Abstract
In a maternal fetal rat model, we investigated the behavioral and neurotoxic effects of fetal exposure to isoflurane. Pregnant rats at gestational day 21 were anesthetized with 1.3% isoflurane for 6 hour. Apoptosis was quantified in the hippocampus and cortex at 2 and 18 hour after exposure in the fetal brain and in the postnatal day 5 (P5) pup brain. Spatial memory and learning of the fetal exposed pups were examined with the Morris Water Maze at juvenile and adult ages. Rat fetal exposure to isoflurane at pregnancy day 21 through maternal anesthesia significantly decreased spontaneous apoptosis in the hippocampal CA1 region and in the retrosplenial cortex at 2 hour after exposure, but not at 18 hour or at P5. Fetal exposure to isoflurane did not impair subsequent juvenile or adult postnatal spatial reference memory and learning and, in fact, improved spatial memory in the juvenile rat. These results show that isoflurane exposure during late pregnancy is not neurotoxic to the fetal brain and does not impair memory and learning in the juvenile or adult rat.
Keywords: anesthetics, fetus, developing brain, apoptosis, memory, learning
1. Introduction
Anesthetics cause neurotoxicity in a concentration and time dependent manner in in vitro neuronal models (Chang and Chou, 2001; Eckenhoff et al., 2004; Kim et al., 2006; Kvolik et al., 2005; Loop et al., 2005; Matsuoka et al., 2001; Wei et al., 2005; Wise-Faberowski et al., 2005; Xie et al., 2006; Xie et al., 2007). Relatively fewer studies have investigated the neurotoxic effects of anesthetics in in vivo models. Isoflurane exposure at a clinically relevant concentration (0.75%) for 6 hours during postnatal development in rats caused persistent memory and learning deficits, which was associated with widespread neuronal apoptosis (Jevtovic-Todorovic et al., 2003; Yon et al., 2005). Neurons in the developing brain are specifically vulnerable to isoflurane neurotoxicity (Jevtovic-Todorovic et al., 2003). However, the mechanisms for isoflurane neurotoxicity are unknown.
Since anesthetics easily cross the placenta, the developing fetal brain will be exposed to inhaled anesthetics, such as isoflurane, when pregnant women require surgery. In some cases, such as fetal surgery to correct various congenital malformations during mid-gestation (18-25 weeks) (Myers et al., 2002), the fetal brain can be exposed to 2-3 times (2.5-3 Minimal Alveolar Concentration (MAC)) higher than normal concentrations of inhaled anesthetics, in order to relax uterine smooth muscle and provide adequate anesthesia (Cauldwell, 2002; Myers et al., 2002). Although fetal surgery is relatively new, it is a rapidly growing and evolving area, and may become standard therapy for most disabling malformations that are currently treated in young infants (Goldsmith et al., 1999; Myers et al., 2002). Because most fetal surgeries in humans are performed during mid-gestation, it is important and urgent to know if the anesthetics used cause damage to the developing brain and subsequent postnatal memory problems and learning disabilities.
The aim of the current study was to determine whether exposure to clinically relevant concentrations of isoflurane during prenatal development causes neuronal apoptosis and postnatal learning and memory deficits.
2. Methods
2.1 Animals
Institute of Animal Care and Use Committee (IACUC) at the University of Pennsylvania has approved all experimental procedures and protocols used in this study. All efforts were made to minimize the number of animals used and their suffering. Sprague Dawley pregnant rats (Charles River Laboratories, Inc Wilmington, MA) were housed in polypropylene cages and the room temperature was maintained at 22°C, with a 12-hour light-dark cycle. Pregnant rats at gestation day 21 (E21) were used for all experiments because it is approximately corresponding to the mid-gestation in human beings according to the theory of brain growth spurt (Dobbing and Sands, 1979; Jevtovic-Todorovic et al., 2003), and is a common time for most fetal surgeries (18-25 week) (Myers et al., 2002).
We have designed following three related studies: 1. pilot study; 2. neurodegeneration study; 3. finally a behavioral study. A pilot study was first conducted to find the highest concentration of isoflurane not accompanied by significant arterial blood gas (ABG) and mean arterial blood pressure (MABP) changes in the mothers. A neurodegeneration study was used to determine the appearance of apoptosis by detection of caspase-3 and TUNEL (Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling) positive cells in the fetal brain (2 and 18 hour post-exposure) or neonatal brain at postnatal day 5 (P5). We have chosen the above time points to detect apoptosis in fetal or newborn brains, based on previously published work (Jevtovic-Todorovic et al., 2003). The behavioral study was performed to investigate the effects of fetal exposure to isoflurane on postnatal memory and learning. The pregnant rats used in each study were not reused in the other two studies. Within each study described above, animals were randomly divided into either isoflurane treatment or sham control groups. Pregnant rats in the isoflurane treatment groups inhaled isoflurane for 6 hour while those in the sham control group only inhaled a carrier gas (30% oxygen, balanced with nitrogen) for 6 hour under the same experimental conditions. The distribution of pregnant rats and pups in all three groups is illustrated in Figure 1.
Figure 1. Nomogram illustrating distribution of pregnant mother and their fetus or postnatal rats among different studies.
2.2 Anesthetic Exposure
Isoflurane is used clinically at a wide range of concentrations (about 0.2%-3%), depending on the presence of other kinds of anesthetics or narcotics and the type and duration of surgery. As isoflurane neurotoxicity is concentration-dependent (Jevtovic-Todorovic et al., 2003; Wei et al., 2005), a primary goal of this study was to investigate if the highest isoflurane concentration used clinically is harmful to the fetal brain. Due to our concern that the physiological side effects of these drugs would contaminate the interpretation, we conducted a pilot study to determine the highest anesthetic concentration we could use without invasive support (tracheal intubation and ventilation) that would not significantly affect arterial blood gas (ABG) and mean arterial blood pressure (MABP) in the mothers, and then used this concentration in the subsequent formal study. We wanted to avoid the tracheal intubation, as it could possibly affect the hemodynamics of pregnant rats and the apoptosis in the fetus brains. In addition, this makes it more difficult to set up the sham control groups without anesthesia. In the pilot study, five pregnant rats were initially anesthetized with 2% isoflurane in 30% oxygen via a snout cone for approximately 1 hour and the right femoral artery was catheterized for blood sample collection and measurement of MABP by a pressure transducer/amplifier (AD Instruments Inc., Colorado Springs, CO, U.S.A.). The rats were recovered for 2 hours, and then exposed to isoflurane starting at 1.5% in a humidified carrier gas of 30% oxygen, balance nitrogen for 6 hour in a monitored chamber in hood. The pregnant rats breathed spontaneously without intubation or other support while being warmed using a deltaphase isothermal pad (Braintree Scientific Inc, Braintree, MA, U.S.A.). The rectal temperature was maintained (Fisher Scientific, Pittsburgh, PA, U.S.A.) at 37±0.5°C. We monitored isoflurane concentration in the chamber using IR absorbance (Ohmeda 5330, Detex-Ohmeda, Louisville, CO, U.S.A.). Arterial blood (0.1 ml) from previously placed femoral arterial catheter was collected and ABG determined every 2 hours for up to 6 hours by an ABG analyzer (Nova Biomedical, Waltham, MA, U.S.A.). Blood glucose was simultaneously measured with a glucometer (ACCU-CHECK Advantage, Roche Diagnostics Corporations, Indianapolis, IN, U.S.A.). Control rats were exposed only to humidified 30% O2 balanced by N2 (carrier gas for isoflurane in the treatment group) for 6 hour in a same chamber under same experimental condition as in the treatment group. Because one pregnant rat treated with 1.5% isoflurane showed obvious acidemia (which reversed after termination of anesthesia), we decreased the isoflurane concentration to 1.3%, and subsequently found no significant changes in the ABG or MABP between the treatment group and the sham control group (Table 1). Therefore, 1.3% isoflurane was used in the ensuing neurodegeneration and behavioral studies.
Table 1. 1.3% Isoflurane did not affect arterial blood pressure and blood gas significantly.
| Baseline | 2 hr | 4 hr | 6 hr | |||||
|---|---|---|---|---|---|---|---|---|
| Control | 1.3% Iso | Control | 1.3% Iso | Control | 1.3% Iso | Control | 1.3% Iso | |
| pH | 7.46±0.02 | 7.46±0.03 | 7.47±0.01 | 7.44±0.02 | 7.45±0.01 | 7.39±0.01 | 7.49±0.00 | 7.39±0.02 |
| PaCO2 | 36.7±2.80 | 32.8±0.64 | 33.4±1.39 | 37.2±4.33 | 35.8±3.43 | 39.9±4.95 | 37.5±0.90 | 36.6±2.20 |
| PaO2 | 158±6.98 | 162±4.67 | 149±9.08 | 159±2.12 | 166±5.45 | 156±8.67 | 163±5.45 | 154±9.43 |
| HCO3- | 25.6±2.38 | 23.3±1.57 | 24.4±1.15 | 25.5±2.02 | 25.2±2.84 | 24.2±2.53 | 28.3±2.70 | 23±2.50 |
| MABP | 117±7.42 | 119±0.56 | 106±9.61 | 90±6.67 | 102±4.06 | 89±6.68 | 106±4.81 | 89±7.33 |
Values are mean±SD. N=4 for each group. Iso means isoflurane. MABP means mean arterial blood pressure.
In the behavioral study, pregnant rats were treated with 1.3% isoflurane (n=8) or carrier gas (sham controls, n=7) for 6 hours. The monitoring was the same as that in the pilot study except that femoral artery catheters were not placed. After the exposures, the animals were returned to their cages and the rat pups were delivered naturally. Four rat pups from each pregnant mother were raised to P28 (Juvenile) and P118 (adult), and then used to determine memory and learning ability with a Morris Water Maze (MWM). Two rat pups from the control group and one from the isoflurane group died unexpectedly, so that there are total of 26 and 31 rat pups in the control and isoflurane treatment groups respectively (Figure 1).
In the fetal brain apoptosis study, pregnant rats were treated with either 1.3% isoflurane or carrier gas for 6 hour. At 2 and 18 hour after exposures, the rat pups were delivered by C-section under sodium pentobarbital (100mg/kg, i.p.) anesthesia. The fetal brains were removed and snap frozen for immunohistochemical analysis. Two fetal brains from each pregnant rat were studied. In addition, newborn brains from the rat pups born to the pregnant rats in behavioral study group (One pup from each pregnant rat, treatment n=8, control n=7) were also obtained at postnatal day 5 and prepared for the apoptosis study at P5 (Figure 1).
2.3 Measurement of isoflurane concentration in the brain tissues
To confirm that the isoflurane concentration in the fetal brain correlated with the inhaled concentration and brain concentration in the pregnant mothers, we measured the brain isoflurane concentrations in the fetus and the mother simultaneously in one rat. Briefly, after the pregnant rat was exposed to 1.3% isoflurane for 6 hour, the brains of both mother and fetuses were removed and the brain tissue was immediately placed into 4 ml of 0.02 M phosphate buffer (with 1 mM halothane as internal standard) and homogenized in a glass homogenizer. The homogenate was centrifuged (30,000g at 4°C for 30 min), the supernatant collected and then loaded onto C18 cartridge that had been conditioned with 2 ml methanol and washed with water, for solid phase extraction. The final sample was eluted with 0.5 ml solution of methanol and 2-propanol (vol:vol; 2:1) with 0.1% trifluoroacetic acid. All procedures were performed in the cool room (4°C). A 250 μl aliquot of each final elute was injected into High Performance Liquid Chromatography (HPLC) system, equipped with a refractive index monitor, for quantitation.
2.4 Tissue preparation
After isoflurane or carrier gas alone (control) treatment, pregnant rats were anesthetized with sodium pentobarbital intraperitoneally (i.p. 100mg/kg) at either 2 or 18 hours after the end of isoflurane exposure, and the fetuses removed by Cesarean section. Likewise, postnatal pups at day 5 (P5) were given the same dose of sodium pentobarbital. All fetuses and pups were then perfused transcardially with ice-cold normal saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were then removed and post-fixed overnight in the same fixative at 4°C, and cryoprotected in 30% (wt/vol) sucrose in 0.1 M phosphate buffer (pH 7.4) at 4°C for 24 hour. Thereafter, the brains were frozen in isopentane at -20°C and stored at -80°C until use. Serial coronal sections (10 μm) were cut in a cryostat (Dolbey-Jamison Optical Company, Inc., Pottstown, PA, U.S.A.), mounted on gelatin-coated slides and stored at -80°C. Coronal brain sections from the same brain corresponding to figure 96 of the rat fetal brain atlas (Paxinos et al., 1990) were chosen for detection of apoptosis by caspase-3 immunohistochemistry and TUNEL staining. In the initial examination of brains sections from the neurodegeneration study, we noticed that apoptosis was most apparent in the hippocampus CA1 region and the retrosplenial cortex, and thus we chose these two brain regions to quantify apoptosis.
2.5 Immunohistochemistry for caspase-3
Caspase-3 positive cells were detected using immunohistochemical methods described previously (Gown and Willingham, 2002). Briefly, brain sections were first incubated in 3% hydrogen peroxide in methanol for 20 minutes to quench endogenous peroxidase activity. Sections were then incubated with blocking solution containing 10% normal goat serum in 0.1% phosphate buffered saline with 0.1% Tween 20 (PBST) for 1 hour at room temperature after washing with 0.1% PBST. The anti-activated caspase-3 primary antibody (1/200, Cell Signaling Technology, Inc Danvers, MA, U.S.A.) was then applied in blocking solution and incubated at 4°C overnight. Tissue sections were biotinylated with goat anti-rabbit antibody (1/200, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, U.S.A) in 0.1% PBST for 40 minutes, followed by incubation with the avidin-biotinylated peroxidase complex (Vectostain ABC-Kit, Vector Lab, Burlingame, CA, U.S.A.) for 40 minutes. Tissue sections were colorized with diaminobenzidine (DAB, Vector Laboratories, Burlingame, CA, U.S.A.) for 8 minutes and counterstained with modified hematoxylin. Negative control sections were incubated in blocking solution that did not contain primary antibody. Images were acquired and assessed at 200X using IP lab 7.0 software linked to Olympus IX70 microscope (Olympus corporation, Japan) equipped with a Cooke SensiCam camera (Cooke corporation, Romulus, MI, U.S.A.). Three brain tissue sections at 10 μm corresponding to the Atlas of the Developing Rat Brain, Figure 96 (Paxinos et al., 1990) were chosen from each animal and analyzed for caspase-3 positive cells in the two brain regions. Two persons blinded to the treatments counted the total number of caspase-3 positive cells in the hippocampal CA1 region and retrosplenial cortex. The areas of entire hippocampal CA1 region and retrosplenical cortex were defined according to the Atlas of the Developing Rat Brain, Figure 96 (Paxinos et al., 1990) and the area measured using IPLab Suite v3.7 imaging processing and analysis software (Biovision Technologies, Exton, PA www.BioVis.com.). The density of caspase-3 positive cells in a particular brain region was calculated by dividing the number of caspase-3 positive cells by the area of that brain region.
2.6 TUNEL for DNA fragmentation
Three brain sections (10 μm) adjacent to the sections used for caspase-3 detection were used for TUNEL staining using the DeadEnd™ Colorimetric TUNEL System Kit (Promega Corporation, Madison, WI, U.S.A.) according to manufacturers protocol (Gavrieli et al., 1992). Briefly, sections were permeabilized by proteinase K solution (20 μg/ml) for 8 minutes, incubated in equilibration buffer for 10 minutes and the terminal deoxynucleotidyl transferase (TdT) and biotinylated nucleotide were added to the section and incubated in a humidified chamber at 37°C for 1 hour. The reaction was then stopped, followed by incubation with horseradish peroxidase-labeled streptavidin, colorization with DAB/ H2O2 and counterstained with modified hematoxylin. For positive-controls, the tissue sections were first treated with DNase I (1,000 U/ml, pH 7.6) for 10 minutes at the room temperature to initiate breakdown of DNA. Incubation of sections in reaction buffer without TdT provided negative controls. Images were acquired, and TUNEL quantitation performed as described above for caspase-3.
2.7 Spatial reference memory and learning performance
Morris Water Maze (MWM)
Pregnant rats were allowed to deliver after the isoflurane treatment and 4 pups per litter (2 females and 2 males) were raised. The body weights of the rat pups were recorded at P0, P3, P5, P11, P17 and P28 to determine growth rate. We determined spatial reference memory and learning with the MWM as reported previously with some modification (Jevtovic-Todorovic et al., 2003). A schematic of the experimental paradigm is shown in Figure 2. A round, fiberglass pool, 150 cm in diameter and 60 cm in height, was filled with water to a height of 1.5 cm above the top of the movable clear 15 cm diameter platform. The pool was located in a room with numerous visual cues (including computers, posters and desks) that remained constant during the studies. Water was kept at 20°C and opacified with titanium dioxide throughout all training and testing. A video tracking system recorded the swimming motions of animals and the data were analyzed using motion-detection software for the MWM (Actimetrics software, Evanston, IL, U.S.A.). After every trial, each rat was placed in a holding cage, under an infrared heat lamp, before returning to its regular cage.
Figure 2. Schematic time-line of Morris Water Maze tests paradigm.
E21, pregnant rats at gestational day 21. P0, postnatal day 0.
Cued trials
The cued trials were performed only for postnatal rats at P28 and P29 (28 rats in control group and 31 rats in treatment group) to determine whether any non-cognitive performance impairments (e.g. visual impairments and/or swimming difficulties) were present, which might affect performance on the place or probe trials. A white curtain surrounded the pool to prevent confounding visual cues. All rats received 4 trials per day. On each trial, rats were placed in a fixed position of the swimming pool facing the wall and were allowed to swim to a platform with a rod (cue) 20 cm above water level randomly placed in any of the four quadrants of the swimming pool. They were allotted 60s to find the platform upon which they sat for 30s before being removed from the pool. If a rat did not find the platform within 60s, the rat was gently guided to the platform and allowed to remain there for 30s. The time for each rat to reach the cued platform and the swim speed was recorded and the data at P28/29 were analyzed.
Place trials
After completion of cued trials, we used the same rats to perform the place trials to determine the rat's ability to learn the spatial relationship between distant cues and the escape platform (submerged, no cue rod), which remained in the same location for all place trials. The starting points were random for each rat. The time to reach the platform was recorded for each trial. The less time it took a rat to reach the platform, the better the learning ability. The juvenile rats (P32) received two blocks of trials (two trials per block with 30 seconds apart, 60 seconds maximum for each trial and 2 hour rest between blocks) each day for 5 days. The adult rats (P115) received only one block of trials each day for 5 days using a new platform location in an effort to increase task difficulty and improve test sensitivity.
Probe trials
Probe trials were conducted after the last place trials for the juveniles (P36) and adults (P119) to evaluate memory retention capabilities. After all rats completed the last place trial on the fifth day, the platform was removed from the water maze and rat was started to swim in the quadrant opposite to one the platform was placed before. The rats were allowed to swim for 60 seconds during each probe trial and the time of rats spent in each quadrant was recorded. The percentage of the swimming time spent in the target (probe) quadrant where the platform was placed before was calculated. The time spent in the target quadrant compared to other quadrants was an indication of memory retention.
Learning to reach criterion test
After the last probe test for the adult rats, the animals performed the learning to reach criterion test during the next nine days as described previously (Chen et al., 2000). The experimental procedure was similar to the place trial except that the platform location was changed. For each rat, the platform was moved between nine different locations set up by the computer. Each rat received up to eight trials per day. In order to advance to the next platform location, each rat had to reach the criterion of three successive trials with escape latency of 20 seconds or less. If a rat reached criterion in 8 or less trials, a new platform location would be selected the following day. The numbers of learned platforms and the number of trials used to reach the criteria were recorded and compared. The number of platforms learned and the number of trials to reach criterion indicated the learning ability of the rats.
2.8 Statistical Analysis
To reduce variance from different size litters, we averaged the data from all fetal or postnatal rats from the same mother and considered it as a single sample. Results of weight gain of postnatal rat pups, ABG and MABP of pregnant rats and place trials of postnatal rats were analyzed using 2-way ANOVA for repeated measurements. Data for immunohistochemistry, TUNEL and other behavioral studies were analyzed using student t test for comparison of two groups or by ANOVA followed by Fisher's post hoc multiple comparison tests for those with more than two groups. In all experiments, difference were considered statistically significant at P<0.05.
3. Results
3.1 Comparison of basic physiological variables and brain isoflurane concentrations between 1.3% Isoflurane treatment and sham control groups
In the pilot study, the pregnant rats at E21, initially exposed to 1.5% isoflurane for 3 hour, developed respiratory acidosis (PaCO2 increased from 32 to 55 mmHg) and hypoxia (PaO2 decreased from 179 to 82 mmHg). When the isoflurane concentration was reduced to 1.3%, for up to 6 hour (2, 4 and 6 hour), there were no significant changes in any of the ABG variables as compared to the controls (Table 1). MABP decreased slightly beginning at 2 hour compared to the same animal's baseline, but was not significantly different from the control group. Therefore, 1.3% isoflurane for 6 hour was used in the subsequent formal study. In addition, there were no significant differences in the blood glucose levels before and after exposure in both the control group (113±28 vs. 116±29 mg/dl, n=4, P>0.05) and the 1.3% isoflurane treatment group (117± 17 vs. 118±14 mg/dl, n=8, P>0.05). In the behavioral study, there were no significant differences between the two groups on growth rate measured by weight gain in rats from postnatal day 0 (P0) to P28 (Data not shown). The concentration of isoflurane in the brain of a pregnant rat was 0.42 μmol/g after exposure to 1.3% isoflurane for 6 hour, which was indistinguishable from that of the fetal brain (0.40 μmol/g) measured at the same time.
3.2 Isoflurane inhibited spontaneous neuronal apoptosis in fetus brains
We determined the degree of apoptosis by counting caspase-3 positive and TUNEL-positive cells in different brain regions at 2 hour and 18 hour after isoflurane treatment and then at postnatal day 5. There was spontaneous apoptosis represented by caspase-3 positive or TUNEL positive cells in the developing fetal brain (Figure 3 and 4). The caspase-3 positive cells were concentrated in the dorsal midline of the fetal brain along its rostral-caudal axis. There were no significant differences between control and treatment groups in the areas of hippocampus CA1 region and the retrosplenial cortex (data not shown) determined at 2, 18 hour and P5. Compared to the sham control group, 1.3% isoflurane treatment significantly decreased spontaneous apoptosis determined by the density of apoptotic cells in both the hippocampus CA1 region and in retrosplenial cortex at 2 hour after treatment, but no differences were seen at 18 hour after treatment or at P5 (Figure 3 B, C and Figure 4 B, C). Isoflurane treatment significantly decreased the density of caspase-3 and TUNEL positive cells by 80% (P<0.001) and 81% (P<0.001) respectively in the hippocampal CA1 region (Figure 3 B, C) and by 82% (P<0.001) and 87% (P<0.01) respectively in retrosplenial cortex (Figure 4 B, C) at 2 hour after isoflurane treatment.
Figure 3. In utero isoflurane inhibited spontaneous apoptosis in the hippocampus CA1 determined 2 hours after treatment.

A. Arrows indicate caspase-3 positive cells and TUNEL-labeled cells in the hippocampus CA1 region of normal control fetal brains (Aa and Ac) or 2 hour after isoflurane treatment (Ab and Ad) at E21. B and C. The comparison between the density of caspase-3 positive cells (B) and TUNEL-positive cells (C) in the hippocampus CA1 region at different times after isoflurane treatment. Data represents mean±SE of 12-14 fetus rat brains from 5-6 pregnant mothers in either the control group or the isoflurane treatment group at 2 and 18 hour after isoflurane treatment (See Figure 1 for the detailed distribution of rats). 7 postnatal rats from 7 pregnant mothers (n=7) and 8 postnatal rats from 8 pregnant mothers (n=8) of behavioral study were used for neurodegeneration study at P5 (See Figure 1). Data represents mean±SE. ***P<0.001 versus control. Iso=isoflurane. Scale bar=50 μm.
Figure 4. Isoflurane inhibited spontaneous apoptosis in the retrosplenial cortex of fetal brains 2 hours after treatment.

A. Arrows indicate caspase-3 positive cells and TUNEL-labeled cells in the retrosplenial cortex of normal control fetal brains (Aa and Ac) or 2 hour after isoflurane treatment (Ab and Ad) at E21. The density of caspase-3 positive cells (B) and TUNEL-positive cells (C) in the retrosplenial cortex area at different time after isoflurane treatment were compared between control and 1.3% isoflurane treatment group. Data represents mean±SE of 12-14 postnatal rats from 5-6 pregnant mothers in either control group or the isoflurane treatment group at 2 and 18 hours after isoflurane treatment (See Figure 1 for the detailed distribution of rats). 7 postnatal rats from 7 pregnant mothers (n=7) and 8 postnatal rats from 8 pregnant mothers (n=8) of behavioral study were used for neurodegeneration study at P5 (See Figure 1). Data represents mean±SE. *** P<0.001, ** P<0.01, compared to control. Iso=isoflurane. Scale bar=50 μm.
3.3 Effect of fetal exposure to isoflurane on memory and learning
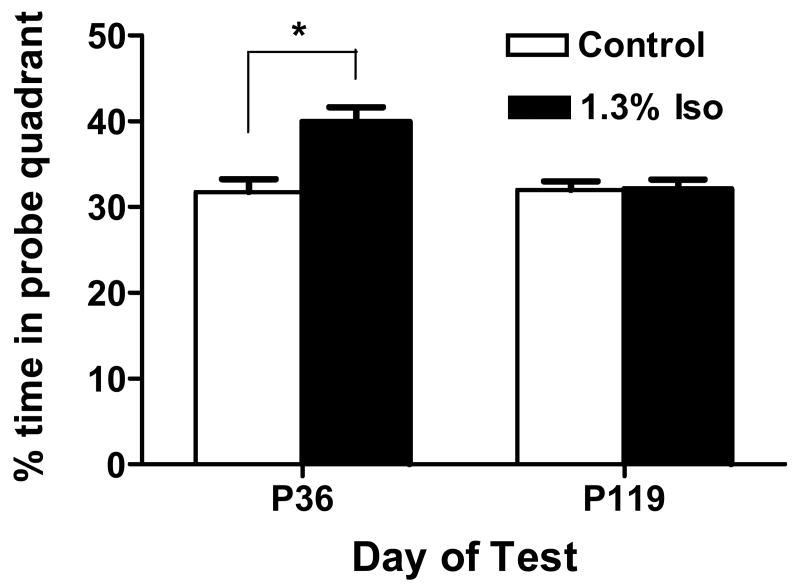
Using the MWM, we examined the effect of prenatal isoflurane exposure on the memory and learning ability in postnatal rats at different ages. In the cued trials, there was no significant difference in the latency of swimming to a visible platform between the control group of 26 postnatal rats from 7 pregnant mothers (n=7) and the isoflurane treatment group of 31 postnatal rats from eight pregnant mothers (n=8) (18.5±1.86s vs. 18±2.22s, P>0.05). When we compared the spatial reference learning ability in the same animals using the place trials, the escape latency to platform was analyzed with 2 factor ANOVA with treatment as a between subjects factor and block as a repeated measure. This analysis yields a main difference in block (P<0.0001 at P32-36 and P115-119). However, neither the main effect of treatment nor the interaction between treatment and block were significant at P32-36 or at P115-119 (Figure 5, A, B). The results indicated that the performance during the place trials improved as training progressed but the there was no significant difference between the two groups at juvenile (P32-36) or at adult (P115-119) ages. We further tested the learning ability in the same adult rats using a more rigorous protocol – the learning to reach criterion test. They all performed equally well in learning as shown in the numbers of platform reached (Figure 5C) and took the same number of trials to reach criterion at each platform (Figure 5D), suggesting a similar learning ability between the two groups. After the place test, the same postnatal rats were used in a probe test to determine memory retention. The juvenile postnatal rats born to the mothers treated with 1.3% isoflurane for 6 hour had significantly improved retention of memory (P<0.05) by spending a greater percentage of time swimming in the probe quadrant as compared with the corresponding control animals (Figure 6). The swim speed in both groups was not different (data not shown), further indicating the improvement of retention memory was not caused by the rats' swimming ability as showed in cued trials. However, when the probe test was repeated in adult rats (P119), there was no significant difference in the percentage of time spent in the probe quadrant between the two groups (Figure 6). These data suggest that any improvement in memory during juvenile age did not last into the adult age.
Figure 5. Isoflurane in utero did not affect postnatal learning ability in juvenile or adult rats.
Spatial learning and memory performance was determined using the Morris Water Maze place test paradigm in postnatal juvenile (A) and adult (B) rats and using the learning to reach criteria test in the P120-128 adult (C, D). Data represents mean±SE of 26 postnatal rats from seven pregnant mothers (n=7) in the control group or 31 postnatal rats from eight pregnant mothers (n=8) in the isoflurane treatment group. Iso=isoflurane.
Figure 6. Isoflurane significantly increased spatial retention memory in postnatal juvenile but not adult rats.
Probe test was performed at postnatal day 36 (P36) and 119 (P119) after the last place trial. Data represents mean±SE of 26 postnatal rats born from seven pregnant mothers (n=7) in the control group or 31 postnatal rats born from eight pregnant mothers (n=8) in the isoflurane treatment group. * P<0.05 compared to control. Iso=isoflurane.
Discussion
Our initial hypothesis was that isoflurane exposure during pregnancy would cause apoptosis in the fetal brain and subsequent postnatal memory and learning disabilities. This hypothesis was based on recent work which showed isoflurane exposure during early postnatal development resulted in an increase in apoptosis and subsequent behavioral impairment (Jevtovic-Todorovic et al., 2003). In contrast, we found that isoflurane exposure during late pregnancy transiently inhibited spontaneous apoptosis in the fetal brain and does not impair memory and learning in the juvenile or adult rat.
The fact that isoflurane did not induce apoptosis in the prenatal rat brain is consistent with a recent study (McClaine et al., 2005), showing no evidence for a neurotoxic effect of the combination of isoflurane/midazolam/sodium thiopental exposure to fetal sheep. Spontaneous apoptosis in the fetal developing brains may be a normal process that shapes the brains as it matures. It seemed that spontaneous apoptosis significantly decreased as brain matured especially at postnatal 5 (Figure 3 and 4), which was consistent with other report (White and Barone, 2001). The inhbitioin of spontaneous apoptosis in the fetus developing brains by isoflurane determined at 2 hr after treatment was not expected. Although the significance of the transient inhibition of spontaneous apoptosis by isoflurane is unknown from this study, we speculate this will not affect brain development significantly. Our observation of postnatal rats in the behavioral study did not notice obvious physical or behavioral difference between control and isoflurane treatment groups, except the transient memory improvement in the isoflurane treated rats. Nevertheless, the results from this study suggest that isoflurane exposure to pregnant rats does not induce apoptosis in the fetus developing brains and not to impair the memory and learning of their offspring.
Our results would appear to be at odds of those observed by Jevtovic-Todorovic (Jevtovic-Todorovic et al., 2003). However, in that study, neonates and not fetuses were exposed to isoflurane, and the interpretation was that vulnerability of isoflurane-induced apoptosis in the developing brains is correlated to period of synaptogenesis (Jevtovic-Todorovic et al., 2003; Yon et al., 2005). If true, then the fetal rat is not expected to be as vulnerable as the neonatal rat, which is consistent with the results of our study. However, it should be noted that although vulnerability to isoflurane-induced apoptosis seems to coincide with the period of synaptogenesis (Hansen et al., 2004; Ikonomidou et al., 2001; Jevtovic-Todorovic et al., 2003), there exists no direct evidence showing that synaptogenesis is itself altered, or is causally linked to the subsequent cognitive changes. Toxic effects of isoflurane have also been found in the adult and aged brains (Culley et al., 2003; Culley et al., 2004), when of course little in the way of synaptogenesis is occurring.
Isoflurane has been long considered to be cytoprotective against ischemia in heart and brain (Sakai et al., 2007; Warner, 2000). In previous studies, we and others (Kudo et al., 2001; Wei et al., 2005) have shown that the concentration and time required for isoflurane to induce apoptosis in cultured neurons was greater than that used clinically in patients. It is possible that sub-apoptotic exposures to isoflurane induce protection, analogous to that induced by hypoxia. Accordingly, our preliminary unpublished data have shown that preconditioning of rat primary cortical neurons with 2 MAC isoflurane for 1 hour abolished the neurotoxicity induced by a subsequent exposure to 2 MAC isoflurane for 24 hr. Thus, it is possible that our use of isoflurane at low “clinical” concentration in the pregnant rat may precondition the fetal brain against spontaneous apoptosis in the current study. Therefore, it remains possible that the higher anesthetic concentrations used in fetal surgery (∼3% isoflurane) may produce neurotoxicity in the fetal brain.
Other limitations of this study are that we did not expose the mother/fetus to isoflurane at other time points during the pregnancy, so it is possible that we missed the time point when fetus might be more vulnerable to isoflurane neurotoxicity. Further, we avoided tracheal intubation in this study in an attempt to minimize confounding variables. This prevented us from testing the effects of isoflurane at concentrations up to 3% often used during fetal surgery in humans (Cauldwell, 2002; Myers et al., 2002).
In summary, prenatal exposure to isoflurane at a concentration commonly used for the maintenance of general anesthesia during late pregnancy in rats does not appear to be neurotoxic to the fetal brain and does not impair memory and learning in the postnatal juvenile or adult rat.
Acknowledgments
We acknowledge discussions with Drs. Roderic Eckenhoff, Randall Pittman and Maryellen Eckenhoff and the technical support of Dr. Min Li, the Department of Anesthesiology and Critical Care, University of Pennsylvania. We thank editorial assistance from Dr. Alex Loeb. This study was supported by March of Dimes Birth Defects Foundation Research Grant (12-FY05-62, PI: Huafeng Wei), and partially supported by the National Institute of General Medical Science (NIGMS) K08 grant (1-K08-GM-073224-01, PI: Huafeng Wei), and the Research Fund at the Department of Anesthesiology and Critical Care, University of Pennsylvania (PI: Huafeng Wei).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Cauldwell CB. Anesthesia for fetal surgery. Anesthesiology Clinics of North America. 2002;20:211–226. doi: 10.1016/s0889-8537(03)00062-2. [DOI] [PubMed] [Google Scholar]
- Chang YC, Chou MY. Cytotoxicity of halothane on human gingival fibroblast cultures in vitro. Journal of Endodontics. 2001;27:82–84. doi: 10.1097/00004770-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RG. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- Culley DJ, Baxter M, Yukhananov R, Crosby G. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth Analg. 2003;96:1004–9. doi: 10.1213/01.ANE.0000052712.67573.12. [DOI] [PubMed] [Google Scholar]
- Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100:309–314. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Human Development. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Bensasson SA. Identification of Programmed Cell-Death Insitu Via Specific Labeling of Nuclear-Dna Fragmentation. Journal of Cell Biology. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith MF, Straus SE, Kupfer C, Lenfant C, Collins F, Hodes RJ, Gordis E, Fauci AS, Alexander D, Battey JF, Slavkin HC, Leshner AI, Olden K, Hyman SE, Fischbach GD. 2020 vision: NIH heads foresee the future. Jama-Journal of the American Medical Association. 1999;282:2287–2290. doi: 10.1001/jama.282.24.2287. [DOI] [PubMed] [Google Scholar]
- Gown AM, Willingham MC. Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. Journal of Histochemistry and Cytochemistry. 2002;50:449–454. doi: 10.1177/002215540205000401. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Briem T, Dzietko M, Sifringer M, Voss A, Rzeski W, Zdzisinska B, Thor F, Heumann R, Stepulak A, Bittigau P, Ikonomidou C. Mechanisms leading to disseminated apoptosis following NMDA receptor blockade in the developing rat brain. Neurobiology of Disease. 2004;16:440–453. doi: 10.1016/j.nbd.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Koch C, Genz K, Hoerster F, Felderhoff-Mueser U, Tenkova T, Dikranian K, Olney JW. Neurotransmitters and apoptosis in the developing brain. Biochemical Pharmacology. 2001;62:401–405. doi: 10.1016/s0006-2952(01)00696-7. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. Journal of Neuroscience. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Oh E, Im H, Mun J, Yang M, Khim JY, Lee E, Lim SH, Kong MH, Lee M, Sul D. Oxidative damages in the DNA, lipids, and proteins of rats exposed to isofluranes and alcohols. Toxicology. 2006;220:169–178. doi: 10.1016/j.tox.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Kudo M, Aono M, Lee Y, Massey G, Pearlstein RD, Warner DS. Effects of volatile anesthetics on N-methyl-D-aspartate excitotoxicity in primary rat neuronal-glial cultures. Anesthesiology. 2001;95:756–765. doi: 10.1097/00000542-200109000-00031. [DOI] [PubMed] [Google Scholar]
- Kvolik S, Glavas-Obrovac L, Bares V, Karner I. Effects of inhalation anesthetics halothane, sevoflurane, and isoflurane on human cell lines. Life Sciences. 2005;77:2369–2383. doi: 10.1016/j.lfs.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Loop T, Dovi-Akue D, Frick M, Roesslein M, Egger L, Humar M, Hoetzel A, Schmidt R, Borner C, Pahl HL, Geiger KK, Pannen BH. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102:1147–1157. doi: 10.1097/00000542-200506000-00014. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Kurosawa S, Horinouchi T, Kato M, Hashimoto Y. Inhalation anesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology. 2001;95:1467–1472. doi: 10.1097/00000542-200112000-00028. [DOI] [PubMed] [Google Scholar]
- McClaine RJ, Uemura K, de la Fuente SG, Manson RJ, Booth JV, White WD, Campbell KA, McClaine DJ, Benni PB, Eubanks WS, Reynolds JD. General anesthesia improves fetal cerebral oxygenation without evidence of subsequent neuronal injury. J Cerebral Blood Flow Metab. 2005;25:1060–1069. doi: 10.1038/sj.jcbfm.9600094. [DOI] [PubMed] [Google Scholar]
- Myers LB, Cohen D, Galinkin J, Gaiser R, Kurth CD. Anaesthesia for fetal surgery. Paediatric Anaesthesia. 2002;12:569–578. doi: 10.1046/j.1460-9592.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Tork I, Teccot LH, Valentino K. Atlas of the developing rat brain. Academic Press; 1990. [Google Scholar]
- Sakai H, Sheng H, Yates RB, Ishida K, Pearlstein RD, Warner DS. Isoflurane provides long-term protection against focal cerebral ischemia in the rat. Anesthesiology. 2007;106:92–99. doi: 10.1097/00000542-200701000-00017. [DOI] [PubMed] [Google Scholar]
- Warner DS. Isoflurane neuroprotection - A passing fantasy, again? Anesthesiology. 2000;92:1226–1228. [PubMed] [Google Scholar]
- Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Research. 2005;1037:139–147. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- White LD, Barone S. Qualitative and quantitative estimates of apoptosis from birth to senescence in the rat brain. Cell Death and Differentiation. 2001;8:345–356. doi: 10.1038/sj.cdd.4400816. [DOI] [PubMed] [Google Scholar]
- Wise-Faberowski L, Zhang H, Ing R, Pearlstein RD, Warner DS. Isoflurane-induced neuronal degeneration: an evaluation in organotypic hippocampal slice cultures. Anesthesia and Analgesia. 2005;101:651–657. doi: 10.1213/01.ane.0000167382.79889.7c. [DOI] [PubMed] [Google Scholar]
- Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104:988–994. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J of Neuroscience. 2007;27:1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]