Abstract
While the origins and developmental course of self-injurious behavior (SIB) remain relatively unknown, recent studies suggest a biological imbalance may potentiate or provoke the contagious recurrence of SIB patterns in individuals with severe developmental disabilities (DD). Evidence from several laboratories indicates that functioning, relations, and processing of a stress-related molecule, proopiomelanocortin, (POMC), may be perturbed among certain subgroups of individuals exhibiting SIB. The current investigation employed a unique time-pattern analysis program (THEME) to examine whether recurrent temporal patterns (T-patterns) of SIB were related to morning levels of two POMC-derived hormones: β-endorphin (βE) and adrenocorticotropic hormone (ACTH). THEME was used to quantify highly significant (nonrandom) T-patterns that included SIB within a dataset of in-situ observational recordings spanning 8 days (~40 hours) in 25 subjects with DD. Pearson’s product-moment analyses revealed highly significant correlations between the percentage of T-patterns containing SIB and basal levels of both βE and ACTH, which were not found with any other “control” T-patterns. These findings support the hypothesis that the recurrent temporal patterning of SIB represents a unique behavioral phenotype directly related to perturbed levels of POMC-derived stress hormones in certain individuals with severe DD.
Keywords: self-injurious behavior, developmental disabilities, temporal pattern analysis, proopiomelanocortin, ACTH, β-endorphin
1. Introduction
Self-injurious behavior (SIB) is among the most troubling and persistent maladaptive behaviors observed in individuals with severe developmental disabilities (DD) and may be interpreted as a key manifestation of psychiatric co-morbidity. Estimations of the prevalence of SIB have been reported to be as high as 66% among institutionalized individuals with severe DD (Schroeder et al., 1978; Rojahn, 1984; Griffin et al., 1986). Although recent years have seen a dramatic increase in research on the topic, there still exists no clear consensus regarding the transitory causes or ‘triggers’ (social or biological) or developmental course of SIB in this population. A primary obstacle to understanding and treating SIB is the absence of reliable and effective methods for quantifying the complex, recurrent patterns of SIB across varying settings, such that relations with a variety of factors may be empirically assessed. Indeed, the topographies and circumstances surrounding recurrences of SIB vary so considerably from one individual to the next that establishing a metric or analytical technique to reliably assess severity, change, or temporal contingencies is a major methodological impediment to fully understanding the nature of the disorder (Schroeder et al., 2002; Symons et al., 2005).
Sackett (1979) provided a detailed description of the application of lag sequential analyses to directly address the complexity and constraints of existing methods for identifying contingent relations across time in multivariate observational data. The conceptual basis for lag analyses derives from the quantitative methods of auto- and cross-correlation. When applied to qualitative behavioral data, the lag principle examines the conditional (or transitional) probability that a criterion event of interest will be sequentially followed by another event of interest (event lag), or that any observed event will fall within a specified temporal window in relation to the criterion event (time lag) (for review, see Bakeman and Gottman, 1997). Of particular relevance to the current investigation are studies that have applied this method of analysis to maladaptive or challenging behaviors such as SIB.
In a cross-validational comparison of time-based lag sequential analyses with traditional, experimental (functional) analyses, Emerson et al. (1995) found a high degree of consistency between the two approaches (86% agreement in the identification of behavioral processes underlying SIB). These results were interpreted as lending support to the external validity and overall viability of time-based lag analyses for exploring the mechanisms and contextual contingencies underlying SIB in DD populations.
In the first reported application of sequential analyses to examine whether contextual contingencies of SIB change with pharmacological treatment, Symons et al., (2001) employed an event-based lag approach to assess the sequential dependencies between SIB and antecedent staff instructional behavior during opiate-antagonist (Naltrexone) and placebo administration. Though their conclusions were limited by a low number of subjects (n=4), they did report reductions in the rate of SIB and an increase in the sequential dependencies between staff behavior and SIB during Naltrexone treatment. They suggest that, because SIB may be maintained by multiple motivating events that are both behaviorally and biologically based, treatment with Naltrexone may serve to selectively reduce SIB that is opioidergically mediated, leaving socially mediated SIB unchanged, thereby increasing the relative sequential dependencies between SIB and social environment.
Recently, researchers from our project team (Marion et al., 2003) employed time-based lag sequential analyses to examine whether successive episodes of SIB were sequentially dependent. Sequential dependencies were determined by calculating the conditional probabilities that a match event followed a criterion event within four windows of time: 2, 10, 30, and 60 seconds. The results indicated that the only, highly significant, sequential predictor of a SIB event was another, antecedent SIB event. There was no evidence that SIB was conditional (sequentially dependent) on environmental events or on other observationally recorded behaviors within these temporal windows. Furthermore, the method of analysis controlled for chance pairings of events and revealed that the sequential patterns of SIB were independent of frequency or rate of occurrence. Additionally, the conclusion that SIB occur in sequentially related “bouts” was also confirmed using survival analyses to quantify the temporal distribution of SIB patterns (Kroeker et al., 2004). These results suggest that, within some individuals with severe DD, SIB follows “contagious” temporal distribution patterns, which may represent a unique behavioral phenotype that is maintained by biological rather than social or environmental factors.
In a related project, Sandman et al. (2002) found these contagious patterns of SIB reached highest conditional probabilities in subjects who exhibited a disregulation (“uncoupling”) of the proopiomelanocortin (POMC) system, as characterized by elevated morning (basal) levels of β-endorphin (βE) relative to basal levels of adrenocorticotropic hormone (ACTH). These two hormones are POMC-derived neuropeptides that are involved in the stress response, as part of the hypothalamic-pituitary-adrenal axis (ACTH), and in the modulation of pain and pleasure because of their affinity for the opiate receptors (βE).
The purpose of the current investigation was to examine whether the relation between the POMC system and recurrent patterns of SIB could be extended by employing a fundamentally different method of analysis on our in-situ observational data. An inherent limitation of lag sequential analyses is that the temporal windows or variables of interest must be specified a priori. Thus, a limiting assumption of this method is that the behavioral patterns of interest will follow a sequential distribution. The consequence of such an assumption, is that it may discount the possibility that the behavior may be patterned according to nonsequential, or non-obvious, temporal distributions. Furthermore, Sackett (1979) cautions that lag sequential methods are vulnerable to “capitalization on chance”, meaning that as the number of observations collected increases sufficiently, so too will the probabilities of finding significant sequential dependencies. While Bakeman and Gottman (1997) provide detailed methods for controlling such Type 1 errors, they caution that this is an issue of concern whenever sequential analytic methods are employed.
In order to address such concerns, the current study utilized a unique, probabilistic, temporal pattern analysis program known as THEME™ (PatternVision Ltd and Noldus Information Technology bv). As developed by Magnusson (1996, 2000), THEME provides a statistical method of detecting temporal patterns (T-patterns) of related behavioral events that may not be obvious to a trained observer or identifiable by traditional sequential methods. The pattern detection algorithm first identifies significant (non-random) recurrences of any two events within a similar temporal configuration (critical interval) in a real-time behavioral record and then proceeds to identify hierarchical relations with any other antecedent or subsequent events, both sequential and nonsequential in distribution.
T-patterns “grow” in complexity as simple patterns are incorporated into larger patterns, and are retained for further analysis according to whether they meet the search parameters specified by the user. Among these parameters are the probability that a given T-pattern will occur in a Poisson distribution of the current record, the transitional probability that component patterns must possess to be included in a larger pattern, and the minimum number of instances that detected patterns recur across the record. Thus, the search algorithm detects highly significant, hierarchically arranged T-patterns that are composed of statistically related behavioral events that repeatedly appear in the same, relatively invariant, temporal configuration regardless of whether they are sequentially or nonsequentially distributed. Hence, the major advantages of this method are that it is not constrained by implicit assumptions about the distribution of the behaviors of interest and allows the user to select the relevant probability levels to be tested against a random distribution of the actual behavioral record, thereby providing programmatic control of vulnerability to chance findings.
Several recent studies have utilized such methods to examine the relations between overt behavioral patterns and underlying regulatory mechanisms. Lyon and Kemp (2004) described the effects of various antipsychotic medications on patients with schizophrenia and mania showing abnormal (perseverative) temporal patterning of behavioral responses on a computerized two-choice guessing task. These abnormal patterns were interpreted as providing insight into the underlying cognitive processes that are characteristically disturbed in individuals with these disorders, and have also been suggested to aid in diagnostic specificity (Lyon, Lyon & Magnusson, 1994) and as surrogate dynamical markers of prenatal stressor effects (Lyon and Kemp, 2003). Similarly, in a study examining stereotypic locomotor activity in mice, Bonasera et al (2006) reported that “T-pattern analysis is a versatile and robust pattern detection and quantification algorithm that may augment currently available behavioral phenotyping strategies.” Furthermore, Hirschenhauser and Frigerio (2005) recently described the use of time-pattern analyses to examine the temporal relations between peak levels of testosterone and progesterone and sexual behavior among healthy males. They suggest that the method warrants further applications to investigate the interaction between hormones and behavior and therefore should be regarded as an important emerging tool in the field of behavioral endocrinology.
The current investigation was designed to evaluate whether the application of temporal pattern analyses could further elucidate the hypothesized relations between disturbed basal levels of two POMC-derived hormones (βE and ACTH) and a unique behavioral presentation of temporally patterned recurrences of self-injury.
2. Methods
2.1. Subjects
The 25 participants (13 male, 12 female; mean age=40.5 years) were drawn from the population of a 900-bed residential facility for individuals with severe developmental disabilities. Study candidates were referred because of self-injury or agitation identified by primary care or residential staff members (familiar with each individual). Selection was based upon a thorough review by the research staff of charted behavior counts routinely recorded by residential staff. Informed consent was obtained from conservators and guardians of study participants and, where possible, from the individuals themselves (i.e., when cognitive and communicative skills did not prevent understanding the nature and implication of the study). The method of subject consent and the protocol were reviewed and approved by ethics oversight committees of the University of California, Irvine (UCI Institutional Review Board) and the State of California (Committee for the Protection of Human Subjects). All participants were over 16 years of age, and did not have a diagnosed medical condition that could have been responsible for their maladaptive behavior. The prevalence of DSM-IV Axis I disorders was: Autistic Disorder, 32%; Mood Disorders, 26%; Stereotypic Movement Disorder, 19%; PICA, 10%; Impulse-Control Disorder, 10%; Thought Disorder, 3%. The distribution of Axis II disorders was: Profound Mental Retardation, 74%; Severe Mental Retardation, 10%; and Mild Mental Retardation, 3%. All individuals exhibiting SIB or agitation previously had been exposed to repeated behavioral and pharmacological interventions with either limited or no success.
2.2. Biological Measures
Blood samples (10 ml/draw) were collected via antecubital venipuncture in the morning (8 AM), immediately transferred into EDTA (purple top) Vacutainers, and chilled on ice. Samples were centrifuged at 2000 × g (15 min.) and the plasma was decanted into polypropylene tubes containing 500 KIU/ml aprotinin (Sigma; St. Louis, MO). The samples were stored at -70° C until assayed. Samples were collected on one day during the two-week period during which the observational data were collected. Previous studies have established the reliability of AM levels of βE and ACTH across 6 to 9 months, as would be indicative of trait-specific basal levels (Sandman et al, 2002).
2.2.1. β-Endorphin (βE)
Plasma levels of βE were determined by a commercially available direct solid phase two-site immunoradiometric assay (IRMA; Nichols Institute; San Juan Capistrano, CA, USA). The βE assay incorporated two antibodies with high affinity and specificity for N-terminal and C-terminal regions of the βE1-31 molecule. Both antibodies bound βE without competition or steric interference and formed a sandwich complex between the immobilized βE antibody on the plastic bead and 125I-labeled βE antibody. The antiserum has 16% cross-reactivity with beta-lipotropin at 500 pg/ml and has <0.01% cross-reactivity with related opiates at 5 μg/ml, as listed in the manufacturer’s information sheet. Samples were assayed in duplicate (200 μl per assay tube). 125I-anti-βE (rabbit) solution (100 μl) was added to each tube and vortexed. The reaction was initiated by adding one anti-βE (rabbit) coated polystyrene bead to the assay tube followed by a stationary incubation at room temperature for 20 ± 1 hours. The beads were then washed twice with phosphate buffered saline and aspirated to dryness. The labeled antibody complex bound to the solid phase was measured using an ICN Biomedical (formerly Micromedic) Isoflex Gamma Counter. All plasma βE values were calculated using a four-parameter logistics program (Rodbard and Hutt, 1974) obtained from a modified standard curve which included two additional low level standards. The calculated assay sensitivity using this method was 5.7 pg/ml with intra- and inter-assay variations of 4.2% and 8.3%, respectively.
2.2.2. Adrenocorticotropic Hormone (ACTH)
Levels of ACTH were measured directly in plasma using a commercial immunoradiometric assay (IRMA; Nichols Institute; San Juan Capistrano, CA, USA). The ACTH immunoassay measures intact ACTH in a radiolabeled soluble sandwich complex bound to two antibodies with high affinity and specificity for ACTH coupled to a solid bead matrix. The antiserum employed has <0.001% cross-reactivity with βE and ACTH fragments, and the detection limits of the ACTH IRMA were reported as 1.0 pg/ml in the manufacturer’s information sheet. Briefly, duplicate samples (200 μl per assay tube) were combined with ACTH 125I-antibody solution (100 μl), vortexed , and incubated at room temperature for 20 ± 1 hours after the addition of an avidin-coated bead. The solid matrix was washed with buffered surfactant in phosphate-buffered saline to remove unbound components and the bound radiolabeled antibody complex was quantified using an ICN Biomedical (formerly Micromedic) Isoflex Gamma Counter. Concentrations of ACTH for each sample were determined directly from the standard curve computed by a four-parameter logistics program (Rodbard and Hutt, 1974). The intra- and inter-assay coefficients of variance were 4.4% and 10.8%, respectively.
2.3. Observational Procedures
Individual participants were observed by research staff throughout their regular daily routines, in situ, with minimal intrusion. Forty hours of data were collected in five-hour epochs each day (two and a half hours in the morning [9:30 AM to 12:00 PM] and afternoon [1:00 PM to 3:30 PM]) for each subject in a contiguous two-week period. Twenty categories of events were recorded using a novel palm-top computer-assisted observational system (The Observer®; Noldus Information Technology bv) that was used to digitally capture the time at which each behavioral and environmental event occurred (e.g. SIB, agitation, staff behavior, etc; Sandman et al., 2000b).
Observation of a large number of self-injurious individuals with varying behavioral topography required a coding strategy with a broad selection of the most salient features observed in the field and informed by previous research from this laboratory (Sandman et al., 2000b). SIB was composed of the following manifestations: hits self with open hand or fist (62.29%, ±35.76 of the total number of SIB events), bites self (8.01%, ±19.03), bangs head with or against an object (15.62%, ±25.69), any other self-inflicted harmful behavior (e.g., picking lesions; 14.00% ±28.09). As described elsewhere (Marion et al., 2003), staff interactions, peer interactions, stereotypies, staff proximity, agitation and restraint were clearly defined and recorded as discrete events. Specifically, agitated behavior (AB) was operationally defined as “behaviors that represented increased levels of activity or arousal but did not result in self injury, such as tempter tantrums, hyperactivity, pacing, etc”.
The observational data across eight days were collapsed into single data files for each of the 25 subjects. The files were spliced together, for each individual, by adding an artificial time gap to mark ‘hour’ and ‘day’ breaks between observational sessions. These gap events were then programmatically excluded from further analyses in THEME, so as to prohibit these artificial durations from contributing to the search algorithm and to negate any possibility of inclusion in the T-patterns of observed behaviors.
2.3.1. Interobserver Agreement
All research staff were trained to observe behavior and to utilize the software and hardware while viewing video vignettes until they achieved 100% agreement with a master observer. Training continued in vivo until the accuracy of the new observers’ data recording achieved an acceptable (above 85%) level of agreement with previously trained research personnel. Inter-rater reliability during data collection was established by comparing records of two observers simultaneously, but independently, recording the behavior of individuals during 117, 20-minute sessions. Inter-observer agreement was calculated across 4,680, 30-second intervals for each behavioral category (93,600 intervals × event/behaviors). Pearson product-moment correlations were computed between the two sets of recorded observations for each participant. The correlation coefficients were transformed into z-scores so they could be averaged across individuals and converted back to an averaged Pearson r. Pearson product-moment correlations between observer records were highly statistically significant for all categories of recorded events (r’s from 0.83 to 0.97).
2.4. THEME Procedures
The THEME (Version 5.0) search parameters, used to narrow or expand the sensitivity, or exclusivity, of the pattern detection algorithm, were systematically varied to assess their effects on the number of T-patterns identified on a representative file from the dataset. These results were used to determine the optimum combination of settings necessary to proceed with a thorough search on the current dataset. As such, the relevant parameters used to obtain the results discussed below were: 1) Minimum Occurrence = 3 – this specifies that a given pattern must occur a minimum of three times in the record to be included in the results; 2) Significance Level < 0.000001 – this specifies the probability that a given pattern would occur in a random (Poisson) distribution of the current data; 3) Lumping Factor = 0.99 – this specifies the transitional probability necessary for two patterns to be included into a larger pattern without the component patterns being included separately in the results (i.e., if pattern A follows pattern B at least 99% of the time, then pattern AB will replace the two component patterns A and B).
2.5. Data Analyses
The output of the temporal pattern analysis included an overview table containing summary statistics of the various T-patterns discovered in each of the 25 data files included in the project. A T-pattern filter within THEME was also employed to categorize T-patterns based on the events included in their composition. The selection of T-patterns containing self-injurious behavior (SIB), agitated behavior (AB), both SIB and AB, and “control” T-patterns containing non-injurious behavioral stereotypies (STER), and staff far away or absent (SFAR) were all obtained by this method. The overview tables for each of these output files were then combined with the results of the hormone level assays to yield a spreadsheet containing: the total number of T-patterns; the number and percentage of T-patterns containing SIB, AB, both SIB and AB, STER, and SFAR, respectively; and the βE and ACTH levels for each of the 25 subjects. These data were then analyzed using Pearson’s product-moment correlations in SPSS.
3. Results
The mean number of different T-patterns; the mean numbers and percentages of different T-patterns containing SIB, AB, both SIB and AB, STER, and SFAR, respectively; and the mean βE and ACTH levels are presented in Table 1. Of the five behavioral event types examined, T-patterns containing SFAR had the highest relative percentage of total T-patterns (78% ± 12), and T-patterns containing both SIB and AB had the lowest (6% ± 16).
Table 1.
Descriptive statistics of THEME-derived T-patterns and hormone levels (N=25)
| Mean | sd | Min | Max | |
|---|---|---|---|---|
| Total T-patterns | 4748 | 6855 | 150 | 26863 |
| SIB T-patterns | 1487 | 3816 | 0 | 16552 |
| % SIB T-patterns | 15 | 20 | 0 | 79 |
| AB T-patterns | 1342 | 3240 | 0 | 15748 |
| % AB T-patterns | 28 | 28 | 0 | 81 |
| SIB & AB T-patterns | 508 | 1825 | 0 | 8802 |
| % SIB & AB T-patterns | 6 | 16 | 0 | 67 |
| STER T-patterns | 3210 | 6267 | 0 | 23967 |
| % STER T-patterns | 41 | 34 | 0 | 92 |
| SFAR T-patterns | 3698 | 5309 | 85 | 19731 |
| % SFAR T-patterns | 78 | 12 | 56 | 94 |
| AM βE level (pg/ml) | 49.36 | 28.95 | 22.32 | 166.08 |
| AM ACTH level (pg/ml) | 46.38 | 42.52 | 6.59 | 230.56 |
As can be seen in Table 2, the most significant correlations identified were between the percent of T-patterns containing both SIB and AB and AM levels of βE (r = 0.825, P < 0.0005) and ACTH (r = 0.851, P < 0.0005). These correlations are graphically depicted as scatterplots in Figure 1 and Figure 2, respectively. Similarly, the percent of T-patterns containing SIB (without AB) also showed highly significant positive correlations with the same measures, though slightly lower in value. However, the percentage of T-patterns containing AB (without SIB) were not significantly correlated with βE (r = 0.175, P = 0.403) or ACTH (r = 0.187, P = 0.371). None of the other output “control” measures showed significant correlations with these hormone levels either.
Table 2.
Pearson’s r (P) correlations between THEME results, hormone levels, and behavior counts (N=25)
| Total
T-patterns |
% SIB
T-patterns |
% AB
T-patterns |
% SIB & AB
T-Patterns |
% STER
T-patterns |
% SFAR
T-patterns |
|
|---|---|---|---|---|---|---|
| AM βE level | 0.298 (0.147) | 0.794 (<0.000) | 0.175 (0.403) | 0.825 (<0.000) | -0.090 (0.668) | -0.065 (0.759) |
| AM ACTH level | 0.232 (0.265) | 0.793 (<0.000) | 0.187 (0.371) | 0.851 (<0.000) | -0.144 (0.494) | -0.092 (0.660) |
| Total SIB | 0.084 (0.690) | 0.200 (0.337) | 0.065 (0.755) | 0.148 (0.481) | 0.216 (0.228) | 0.043 (0.839) |
| SIB per hour | 0.115 (0.585) | 0.227 (0.274) | 0.068 (0.748) | 0.176 (0.401) | 0.209 (0.243) | 0.029 (0.892) |
| Total Behaviors | 0.573 (0.003) | 0.367 (0.073) | -0.229 (0.270) | 0.288 (0.163) | 0.437 (0.011) | -0.258 (0.213) |
Figure 1.
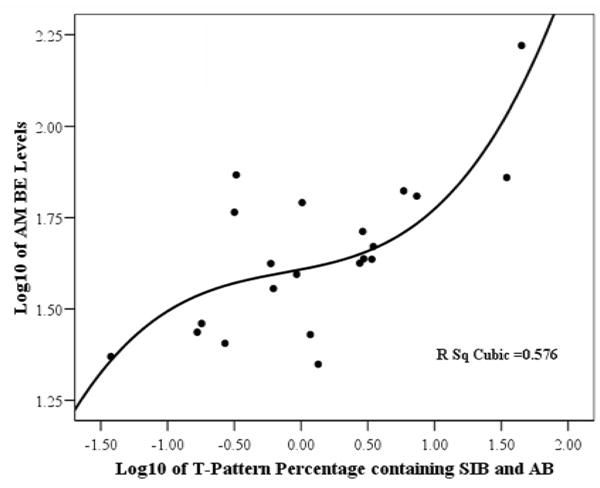
Scatterplot showing the correlation of AM levels of beta-endorphin (βE) with the percentage of T-patterns containing both self-injurious behaviors (SIB) and agitated behaviors (AB). (The data for both axes have been logarithmically transformed.)
Figure 2.
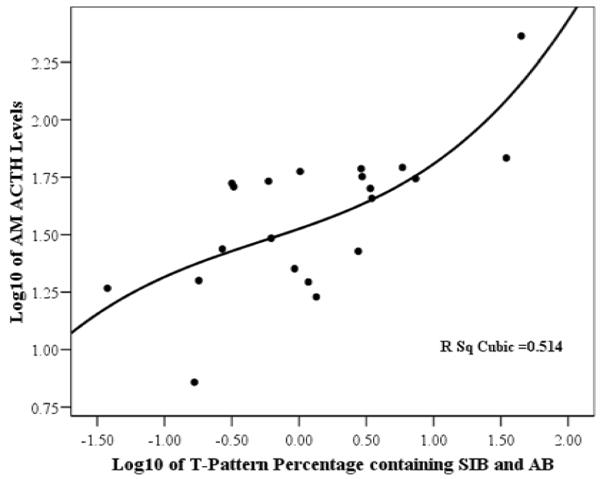
Scatterplot showing the correlation of AM levels of adrenocorticotropic hormone (ACTH) with the percentage of T-patterns containing both self-injurious behaviors (SIB) and agitated behaviors (AB). (The data for both axes have been logarithmically transformed.)
Furthermore, neither the total raw count of SIB nor the rate of SIB per hour correlated with any of the T-pattern measures. The total count of all observed behaviors did, however, correlate positively with the percentage of T-patterns containing stereotyped (but not self-injurious) behaviors (r = 0.437, P = 0.011), and the total number of patterns detected (r = 0.573, P = 0.003). This latter finding suggests that the relative percentage of T-patterns was the most appropriate measure with which to examine the relation of temporal patterning of SIB with the hormone levels, because it provided a control for the fact that the total number of detected T-patterns increased with the total number of behaviors recorded.
4. Discussion
The present results demonstrate that the recurrent temporal patterning of SIB can be statistically quantified across multiple observational sessions. These T-patterns are not linked by sequential proximity but by relatively invariant temporal relations not observable with traditional linear analyses. The total number of T-patterns were significantly correlated with the total number of behaviors coded, however the relative percentages of T-patterns containing SIB were not. Further, the percentages of T-patterns containing SIB were not correlated with either the frequency or total count of SIB observed. Thus, these findings do not appear to be an artifact of more detailed or extensive behavioral coding in those subjects displaying higher rates of SIB, but may instead represent an entirely new metric of the organizational dynamics underlying the expression of these behaviors across time.
These findings represent a unique contribution to the study of SIB, because they highlight the importance of using pattern analyses to identify the temporal contingencies among observationally recorded behaviors, without regard to sequential distribution. While not obvious or identifiable by traditional methods of analysis, temporal patterning may reveal the complex behavioral manifestations of regulatory mechanisms, which serve as control parameters in the emergent ordering of these seemingly disordered or chaotic patterns of behavior. This perspective, as endorsed by Thelen and Smith (1994) and Kelso (1995), may be of critical importance to future investigations of how this mysterious, maladaptive behavior may be nonsequentially organized across time and dynamically regulated by “internal” organizational processes.
Indeed, the current results have demonstrated some of the largest correlations yet observed between incidences of self-injury and a measure of biological derivation. The relative percentage of T-patterns containing SIB were found to have highly significant correlations with basal levels of two POMC-derived, stress-related hormones, particularly when accompanied by observations of AB. Neither the proportion of T-patterns containing non-injurious, agitated or stereotyped behaviors nor those indicating staff absence were found to have significant correlations with these hormone levels. As such, the current results are interpreted as lending support to the hypothesis that disturbances of the POMC system are related to a unique behavioral presentation of recurrent, temporal patterns of SIB.
These findings are in agreement with previous studies which have demonstrated a significant relation between SIB and the stress-related POMC system. Indeed, numerous studies from many laboratories have shown that the functioning, relations, and processing of the POMC system are uncoupled or perturbed in subgroups of self-injuring individuals, resulting in different ratios of βE and ACTH, particularly under conditions of stress (Leboyer et al., 1994, 1999; Gillberg, 1995; Cazzullo et al., 1999; Sandman et al., 1990a, 1990b, 1995, 1997, 1999, 2000a, 2002, 2003).
The current results confirm and extend these findings by demonstrating that the recurrent temporal patterning of SIB may indeed represent a behavioral phenotype which may be maintained by perturbed levels of POMC-derived stress hormones. Without the application of temporal pattern analyses, however, it would not have been possible to demonstrate such high levels of correlation between the clinical presentation of these behavioral patterns and their hypothesized underlying biological mechanisms. None of these previous studies have demonstrated a direct relation between raw counts or rates of SIB and basal levels of βE and ACTH. Previous research has established a relation with the relative ratios of βE and ACTH; however, this is the first clear indication that this unique behavioral measure may be used to reveal a more direct correspondence to trait-specific basal levels. As such, these results may portend the utility of temporal pattern analyses to identify a sub-type of subjects that may respond more beneficially to certain treatment approaches (e.g., Naltrexone) over others.
Quantifying the recurrent patterns of SIB, such that the relation between temporal contingencies and biological or environmental correlates may be assessed, is among the greatest challenges to elucidating the occurrence of this troubling psychiatric symptom among individuals with DD (Schroeder et al., 2002). The current study has demonstrated that computerized in-situ observational recordings combined with temporal pattern analyses offer the possibility of discovering otherwise undetectable physiological correspondences with recurrent patterns of self-injurious behavior. As such, these methods merit further application to investigate the temporal contingencies which may reveal causal relations with a variety of factors and to evaluate the potential efficacy of experimental interventions for this disturbing and persistent psychiatric condition.
Acknowledgments
This work was supported by grant HD31571 from the National Institutes of Child Health and Human Development. The assistance of Enjey Bahng and the student assistants who diligently collected the observational data is also gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakeman R, Gottman JM. Observing interaction: An introduction to sequential analysis. 2. Cambridge University Press; Cambridge, UK: 1997. [Google Scholar]
- Bonasera SJ, Schenk KA, Tecott LH. 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience, 2006; 2006. T-pattern sequential analysis to quantify “route tracing” locomotor stereotypic behavior. Program No. 836.3/KK4. [Google Scholar]
- Cazzullo AG, Musetti MC, Musetti L, Bajo S, Sacerdote P, Panerai A. Beta-endorphin levels in peripheral blood mononuclear cells and long-term naltrexone treatment in autistic children. European Neuropsychopharmacology. 1999;9:361–363. doi: 10.1016/s0924-977x(99)00010-3. [DOI] [PubMed] [Google Scholar]
- Emerson E, Thompson S, Reeves D, Henderson D, Robertson J. Descriptive analysis of multiple response topographies of challenging behavior across two settings. Research in Developmental Disabilities. 1995;16(4):301–329. doi: 10.1016/0891-4222(95)00016-g. [DOI] [PubMed] [Google Scholar]
- Gillberg C. Endogenous opioids and opiate antagonists in autism: Brief review of empirical findings and implications for clinicians. Developmental Medicine and Child Neurology. 1995;37:88–92. doi: 10.1111/j.1469-8749.1995.tb11998.x. [DOI] [PubMed] [Google Scholar]
- Griffin JC, Williams DE, Stark MT, Altmeyer BK, Mason M. Self-injurious behavior: A state-wide prevalence survey of the extent and circumstances. Applied Research in Mental Retardation. 1986;7:105–116. doi: 10.1016/0270-3092(86)90022-6. [DOI] [PubMed] [Google Scholar]
- Hirschenhauser K, Frigerio D. Hidden patterns of male sex hormones and behavior vary with life history. In: Anolli L, Duncan S, Magnusson MS, Riva G, editors. The hidden structure of interaction: From neurons to culture patterns. IOS Press; Amsterdam: 2005. pp. 81–96. [Google Scholar]
- Kelso JAS. Dynamic Patterns: The Self-Organization of Brain and Behavior. The MIT Press; Cambridge, MA: 1995. [Google Scholar]
- Kroeker R, Touchette PE, Engleman L, Sandman CA. Quantifying temporal distributions of self-injurious behavior: defining bouts versus discrete events. American Journal of Mental Retardation. 2004;109(1):1–8. doi: 10.1352/0895-8017(2004)109<1:QTDOSB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Bouvard MP, Recasens C, Philippe A, Guilloud-Bataille M, Bondoux D, Tabuteau F, Dugas M, Panksepp A, Launay JM. Difference between plasma N- and C-terminally directed beta-endorphin immunoreactivity in infantile autism. American Journal of Psychiatry. 1994;151:1797–1801. doi: 10.1176/ajp.151.12.1797. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Philippe A, Bouvard M, Guilloud-Bataille M, Bondoux D, Tabuteau F, Feingold J, Mouren-Simeoni MC, Launay JM. Whole blood serotinin and plasma beta-endorphin in autistic probands and their first degree relatives. Biological Psychiatry. 1999;45:158–163. doi: 10.1016/s0006-3223(97)00532-5. [DOI] [PubMed] [Google Scholar]
- Lyon M, Kemp AS. Excessive complexity in the temporal patterning of behavioral responses of patients born during World War I and II: A dynamical marker for prenatal stress?. Society of Biological Psychiatry Annual Meeting; San Francisco. May 15-17.2003. [Google Scholar]
- Lyon M, Kemp AS. Increased temporal patterns in choice responding and altered cognitive processes in schizophrenia and mania. Psychopharmacology. 2004;172(2):211–219. doi: 10.1007/s00213-003-1646-0. [DOI] [PubMed] [Google Scholar]
- Lyon M, Lyon N, Magnusson MS. The importance of temporal structure in analyzing schizophrenic behavior: some theoretical and diagnostic implications. Schizophrenia Research. 1994;13:45–56. doi: 10.1016/0920-9964(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Magnusson MS. Discovering hidden time patterns in behavior: T-patterns and their detection. Behavior Research Methods, Instruments and Computers. 2000;12(1):93–110. doi: 10.3758/bf03200792. [DOI] [PubMed] [Google Scholar]
- Magnusson MS. Hidden real-time patterns in intra- and inter-individual behavior: description and detection. European Journal of Psychological Assessment. 1996;12(2):112–123. [Google Scholar]
- Marion SD, Touchette PE, Sandman CA. Sequential analysis reveals a unique structure for self-injurious behavior. American Journal of Mental Retardation. 2003;108(5):301–13. doi: 10.1352/0895-8017(2003)108<301:SARAUS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rodbard D, Hutt DM. Radioimmunoassays and Related Procedures in Medicine. Vol. 1. Vienna: International Atomic Energy Agency, Vienna; 1974. pp. 165–192. [Google Scholar]
- Rojahn J. Self-injurious behavior in institutionalized, severely/profoundly retarded adults: Prevalence data and staff agreement. Journal of Behavioral Assessment. 1984;8:12–27. [Google Scholar]
- Sackett GP. The lag sequential analysis of contingency and cyclicity in behavioral interaction research. In: Osofsky JD, editor. Handbook of infant development. Wiley and Sons; New York: 1979. pp. 623–649. [Google Scholar]
- Sandman CA, Barron JL, Chicz-DeMet A, DeMet E. Plasma b-endorphin levels in patients with self-injurious behavior and stereotypy. American Journal of Mental Retardation. 1990a;95:3–10. [PubMed] [Google Scholar]
- Sandman CA, Barron JL, DeMet E, Chicz-DeMet A, Rothenburg S. Opioid peptides and development: Clinical implications. In: Koob GF, Sandman CA, Strand FL, editors. A Decade of Neuropeptides, Past, Present and Future. Annals of the New York Academy of Sciences; New York: 1990b. pp. 91–107. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Hetrick W, Talyor D, Marion S, Chicz-DeMet A. Uncoupling of proopiomelanocortin (POMC) fragments is related to self-injury. Peptides. 2000a;21(6):785–91. doi: 10.1016/s0196-9781(00)00209-6. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Hetrick WP, Taylor DV, Chicz-DeMet A. Dissociation of POMC peptides after self-injury predicts response to centrally acting opiate blockers. American Journal of Mental Retardation. 1997;102:182–199. doi: 10.1352/0895-8017(1997)102<0182:DOPPAS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Hetrick WP. Opiate mechanisms in self-injury. Mental Retardation and Developmental Disabilities Research Reviews. 1995;1:130–136. [Google Scholar]
- Sandman CA, Spence MA, Smith M. Proopiomelanocortin POMC disregulation and response to opiate blockers. Mental Retardation and Developmental Disabilities Research Reviews. 1999;5:314–321. [Google Scholar]
- Sandman CA, Touchette P, Lenjavi M, Marion S, Chicz-DeMet A. Beta-Endorphin and ACTH are dissociated after self-injury in adults with developmental disabilities. American Journal of Mental Retardation. 2003;108(6):414–24. doi: 10.1352/0895-8017(2003)108<414:EAAADA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Touchette P, Ly J, Marion S, Bruinsma Y. Computerized-assessment of treatment effects among individuals with developmental disabilities. In: Thompson T, Felces D, Symons F, editors. Behavioral Observations: Technology and Applications in Developmental Disabilities. Brookes Publishing; Baltimore: 2000b. pp. 271–293. [Google Scholar]
- Sandman CA, Touchette P, Marion S, Lenjavi M, Chicz-DeMet A. Disregulation of proopiomelanocortin and contagious maladaptive behavior. Regulatory Peptides. 2002;108:179–185. doi: 10.1016/s0167-0115(02)00097-6. [DOI] [PubMed] [Google Scholar]
- Schroeder SR, Oster-Granite ML, Thompson T, editors. Self-Injurious Behavior. American Psychological Association; Washington: 2002. [Google Scholar]
- Schroeder SR, Schroeder CS, Smith B, Dalldorf J. Prevalence of self-injurious behaviors in a large state facility for the retarded: A three-year follow-up study. Journal of Autism and Childhood Schizophrenia. 1978;8:261–269. doi: 10.1007/BF01539629. [DOI] [PubMed] [Google Scholar]
- Symons FJ, Sperry LA, Dropik PL, Bodfish JW. The early development of stereotypy and self-injury: A review of research methods. Journal of Intellectual Disabilities Research. 2005;49(2):144–58. doi: 10.1111/j.1365-2788.2004.00632.x. [DOI] [PubMed] [Google Scholar]
- Symons FJ, Tapp J, Wulfsberg A, Sutton KA, Heeth WL, Bodfish JW. Sequential analysis of the effects of naltrexone on the environmental mediation of self-injurious behavior. Experimental and Clinical Psychopharmacology. 2001;9(3):269–76. doi: 10.1037//1064-1297.9.3.269. [DOI] [PubMed] [Google Scholar]
- Thelen E, Smith LB. A Dynamic Systems Approach to the Development of Cognition and Action. The MIT Press; Cambridge, MA: 1994. [Google Scholar]


