.D. Rat serum inhibits progression of alveolar epithelial cells toward the type I cell phenotype in vitro. Am. J. Respir. Cell Mol. Biol. 1995;12(1):50–55. doi: 10.1165/ajrcmb.12.1.7811470. [DOI] [PubMed] [Google Scholar]
- Borok Z., Danto S.I., Lubman R.L., Cao Y., Williams M.C., Crandall E.D. Modulation of t1alpha expression with alveolar epithelial cell phenotype in vitro. Am. J. Physiol. 1998;275(1 Pt 1):L155–L164. doi: 10.1152/ajplung.1998.275.1.L155. [DOI] [PubMed] [Google Scholar]
- Borok Z., Lubman R.L., Danto S.I., Zhang X.L., Zabski S.M., King L.S., Lee D.M., Agre P., Crandall E.D. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: expression of aquaporin 5. Am. J. Respir. Cell Mol. Biol. 1998;18(4):554–561. doi: 10.1165/ajrcmb.18.4.2838. [DOI] [PubMed] [Google Scholar]
- Chan M.C., Cheung C.Y., Chui W.H., Tsao S.W., Nicholls J.M., Chan Y.O., Chan R.W., Long H.T., Poon L.L., Guan Y., Peiris J.S. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar B., Melby P.C., Sarau H.M., Raveendran M., Perla R.P., Marelli-Berg F.M., Dulin N.O., Singh I.S. Chemokine-cytokine cross-talk. The ELR+ CXC chemokine LIX (CXCL5) amplifies a proinflammatory cytokine response via a phosphatidylinositol 3-kinase–NF-kappa B pathway. J. Biol. Chem. 2003;278(7):4675–4686. doi: 10.1074/jbc.M207006200. [DOI] [PubMed] [Google Scholar]
- Chang Y.J., Liu C.Y., Chiang B.L., Chao Y.C., Chen C.C. Induction of IL-8 release in lung cells via activator protein-1 by recombinant baculovirus displaying severe acute respiratory syndrome-coronavirus spike proteins: identification of two functional regions. J. Immunol. 2004;173(12):7602–7614. doi: 10.4049/jimmunol.173.12.7602. [DOI] [PubMed] [Google Scholar]
- Compton S.R., Smith A.L., Gaertner D.J. Comparison of the pathogenicity in rats of rat coronaviruses of different neutralization groups. Lab. Anim. Sci. 1999;49(5):514–518. [PubMed] [Google Scholar]
- Danto S.I., Shannon J.M., Borok Z., Zabski S.M., Crandall E.D. Reversible transdifferentiation of alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 1995;12(5):497–502. doi: 10.1165/ajrcmb.12.5.7742013. [DOI] [PubMed] [Google Scholar]
- De Albuquerque N., Baig E., Ma X., Zhang J., He W., Rowe A., Habal M., Liu M., Shalev I., Downey G.P., Gorczynski R., Butany J., Leibowitz J., Weiss S.R., McGilvray I.D., Phillips M.J., Fish E.N., Levy G.A. Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. J. Virol. 2006;80(21):10382–10394. doi: 10.1128/JVI.00747-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devalaraja R.M., Nanney L.B., Du J., Qian Q., Yu Y., Devalaraja M.N., Richmond A. Delayed wound healing in CXCR2 knockout mice. J. Invest. Dermatol. 2000;115(2):234–244. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Dinarello C.A. Differences between anti-tumor necrosis factor—a monoclonal antibodies and soluble TNF receptors in host defense impairment. J. Rheumatol. 2005;32:40–47. [PubMed] [Google Scholar]
- Dobbs L.G., Mason R.J. Pulmonary alveolar type II cells isolated from rats. Release of phosphatidylcholine in response to beta-adrenergic stimulation. J. Clin. Invest. 1979;63(3):378–387. doi: 10.1172/JCI109313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs L.G., Williams M.C., Brandt A.E. Changes in biochemical characteristics and pattern of lectin binding of alveolar type II cells with time in culture. Biochim. Biophys. Acta. 1985;846(1):155–166. doi: 10.1016/0167-4889(85)90121-1. [DOI] [PubMed] [Google Scholar]
- Dobbs L.G., Williams M.C., Gonzalez R. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim. Biophys. Acta. 1988;970(2):146–156. doi: 10.1016/0167-4889(88)90173-5. [DOI] [PubMed] [Google Scholar]
- Dunstan C.A., Salafranca M.N., Adhikari S., Xia Y., Feng L., Harrison J.K. Identification of two rat genes orthologous to the human interleukin-8 receptors. J. Biol. Chem. 1996;271(51):32770–32776. doi: 10.1074/jbc.271.51.32770. [DOI] [PubMed] [Google Scholar]
- Evans M.J., Cabral L.J., Stephens R.J., Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp. Mol. Pathol. 1975;22(1):142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Faisca P., Desmecht D. Sendai virus, the mouse parainfluenza type 1: a longstanding pathogen that remains up-to-date. Res. Vet. Sci. 2007;82(1):115–125. doi: 10.1016/j.rvsc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Fouchier R.A., Hartwig N.G., Bestebroer T.M., Niemeyer B., de Jong J.C., Simon J.H., Osterhaus A.D. A previously undescribed coronavirus associated with respiratory disease in humans. Proc. Natl. Acad. Sci. U.S.A. 2004;101(16):6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneten S., Scanga C.A., Dveksler G.S., Beauchemin N., Percy D., Holmes K.V. Attachment glycoproteins and receptor specificity of rat coronaviruses. Lab. Anim. Sci. 1996;46(2):159–166. [PubMed] [Google Scholar]
- Gonzalez R., Yang Y.H., Griffin C., Allen L., Tigue Z., Dobbs L. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2005;288:L179–L189. doi: 10.1152/ajplung.00272.2004. [DOI] [PubMed] [Google Scholar]
- Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A., Rimmelzwaan G.F., van Amerongen G., van Riel D., de Jong T., Itamura S., Chan K.H., Tashiro M., Osterhaus A.D. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 2004;10(3):290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson C.A., Jardine A., Edwards M.R., Laza-Stanca V., Johnston S.L. Toll-like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection of human bronchial epithelial cells. J. Virol. 2005;79(19):12273–12279. doi: 10.1128/JVI.79.19.12273-12279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono K.T., Kazi L., Weiss S.R. Both spike and background genes contribute to murine coronavirus neurovirulence. J. Virol. 2006;80(14):6834–6843. doi: 10.1128/JVI.00432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T., Hatakeyama M., Taima K., Ishikawa A., Yamashita K., Yoshida H., Satoh K. Effect of double-stranded RNA on the expression of epithelial neutrophil activating peptide-78/CXCL-5 in human endothelial cells. Inflammation. 2004;28(4):215–219. doi: 10.1023/b:ifla.0000049046.23377.44. [DOI] [PubMed] [Google Scholar]
- Jacoby R.O., Bhatt P.N., Jonas A.M. Pathogenesis of sialodacryoadenitis in gnotobiotic rats. Vet. Pathol. 1975;12(3):196–209. doi: 10.1177/030098587501200305. [DOI] [PubMed] [Google Scholar]
- Jeyaseelan S., Manzer R., Young S.K., Yamamoto M., Akira S., Mason R.J., Worthen G.S. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am. J. Respir. Cell Mol. Biol. 2005;32(6):531–539. doi: 10.1165/rcmb.2005-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima A., Okaniwa A. Antigenic heterogeneity of sialodacryoadenitis virus isolates. J. Vet. Med. Sci. 1991;53(6):1059–1063. doi: 10.1292/jvms.53.1059. [DOI] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kuypers J., Martin E.T., Heugel J., Wright N., Morrow R., Englund J.A. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics. 2007;119(1):e70–e76. doi: 10.1542/peds.2006-1406. [DOI] [PubMed] [Google Scholar]
- Lane T.E., Asensio V.C., Yu N., Paoletti A.D., Campbell I.L., Buchmeier M.J. Dynamic regulation of alpha- and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol. 1998;160(2):970–978. [PubMed] [Google Scholar]
- Law A.H., Lee D.C., Cheung B.K., Yim H.C., Lau A.S. Role for nonstructural protein 1 of severe acute respiratory syndrome coronavirus in chemokine dysregulation. J. Virol. 2007;81(1):416–422. doi: 10.1128/JVI.02336-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londhe V.A., Belperio J.A., Keane M.P., Burdick M.D., Xue Y.Y., Strieter R.M. CXCR2 is critical for dsRNA-induced lung injury: relevance to viral lung infection. J. Inflamm. (Lond.) 2005;2(1):4. doi: 10.1186/1476-9255-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzer R., Wang J., Nishina K., McConville G., Mason R.J. Alveolar epithelial cells secrete chemokines in response to IL-1beta and lipopolysaccharide but not to ozone. Am. J. Respir. Cell Mol. Biol. 2006;34(2):158–166. doi: 10.1165/rcmb.2005-0205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.D., Rochelle L.G., Fischer B.M., Krunkosky T.M., Adler K.B. Airway epithelium as an effector of inflammation: molecular regulation of secondary mediators. Eur. Respir. J. 1997;10(9):2139–2146. doi: 10.1183/09031936.97.10092139. [DOI] [PubMed] [Google Scholar]
- Mason R.J. Biology of alveolar type II cells. Respirology. 2006;11:S12–S15. doi: 10.1111/j.1440-1843.2006.00800.x. (Suppl.) [DOI] [PubMed] [Google Scholar]
- Mason R.J., Lewis M.C., Edeen K.E., McCormick-Shannon K., Nielsen L.D., Shannon J.M. Maintenance of surfactant protein A and D secretion by rat alveolar type II cells in vitro. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2002;282(2):L249–L258. doi: 10.1152/ajplung.00027.2001. [DOI] [PubMed] [Google Scholar]
- McCray P.B., Jr., Pewe L., Wohlford-Lenane C., Hickey M., Manzel L., Shi L., Netland J., Jia H.P., Halabi C., Sigmund C.D., Meyerholz D.K., Kirby P., Look D.C., Perlman S. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007;81(2):813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchjorsen J., Sorensen L.N., Paludan S.R. Expression and function of chemokines during viral infections: from molecular mechanisms to in vivo function. J. Leukoc. Biol. 2003;74(3):331–343. doi: 10.1189/jlb.1102577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.G., Carnell L., Moore H.H. Post-Golgi membrane traffic: brefeldin A inhibits export from distal Golgi compartments to the cell surface but not recycling. J. Cell Biol. 1992;118(2):267–283. doi: 10.1083/jcb.118.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata N., Iwata N., Hasegawa H., Fukushi S., Yokoyama M., Harashima A., Sato Y., Saijo M., Morikawa S., Sata T. Participation of both host and virus factors in induction of severe acute respiratory syndrome (SARS) in F344 rats infected with SARS coronavirus. J. Virol. 2007;81(4):1848–1857. doi: 10.1128/JVI.01967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina K., Zhang F., Nielsen L.D., Edeen K., Wang J., Mason R.J. Expression of CINC-2beta is related to the state of differentiation of alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 2005;33(5):505–512. doi: 10.1165/rcmb.2005-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J.C., Cross S.S., Rowe W.P. Rat coronavirus (RCV): a prevalent, naturally occurring pneumotropic virus of rats. Arch. Gesamte Virusforsch. 1970;31(3):293–302. doi: 10.1007/BF01253764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pene F., Merlat A., Vabret A., Rozenberg F., Buzyn A., Dreyfus F., Cariou A., Freymuth F., Lebon P. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin. Infect. Dis. 2003;37(7):929–932. doi: 10.1086/377612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy D.H., Williams K.L., Paturzo F.X. A comparison of the sensitivity and specificity of sialodacryoadenitis virus, Parker's rat coronavirus, and mouse hepatitis virus-infected cells as a source of antigen for the detection of antibody to rat coronaviruses. Arch. Virol. 1991;119(3–4):175–180. doi: 10.1007/BF01310668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrc K., Berkhout B., van der Hoek L. The novel human coronaviruses NL63 and HKU1. J. Virol. 2007;81(7):3051–3057. doi: 10.1128/JVI.01466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Paddock C., Vogel L., Butler E., Zaki S., Subbarao K. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J. Virol. 2005;79(9):5833–5838. doi: 10.1128/JVI.79.9.5833-5838.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Deming D., Paddock C.D., Cheng A., Yount B., Vogel L., Herman B.D., Sheahan T., Heise M., Genrich G.L., Zaki S.R., Baric R., Subbarao K. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3(1):e5. doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H.F., Bonville C.A., Easton A.J., Domachowske J.B. The pneumonia virus of mice infection model for severe respiratory syncytial virus infection: identifying novel targets for therapeutic intervention. Pharmacol. Ther. 2005;105(1):1–6. doi: 10.1016/j.pharmthera.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Schmitz N., Kurrer M., Bachmann M.F., Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 2005;79(10):6641–6648. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstatter I.U., Chung J., Burger M. IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac signaling pathways. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2001;280(6):L1094–L1103. doi: 10.1152/ajplung.2001.280.6.L1094. [DOI] [PubMed] [Google Scholar]
- Shibata F., Konishi K., Kato H., Komorita N., al-Mokdad M., Fujioka M., Nakagawa H. Recombinant production and biological properties of rat cytokine-induced neutrophil chemoattractants, GRO/CINC-2 alpha, CINC-2 beta and CINC-3. Eur. J. Biochem. 1995;231(2):306–311. doi: 10.1111/j.1432-1033.1995.tb20701.x. [DOI] [PubMed] [Google Scholar]
- Shibata F., Konishi K., Nakagawa H. Identification of a common receptor for three types of rat cytokine-induced neutrophil chemoattractants (CINCs) Cytokine. 2000;12(9):1368–1373. doi: 10.1006/cyto.2000.0739. [DOI] [PubMed] [Google Scholar]
- Smith J.B., Wadleigh D.J., Xia Y.R., Mar R.A., Herschman H.R., Lusis A.J. Cloning and genomic localization of the murine LPS-induced CXC chemokine (LIX) gene, Scyb5. Immunogenetics. 2002;54(8):599–603. doi: 10.1007/s00251-002-0501-5. [DOI] [PubMed] [Google Scholar]
- Starner T.D., Barker C.K., Jia H.P., Kang Y., McCray P.B., Jr. CCL20 is an inducible product of human airway epithelia with innate immune properties. Am. J. Respir. Cell Mol. Biol. 2003;29(5):627–633. doi: 10.1165/rcmb.2002-0272OC. [DOI] [PubMed] [Google Scholar]
- Stievano L., Piovan E., Amadori A. C and CX3C chemokines: cell sources and physiopathological implications. Crit. Rev. Immunol. 2004;24(3):205–228. doi: 10.1615/critrevimmunol.v24.i3.40. [DOI] [PubMed] [Google Scholar]
- Sturman L.S., Holmes K.V., Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J. Virol. 1980;33(1):449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N.L., Chan P.K., Wong C.K., To K.F., Wu A.K., Sung Y.M., Hui D.S., Sung J.J., Lam C.W. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin. Chem. 2005;51(12):2333–2340. doi: 10.1373/clinchem.2005.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirado R., Ortega A., Sarmiento R.E., Gomez B. Interleukin-8 mRNA synthesis and protein secretion are continuously up-regulated by respiratory syncytial virus persistently infected cells. Cell. Immunol. 2005;233(1):61–71. doi: 10.1016/j.cellimm.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Tseng C.T., Huang C., Newman P., Wang N., Narayanan K., Watts D.M., Makino S., Packard M.M., Zaki S.R., Chan T.S., Peters C.J. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human Angiotensin-converting enzyme 2 virus receptor. J. Virol. 2007;81(3):1162–1173. doi: 10.1128/JVI.01702-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui P.T., Kwok M.L., Yuen H., Lai S.T. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg. Infect. Dis. 2003;9(9):1064–1069. doi: 10.3201/eid0909.030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey T.M., Garcia-Sastre A., Taubenberger J.K., Palese P., Swayne D.E., Pantin-Jackwood M.J., Schultz-Cherry S., Solorzano A., Van Rooijen N., Katz J.M., Basler C.F. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J. Virol. 2005;79(23):14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R.B. The role of neutrophils in the pathogenesis of rhinovirus infections. Pediatr. Infect. Dis. J. 1990;9(11):832–835. doi: 10.1097/00006454-199011000-00011. [DOI] [PubMed] [Google Scholar]
- Vanderbilt J.N., Mager E.M., Allen L., Sawa T., Wiener-Kronish J., Gonzalez R., Dobbs L.G. CXC chemokines and their receptors are expressed in type II cells and upregulated following lung injury. Am. J. Respir. Cell Mol. Biol. 2003;29(6):661–668. doi: 10.1165/rcmb.2002-0227OC. [DOI] [PubMed] [Google Scholar]
- van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C., Wertheim-van Dillen P.M., Kaandorp J., Spaargaren J., Berkhout B. Identification of a new human coronavirus. Nat. Med. 2004;10(4):368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.Z., Xu H., Wraith A., Bowden J.J., Alpers J.H., Forsyth K.D. Neutrophils induce damage to respiratory epithelial cells infected with respiratory syncytial virus. Eur. Respir. J. 1998;12(3):612–618. doi: 10.1183/09031936.98.12030612. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang S., Manzer R., McConville G., Mason R.J. Ozone induces oxidative stress in rat alveolar type II and type I-like cells. Free Radical Biol. Med. 2006;40(11):1914–1928. doi: 10.1016/j.freeradbiomed.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69(4):635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.C. Alveolar type I cells: molecular phenotype and development. Annu. Rev. Physiol. 2003;65:669–695. doi: 10.1146/annurev.physiol.65.092101.142446. [DOI] [PubMed] [Google Scholar]
- Wojcinski Z.W., Percy D.H. Sialodacryoadenitis virus-associated lesions in the lower respiratory tract of rats. Vet. Pathol. 1986;23(3):278–286. doi: 10.1177/030098588602300308. [DOI] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y., Wong B.H., Poon R.W., Cai J.J., Luk W.K., Poon L.L., Wong S.S., Guan Y., Peiris J.S., Yuen K.Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005;79(2):884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen Y.T., Liao F., Hsiao C.H., Kao C.L., Chen Y.C., Wu-Hsieh B.A. Modeling the early events of severe acute respiratory syndrome coronavirus infection in vitro. J. Virol. 2006;80(6):2684–2693. doi: 10.1128/JVI.80.6.2684-2693.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Luxon B.A., Casola A., Garofalo R.P., Jamaluddin M., Brasier A.R. Expression of respiratory syncytial virus-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J. Virol. 2001;75(19):9044–9058. doi: 10.1128/JVI.75.19.9044-9058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Stohlman S.A., Hinton D.R., Marten N.W. Neutrophils promote mononuclear cell infiltration during viral-induced encephalitis. J. Immunol. 2003;170(6):3331–3336. doi: 10.4049/jimmunol.170.6.3331. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Tang W., Gwaltney J.M., Jr., Wu Y., Elias J.A. Rhinovirus stimulation of interleukin-8 in vivo and in vitro: role of NF-kappaB. Am. J. Physiol. 1997;273(4 Pt 1):L814–L824. doi: 10.1152/ajplung.1997.273.4.L814. [DOI] [PubMed] [Google Scholar]