Abstract
Introduction
Thrombin or tryptase cleavage of protease-activated receptors (PAR) on human coronary artery endothelial cells (HCAEC) results in activation of a membrane-associated, calcium-independent phospholipase A2 (iPLA2) that selectively hydrolyzes plasmalogen phospholipids. Atherosclerotic plaque rupture results in a coronary ischemic event in which HCAEC in the ischemic area would be exposed to increased thrombin concentrations in addition to tryptase released by activated mast cells present in the plaque.
Materials and Methods
HCAEC were stimulated with thrombin or tryptase in the absence or presence of bromoenol lactone (BEL), a selective iPLA2 inhibitor, and iPLA2 activation, accumulation of biologically active membrane phospholipid-derived metabolites, upregulation of cell surface P-selectin expression and neutrophil adherence were measured.
Results
HCAEC exposed to thrombin or tryptase stimulation demonstrated an increase in iPLA2 activity and arachidonic acid release. Additionally, stimulated HCAEC demonstrated increased platelet-activating factor (PAF) production and cell surface P-selectin expression, resulting in increased adhesion of neutrophils to HCAEC monolayers. Pretreatment with bromoenol lactone to inhibit iPLA2, blocked membrane phospholipid-derived metabolite production, increased cell surface P-selectin expression and neutrophil adherence.
Conclusions
The similar biochemical and cellular responses in HCAEC exposed to thrombin or tryptase stimulation suggest that the cleavage of two separate PAR serve to extend the range of proteases to which the cells respond rather than resulting in separate intracellular events. This suggests that in conditions such as thrombosis and atherosclerosis that multiple mechanisms can activate the inflammatory response.
Keywords: thrombin, tryptase, inflammation, endothelium, protease activated recepotors, atherosclerosis
The PAR represent a family of G-protein coupled receptors that are activated by proteolytic cleavage of their N-terminus [see 1 for review]. Recent evidence suggests that interaction of proteases with PAR have far-reaching implications in diversified cellular responses, particularly in inflammation and host defense [2,3]. PAR couple to multiple intracellular signaling pathways that are related to growth and inflammation including activation of phospholipases and MAP kinases [1]. PAR may play important roles in both the acute anti-inflammatory and chronic inflammatory behavior of both endothelial and epithelial cells that form the defensive barriers of the body [2]. We have previously demonstrated that thrombin (activates PAR-1) and tryptase (activates PAR-2) stimulation of endothelial cells results in activation of phospholipase A2 (PLA2) [4,5] These data agree with a previously published study that suggest that the presence of multiple PARs on the endothelial cell surface serve to extend the number of proteases to which the cells respond rather then being coupled to different intracellular responses [6].
Myocardial infarction and the development of thrombotic coronary artery occlusion are associated with the presence of the serine proteases thrombin and tryptase. Thrombin generated at sites of vascular injury is the most potent activator of blood platelets [7,8] and its action on inflammatory cells has been well characterized, serving as a chemotactic agent for monocytes [9] and a mitogenic for both lymphocytes [10] and vascular smooth muscle cells [11,12]. Thrombin activation of the vascular endothelium occurring in response to vascular injury or wounding can be beneficial in the repair process, but has the potential to mediate a prolonged inflammatory response and proliferative cellular events in the blood vessel wall, such as those that occur in atherosclerosis and restenosis [13]. Similarly, increased numbers of degranulated mast cells have been found in the adventitia of infarct-related coronary arteries [14] and the mediators released from these granules, including tryptase, are mitogens and co-mitogens for human fibroblasts, stimulating collagen synthesis [15]. Though these studies demonstrate the presence of either thrombin or tryptase associated with atherosclerosis, a defined role has yet to be established for these proteases.
Materials and Methods
Reagents
Human tryptase (200 μg/mL recombinant skin β tryptase with 0.5 mg/mL heparin) was purchased from Promega Corporation, Madison, WI. BEL was obtained from Cayman Chemical, Ann Arbor, MI. Goat anti-P-selectin antibody and horse raddish peroxidase-conjugated rabbit anti-goat antibody were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. [3H] arachidonic acid and [3H] acetic acid were obtained from Perkin Elmer Life Sciences, Boston, MA. AACOCF3 was purchased from Calbiochem, La Jolla, CA. PX-18 was a gift from Richard Berney (Richard Berney Associates, LLC), Bethesda, MD. All other reagents were purchased from Sigma Chemical, St. Louis, MO.
Culture of Endothelial Cells
HCAEC were obtained from Cambrex (Walkersville, MD). Cells were grown to confluence, as determined by visual examination utilizing an inverted light microscope. Cells were cultured in EGM-2MV medium from Cambrex (Walkersville, MD) and incubated at 37°C, 95% O2/5% CO2. To passage cells, the Sub-culture Reagent Pack (Cambrex, Walkersville, MD) was used. Approximately 3×105 of cells in 2 mL of EGM-2MV medium were placed in each well of a 6 well plate. Unless otherwise stated, cells from passages 3–4 were used for experiments.
Thrombin or Tryptase Stimulation
Thrombin or tryptase were diluted with medium (for assay of iPLA2 activity, arachidonic acid release, resistance measurements, and neutrophil adhesion), or Hanks’ balanced salts solution (for assay of PAF production and P-selectin surface expression) to the working concentration. Thrombin or tryptase was added to the cell culture plate and the plate gently rotated to ensure thorough mixing and even distribution of stimulant across the HCAEC monolayer.
Measurement of PLA2 Activity
At the end of the stimulation period, the media was removed from the HCAEC monolayer and replaced with ice-cold PLA2 buffer containing: 250 mM sucrose, 10 mM KCl, 10 mM imidazole, 5 mM EDTA, 2 mM dithiothreitol, with 10% glycerol (pH=7.8). The cells were removed from the tissue culture plate by scraping and the suspension was sonicated on ice for 3 bursts of 10 sec. The sonicate was centrifuged at 14,000 x g for 10 minutes. The supernatant was then centrifuged at 100,000 x g for 60 minutes to separate the membrane fraction (pellet) from the cytosolic fraction (supernatant). The membrane fraction, consisting of microsomes including vesicles of cellular membrane and endoplasmic reticulum [16], was washed twice by resuspending in PLA2 assay buffer and centrifuging at 100,000 x g for 60 minutes.
PLA2 activity in the cell sonicates was assessed by incubating enzyme (50 μg protein) with 100 μM (16:0, [3H]18:1) plasmenylcholine substrate in assay buffer containing 10 mM Tris 10% glycerol and 4mM EGTA, pH = 7.0 at 37°C for 5 mins in a total volume of 200 μl. Reactions were initiated by adding the radiolabeled phospholipid substrate as a concentrated stock solution in ethanol. Reactions were terminated by the addition of 100 μl butanol and released radiolabeled fatty acid isolated by application of 25 μl of the butanol phase to channeled Silica Gel G plates, development in petroleum ether/diethyl ether/acetic acid (70/30/1, v/v) and subsequent quantification by liquid scintillation spectrometry.
Arachidonic Acid Release
Arachidonic acid release was determined by measuring the amount of [3H]-arachidonic acid released into the surrounding medium from HCAEC pre-labeled with 3 μCi of [3H]-arachidonic acid per 34 mm culture dish for 18 h. Following incubation, HCAEC were washed three times with Tyrode’s solution containing 3.6% bovine serum albumin (BSA) to remove unincorporated [3H]-arachidonic acid. Endothelial cells were incubated at 37°C for 15 mins prior to implementation of the experimental conditions. At the end of the stimulation period, the surrounding medium was removed to a scintillation vial and represented the amount of radiolabeled arachidonic acid released from the HCAEC during the stimulation interval. The amount of radiolabeled arachidonic acid remaining in the endothelial monolayer was measured by adding 1 mL of 10% sodium dodecyl sulfate, removing the cells from the culture well by scraping, and adding them to a scintillation vial. Radioactivity in both the surrounding medium and endothelial cells was quantified by liquid scintillation spectrometry.
Measurement of PAF Production
HCAEC grown in 34mm culture dishes were washed twice with Hanks’ balanced salts solution containing: NaCl 135 mM, MgSO4 0.8 mM, HEPES (pH=7.4) 10 mM, CaCl2 1.2 mM, KCl 5.4 mM, KH2PO4 0.4 mM, Na2HPO4 0.3 mM and glucose 6.6 mM and incubated with 10 μCi [3H] acetic acid/well for 20 mins. After 10 mins stimulation with thrombin or tryptase, lipids were extracted from the cells by the method of Bligh and Dyer [17]. The chloroform layer was concentrated by evaporation under N2, applied to a silica gel 60 thin layer chromatography plate, and developed in chloroform/methanol/acetic acid/water (50/25/8/4 vol/vol). The region corresponding to PAF was scraped and radioactivity quantified using liquid scintillation spectrometry. Loss of PAF during extraction and chromatography was corrected by adding a known amount of [14C]PAF as an internal standard.
P-selectin Surface Expression Assay
HCAEC, grown to confluence in 16 mm culture dishes, were incubated with thrombin or tryptase in Hanks’ buffer for 5 minutes at 37°C in 95% O2/5% CO2. At the end of incubations, buffer was quickly removed and cells were immediately fixed with 1% paraformaldehyde and incubated overnight at 4°C. Cells were then washed 3 times with phosphate buffered saline (PBS) and then blocked with Tris-buffered saline containing 0.1% Tween (vol/vol) supplemented with 0.8% BSA (wt/vol) and 0.5% fish gelatin (wt/vol) for 1 h at 24°C. Primary goat polyclonal antibody (1:50) for P-selectin was used before treatment with horseradish peroxidase-conjugated rabbit anti-goat secondary antibody (1:5000). Subsequently, each well was incubated in the dark with the 3,3′, 5,5′-tetramethylbenzidine liquid substrate system. Reactions were stopped by the addition of sulfuric acid, and color development was measured with a microtiter plate spectrophotometer at 450 nm.
Isolation of Neutrophils from Human Umbilical Cord Blood
Units of human umbilical cord blood were obtained from the St. Louis Cord Blood Bank. Units were pooled and 25 mL of the pooled product layered over 25 mL of Polymorphprep (Axis-Shield PoC AS, Oslo, Norway) in a 50 mL conical tube. Tubes were spun at 500xg for 30 min at 20°C with no brake. The top band at the sample/medium interface consisting of mononuclear cells and the lower band of polymorphonuclear cells were removed to a clean 50 mL conical tube. An equal volume of 0.5 N Hanks was added to the cells in the 50 mL tube. Normal Hanks was added to bring the total volume to 50 mL. Tubes were spun at 400xg for 10 mins at 4°C. Supernatant was discarded and the cell pellet re-suspended with 3 mL of 0.2% NaCl and incubated for 3 mins at room temperature. 3 mL of cold 1.6% NaCl was added and the solution transferred to a 15 mL conical tube. Ice cold normal Hanks was added to bring the total volume to 15 mL. Cells were centrifuged at 175xg for 10 mins at 4°C. Supernatant was removed and cells re-suspended in 5 mL of ice cold Hanks. An aliquot was taken for a cell count utilizing a hemacytometer. Cells were centrifuged at 175xg for 10 mins and the supernatant discarded. Neutrophils were re-suspended in minimal essential medium (MEM) + 10% fetal calf serum (FCS) at 1×106 cells/mL. This protocol is approved by the Saint Louis University Institutional Review Board.
Neutrophil Adherence Assay
HCAEC were grown to confluence on a 12 -well plate. Cells were washed twice with MEM + 10%FCS. 0.5 mL of neutrophils suspension (5×105 cells) in MEM + 10%FCS. Following a 10 mins pretreatment with BEL, PX-18, or AACOCF3, either thrombin or tryptase were added to each of the wells and incubated for 10 mins at room temperature. Media and unbound neutrophils were removed and discarded. Plates were washed twice with pre-warmed Dulbecco’s PBS. 1 ml of 0.2% Trition X-100 was added to each well to lyse adherent neutrophils and HCAEC. Cell lysates were scraped from the plate and transferred to an Eppendorf tube. A 0.5 mL aliquot of neutrophil suspension was added to 0.5 mL of 0.2% Triton X-100 and used as the theoretical maximal binding sample. 0.5 mL of dH20 and 0.5 mL of 0.2% Triton X-100 was used as the reference blank. Samples, theoretical maximal binding, and blank were sonicated (550 Sonic Dismembrator, Fisher Scientific, Pittsburgh, PA) for 10 sec. To measure neutrophil peroxidase activity, 400 μL of cell lysate was transferred to a glass tube and 1 mL of PBS, 1200 μL Hanks Buffer + BSA, 200 μL 3,3′-dimethyoxybenzidine, and 200 μL of 0.05% H2O2 added. Cell lysate reaction mixture was incubated for 15 mins at room temperature. 200 μL of 1% NaN3 was added to stop the reaction. The absorbance was then measured using a 4050 UV/Visible Spectrophotometer (Biochrom, Cambridge, England) at 460 nm.
Results
PAR couple to multiple intracellular signaling pathways including activation of phospholipases and MAP kinases [2]. Our laboratory has performed immunoblot analysis utilizing primary antibodies specific for both PAR-1 and PAR-2 to demonstrate the presence of these receptors in HCAEC (data not shown).
We measured iPLA2 activity in HCAEC pretreated with several PLA2 inhibitors prior to stimulation with either thrombin or tryptase. PX-18 (2-[N, N-bis(2-oleoyloxyethyl)amine]-1-ethanesulfonic acid) is an sPLA2 inhibitor with an IC50 of less than 1 μM and demonstrates no measurable inhibition of recombinant cPLA2 (personal communication, Richard Berney Associates, LLC). Arachidonyl trifluoromethyl ketone (AACOCF3) is a tight-binding reversible inhibitor that exhibits slow binding with cPLA2, but not with iPLA2 [18]. Bromoenol lactone (BEL) is a selective iPLA2 inhibitor, which has been demonstrated to have 100-fold selectivity for iPLA2 vs cPLA2 and sPLA2 isoforms[19]. To demonstrate the specificity of the iPLA2 inhibitor utilized in these studies, HCAEC were pretreated with BEL, PX-18, or AACOCF3 prior to stimulation with either thrombin or tryptase. PLA2 activity in the membrane and cytosolic subcellular fractions was measured in the presence of 4 mM EGTA and using (16:0, [3H]18:1) plasmenylcholine as substrate. As seen in Table 1, only BEL is able to inhibit both thrombin and typtase stimulated membrane-associated PLA2 activity to near control levels. Neither PX-18 nor AACOCF3 significantly decrease PLA2 activity in the membrane fraction following either thrombin or tryptase stimulation. These results demonstrate the ability of BEL to selectively and significantly inhibit iPLA2 activity.
TABLE 1.
PLA2 activity (nmol/mg protein/min) in the cytosolic or membrane fraction of HCAEC. Cell were pretreated with PX-18 (2 μM), AACOCF3 (5 μM) or BEL (5 μM) for 10 mins prior to stimulation with either thrombin (1 IU/mL, 2 mins) or tryptase (20 ng/mL, 2 mins). PLA2 activity was measured in the presence of 4 mM EGTA using (16:0, [3H] 18:1) plasmenylcholine substrate. Data represent mean ± SEM for three separate experiments.
| Cytosolic Fraction | Membrane Fraction | |||||
|---|---|---|---|---|---|---|
| Unstimulated | Thrombin | Tryptase | Unstimulated | Thrombin | Tryptase | |
| No Pretreatment | 0.52 ± 0.06 | 0.47 ± 0.10 | 0.54 ± 0.08 | 2.54 ± 0.26 | 8.79 ± 0.71 | 6.37 ± 0.82 |
| PX-18 | 0.45 ± 0.05 | 0.41 ± 0.07 | 0.47 ± 0.08 | 2.39 ± 0.14 | 8.12 ± 0.29 | 7.01 ± 0.31 |
| AACOCF3 | 0.22 ± 0.12 | 0.28 ± 0.09 | 0.26±0.10 | 2.41 ± 0.17 | 8.44 ± 0.74 | 6.53 ± 0.74 |
| BEL | 0.17 ± 0.03 | 0.21 ± 0.04 | 0.22 ± 0.03 | 1.07 ± 0.13 | 3.01 ± 0.44 | 2.79 ± 0.12 |
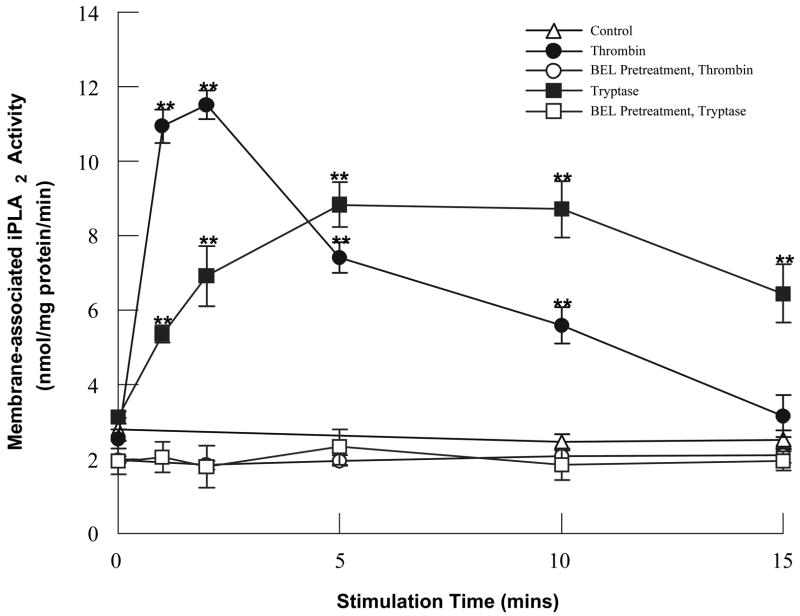
We have compared the time course of activation of HCAEC iPLA2 following incubation with either thrombin (which activates PAR-1) or tryptase (which activates PAR-2) (Fig. 1). While both thrombin and tryptase stimulation lead to increases in iPLA2 activity, the time course of activation is quicker in the presence of thrombin and is more prolonged in the presence of tryptase. Additionally, both thrombin and tryptase stimulated increases in iPLA2 activity are inhibited by pretreatment with BEL. Previous experiments by our laboratory have demonstrated that incubation with the tethered ligand peptides for PAR-1 and PAR-2 activates endothelial cell iPLA2, indicating that the thrombin- and tryptase-stimulated increases in iPLA2 activity occur via activation of PAR [4,5,20].
FIGURE 1.
iPLA2 activation following thrombin (0.1IU/ml) or tryptase (20ng/ml) stimulation in the presence or absence of BEL (5μm, 10 mins) pretreatment. Data represent mean ± SEM for four separate experiments. **p<0.01 when compared to control.
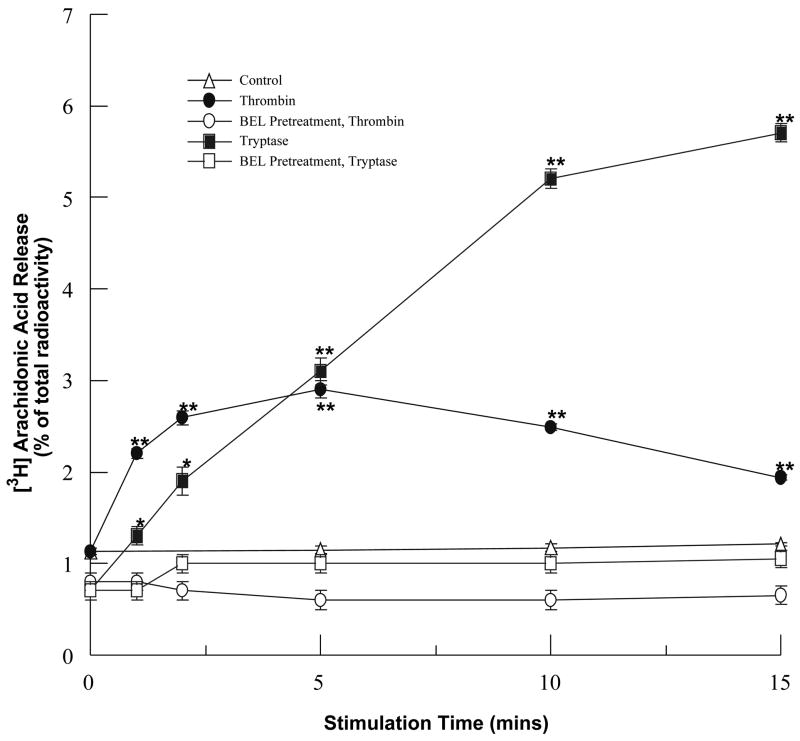
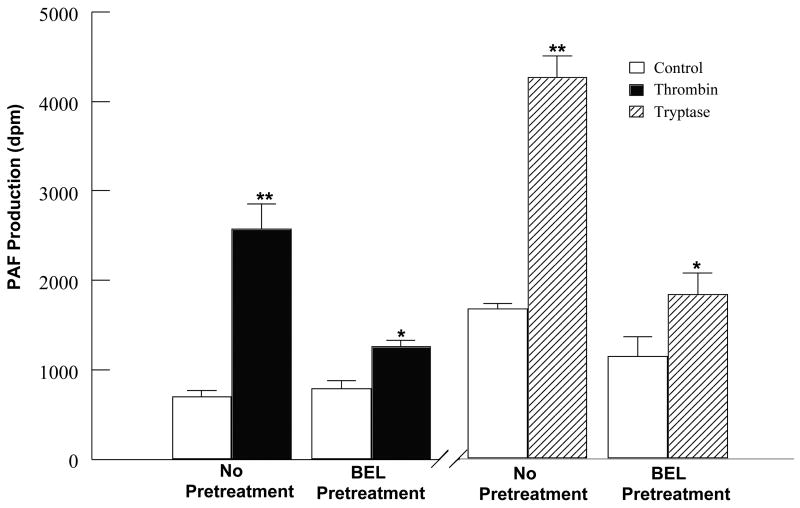
To determine whether thrombin or tryptase-stimulated iPLA2 activity results in accumulation of metabolically active membrane phospholipid-derived metabolites, we measured arachidonic acid release and PAF production in stimulated HCAEC in the absence or presence of BEL pretreatment. Stimulation of HCAEC with thrombin or tryptase induced a significant time-dependent increase in [3H] arachidonic acid release (Fig. 2) and PAF production (Fig. 3). Similar to the pattern of iPLA2 activation, thrombin stimulation causes a more rapid increase in [3H] arachidonic acid release while tryptase induces a greater and more prolonged increase in [3H] arachidonic acid release. Increases in PAF production following a 10min incubation were greater with tryptase than with thrombin, coinciding with the time course of iPLA2 activation upon stimulation with these proteases. These responses were inhibited by pretreatment with BEL demonstrating that production of these metabolites was a result of iPLA2 activation (Figs. 2 and 3). In order to insure that activation of either sPLA2 or cPLA2 was not responsible for increases in PAF produciton cells were pretreated with PX-18 or AACOCF3. Pretreatment with PX-18 had no effect on basal levels of PAF production (884 ± 107 dpm) or PAF production in response to thrombin (2177 ± 512 dpm) or tryptase (4013 ± 476 dpm) stimulation. Similary, AACOCF3 had no effect on basal levels of PAF production (727 ± 96 dpm) or thrombin (2788 ± 347 dpm) or tryptase (4312 ± 601 dpm) stimulated PAF production.
FIGURE 2.
Arachidonic acid release following thrombin (0.1IU/ml) or tryptase (20ng/ml) stimulation in the presence or absence of BEL (5μm, 10 mins) pretreatment. Data represent mean ± SEM for six separate experiments. *p<0.05, **p<0.01 when compared to controls.
FIGURE 3.
PAF production in thrombin (0.1IU/ml) or tryptase (20ng/ml) stimulated HCAEC in the presence or absence of BEL (5μm, 10 mins) pretreatment. Data represents mean + SEM for eight separate experiments. *p<0.05, **p<0.01 when compared to control.
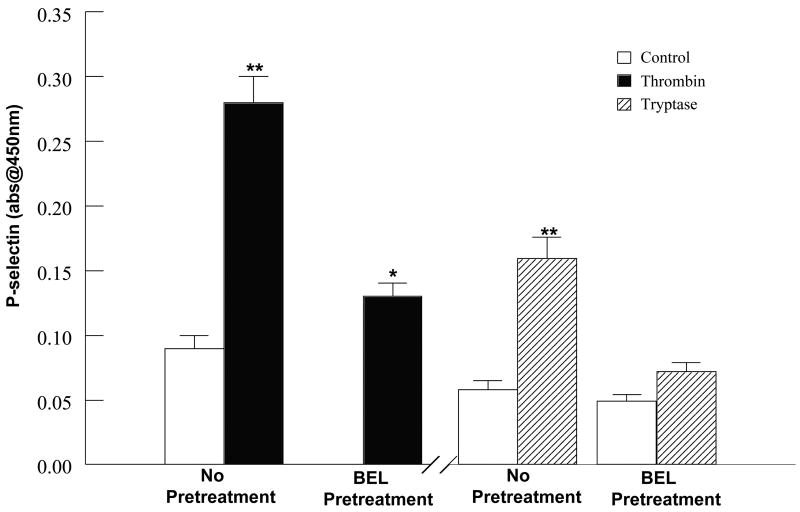
Accompanying the increased PAF production, an increase in HCAEC surface expression of P-selectin was observed following 10 mins stimulation with thrombin or tryptase (Fig. 4). The presence of P-selectin on an activated endothelial cell layer plays an essential role in the initiation of a tentative adhesive interaction between the circulating inflammatory cell and activated endothelial cell monolayer [21]. Subsequently, the enhanced expression of endothelial cell-associated PAF has been shown to cause transient adherence of neutrophils to the endothelial cells [22–24].
FIGURE 4.
Cell surface P-selectin expression following thrombin (0.1IU/ml, 5mins) or tryptase (20ng/ml, 5mins) stimulation in the presence or absence of BEL (5μm, 10 mins) pretreatment. Data represents mean ± SEM for at least six separate cell cultures. **p<0.01 when compared to control.
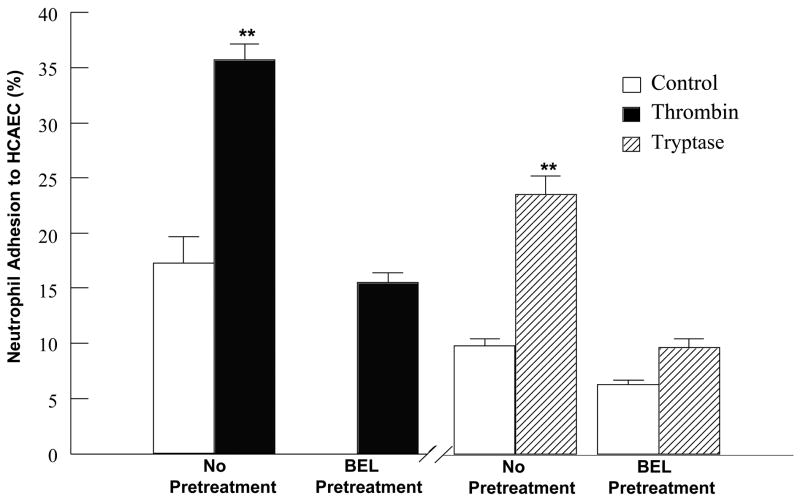
HCAEC were stimulated with either thrombin or tryptase in the absence or presence of BEL pretreatment, prior to a 10 min incubation with neutrophils freshly isolated from human peripheral blood. Adherent neutrophils were detected utilizing a myeloperoxidase activity assay (see [5] for detailed procedure). As shown in Fig. 5, both thrombin and tryptase stimulation of HCAEC monolayers increased neutrophil adherence more than 2-fold over levels of neutrophil adhesion to unstimulated HCAEC. Importantly, pretreatment of the HCAEC with BEL significantly inhibited both thrombin and tryptase induced increases in neutrophil adherence, demonstrating the role of iPLA2 in the regulation of neutrophil adherence. In order to exclude the role of either sPLA2 or cPLA2 in neutrophil adherence, HCAEC were pretreated with PX-18 or AACOCF3 prior to either thrombin or tryptase stimulation and neutrophil adherence measured. Pretreatment with the sPLA2 inhibitor PX-18 had no effect on basal neutrophil adherence (12% ± 3) and did not significantly decrease neutrophil adherence to thrombin- (31% ± 7) or tryptase- (26% ± 4) stimulated HCAEC. Similarly, pretreatment with AACOCF3, a cPLA2 inhibitor, did not effect basal neutrophil adherence (11% ± 4) and failed to inhibit neutrophil adherence to thrombin- (27% ± 8) or tryptase- (19% ± 7) stimulated HCAEC.
FIGURE 5.
Effect of thrombin or tryptase stimulation on the adhesion of neutrophils to HCAEC. This data demonstrates a 2-fold increase in neutrophil adhesion upon stimulation of HCAEC with 0.1IU/ml thrombin for 10 mins. Stimulation with 20ng/ml tryptase for 10 mins caused a 2.4 fold increase in neutrophil adhesion. BEL pretreatment (5 μM, 10 mins) inhibited the tryptase stimulated increase in neutrophil adhesion to a 1.5 fold increase. Results represent the mean + SEM for at least four separate cell cultures. **p<0.01 when compared to control.
Taken together, these results demonstrate activation of iPLA2 via thrombin or tryptase stimulation is capable of inducing inflammatory changes in the endothelium suggesting a possible mechanism for the initiation of the inflammatory response present in coronary endothelium in cardiovascular diseases.
Discussion
In cardiovascular inflammation seen in thrombosis, ischemia, and atherosclerosis, either thrombin or tryptase are known to be elevated, demonstrating their potential to both initiate and propagate the inflammatory response. Maximal activation of iPLA2 and release of arachidonic acid occur slightly earlier when HCAEC are stimulated with thrombin rather than with tryptase, suggesting that while both stimulants ultimately lead to activation of iPLA2, the cell signaling events between iPLA2 and PAR-1 and PAR-2 may be different. Previous experiments by our laboratory examining the signaling events linking PAR-1 to activation of iPLA2 have demonstrated that a member of the novel protein kinase C (PKC) isoenzyme family (calcium-independent, phorbol 12-myristate, 13-acetate dependent) mediates iPLA2 activation following thrombin stimulation of the isolated membrane fraction. Activation of iPLA2 following thrombin stimulation could be the result of any combination of the following three events: 1) direct phosphorylation of iPLA2 by PKC resulting in enhanced catalytic activity 2) modulation of enzyme activity by protein-protein interactions mediated by PKC phosphorylation of an iPLA2 regulatory protein or 3) targeted delivery of iPLA2 to membrane domains enriched in arachidonylated plasmalogen phospholipids, the preferred substrate for HCAEC iPLA2 [4]. Similar studies examining the role of PKC in tryptase mediated iPLA2 activation have yet to be completed.
Experiments in our laboratory examining changes in the electrical resistance of HCAEC monolayers have demonstrated that apical stimulation with thrombin produces a greater decrease in electrical resistance than apical stimulation with tryptase. Interestingly, when HCAEC are basolaterally stimulated with these proteases, tryptase produces a greater decrease in electrical resistance than thrombin. In the process of atherosclerosis/thrombosis, the apical surface of the endothelium would be exposed to thrombin and the basolateral surface would be exposed to tryptase released from mast cell present underlying the HCAEC monolayer. The physiology of an atherosclerotic plaque provides a possible explanation for the differential response of the endothelium to apical versus basolateral stimulation with thrombin and tryptase. The differential response of the endothelium to apical verus basolateral stimulation poses the intriguing possibility that the same intracellular events can be signaled by proteases present on dfferent sides of the endothelium. Additionally, these experiments provide indirect evidence that a sidedness to the expression of PAR-1 and -2 on the endothelium may exist. Current studies in our laboratory are underway to examine the validity of this hypothesis.
Studies by numerous laboratories have identified PLA2 as a critical enzyme in the progression of several cardiovascular diseases. PLA2 are responsible for the hydrolysis of sn-2 esterified fatty acids from membrane phospholipids, resulting in the stoichiometric production of free fatty acid, most notably arachidonic acid, and lysophospholipid. These metabolites are capable of exerting a direct effect on membrane properties and can serve as precursors for biologically active metabolites [25].
Mammalian PLA2s are classified into three main types, secretory, cytosolic, Ca2+-activated and Ca2+-independent [26–28]. While the three types of PLA2s are distinct, they are known to coexist in mammalian cells and may interact with each other. Research in our laboratory and others has shown that the majority of PLA2 activity in both human myocardium [29–33] and coronary vasculature endothelial cells [20,34] occurs in the absence of Ca2+, thus representing iPLA2.
As demonstrated in the studies presented here, activation of iPLA2 via either thrombin or tryptase stimulation leads to arachidonic acid release, PAF production, cell surface P-selectin expression and increased neutrophil adherence, all events contributing to inflammation following vascular injury. These findings demonstrate the significance of iPLA2 in the inflammatory response, emphasizing the possibility of preventing extensive cardiovascular damage by inhibiting iPLA2 mediated inflammation. The activation of iPLA2 in HCAEC and the subsequent production of choline lysophospholipids may, in addition, contribute directly to the initiation of ventricular arrhythmias due to their incorporation into the ischemic myocyte sarcolemma.
Studies by other laboratories have shown that in addition to inhibiting iPLA2, BEL inhibits phosphatidate phosphohydrolase (PAPH) activity, an enzyme whose activation also results in arachidonic acid release [35]. PAPH converts phosphatidic acid, released from membrane phospholipids following hydrolysis by phospholipase D, to diacylglycerol (DAG). Subsequent activation of protein kinase C by DAG can lead to the activation of iPLA2, releasing arachidonic acid as a product of plasmalogen phospholipid hydrolysis. We have previously shown that HCAEC PAPH activity is only significantly decreased at concentrations of BEL greater than 10 μM [36], 2-fold higher than the concentration utilized in these experiments to directly inhibit iPLA2 activity, indicating that HCAEC PAPH activity is not inhibited by 5 μM BEL pretreatment.
Several groups have highlighted the significance of the PAF/PAF-receptor interaction in cell adhesion to, and migration across, the endothelium. Prescott et al. [37] have correlated the adhesion of neutrophils to thrombin-activated endothelium with PAF synthesis and expression on the surface of endothelial cells. Additionally, Kuijpers et al. [38] were able to demonstrate the ability of PAF receptor antagonists to prevent neutrophil migration across cytokine pretreated endothelial cells by approximately 60 percent. As our data indicates, the increase in PAF production (Fig. 3) and P-selectin expression (Fig. 4) in response to thrombin or tryptase is accompanied by an increase in neutrophil adherence (Fig. 5) to the stimulated HCAEC monolayer.
As the studies examining possible factors responsible for either the cause or progression of various cardiovascular diseases have intensified, an increasing amount of information regarding the adverse affects of inflammation in the cardiovascular system has been published. More recent studies have identified inflammation as a risk factor and potential propagative factor for a variety of cardiovascular diseases such as myocarditis, atherosclerosis, and myocardial ischemia. Clearly, the activation of iPLA2 in HCAEC by mediators such as thrombin and tryptase in the progression cardiovascular disease represents an intriguing pathway that could be targeted for therapeutic intervention to alleviate several cardiovascular diseases.
Acknowledgments
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-68588 (JM) and the American Heart Association-Heartland Affiliate (MCW). The authors thank Richard Berney (Richard Berney Associates, LLC) Bethesda, MD for the gift of the sPLA2 inhibitor PX-18 utilized in these studies.
Abbreviations
- AACOCF3
arachidonyl trifluoromethyl ketone
- BEL
bromoenol lactone
- BSA
bovine serum albumin
- DAG
diacylglycerol
- EDTA
ethylenediamine tetraacetic acid
- EGTA
ethylen glycolbis(beta-amino-ethyl ether) tetraacetic acid
- FCS
fetal calf serum
- HCAEC
human coronary artery endothelial cells
- iPLA2
calcium-independent phospholipase A2
- MAP kinase
Mitogen-activated protein kinase
- MEM
minimal essential medium
- PAF
platelet-activating factor
- PAPH
phosphatidate phosphohydrolase
- PAR
protease activated receptor
- PBS
phosphate buffered saline
- PKC
protein kinase C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cocks TM, Moffatt JD. Protease-activated receptors: sentries for inflammation? Trends Pharmacol Sci. 2000;21:103–8. doi: 10.1016/s0165-6147(99)01440-6. [DOI] [PubMed] [Google Scholar]
- 2.Dery O, Corvera CU, Steinhoff M, Bunnett NW. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol. 1998;274:C1429–52. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- 3.Persson CGA, Erjefalt JS, Greiff L, Erjefalt I, Korsgren M, Linden M, Sundler F, Andersson M, Svensson C. Contribution of plasma-derived molecules to mucosal immune defence, disease and repair in the airways. Scand J Immunol. 1998;47:302–13. doi: 10.1046/j.1365-3083.1998.00317.x. [DOI] [PubMed] [Google Scholar]
- 4.Meyer MC, Kell PJ, Creer MH, McHowat J. Calcium-independent phospholipase A2 regulated by protein kinase C activity in human coronary artery endothelial cells. Am J Physiol. 2004;288:C475–82. doi: 10.1152/ajpcell.00306.2004. [DOI] [PubMed] [Google Scholar]
- 5.Meyer MC, Creer MH, McHowat J. Potential role for mast cell tryptase in recruitment of inflammatory cells to endothelium. Am J Physiol. 2005;289:C1485–91. doi: 10.1152/ajpcell.00215.2005. [DOI] [PubMed] [Google Scholar]
- 6.Molino M, Woolkalis MJ, Reavey-Cantwell J, Pratico D, Andrade-Gordon P, Barnathan ES, Brass LF. Endothelial cell thrombin receptors and PAR-2. Two protease-activated receptors located in a single cellular environment. J Biol Chem. 1997;272:11133–41. doi: 10.1074/jbc.272.17.11133. [DOI] [PubMed] [Google Scholar]
- 7.Davey MG, Luscher EF. Activation of a membrane-associated phospholipase A2 during rabbit myocardial ischemia which is highly selective for plasmalogen substrate. Nature. 1967;216:857–8. [PubMed] [Google Scholar]
- 8.Berndt MC, Phillips DR. Platelet membrane proteins: composition and receptor function. In: Gordon JL, editor. Platelets in Biology and Pathology. Amsterdam: Elsevier/North Holland; 1981. pp. 43–74. [Google Scholar]
- 9.Bar-Shavit R, Kahn A, Wilner GD, Fenton JW. Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science. 1983;220:728–31. doi: 10.1126/science.6836310. [DOI] [PubMed] [Google Scholar]
- 10.Chen LB, Teng NNH, Buchanan JM. Mitogenicity of thrombin and surface alterations on mouse splenocytes. Exp Cell Res. 1976;101:41–6. doi: 10.1016/0014-4827(76)90409-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen LB, Buchanan JM. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci USA. 1975;72:131–5. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNamara CA, Sarembock IJ, Gimple LW, Fenton JW, II, Coughlin SR, Owens GK. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J Clin Invest. 1993;91:94–8. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelken NA, Soifer SJ, O’Keefe J, Vu TKH, Charo IF, Coughlin SR. Thrombin receptor expression in normal and atherosclerotic human arteries. J Clin Invest. 1992;90:1614–21. doi: 10.1172/JCI116031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laine P, Kaartinen M, Pentillä A, Panula P, Paavonen T, Kovanen P. Association between myocardial infarction and the mast cells in the adventitia of the infarct-related coronary artery. Circulation. 1999;99:361–9. doi: 10.1161/01.cir.99.3.361. [DOI] [PubMed] [Google Scholar]
- 15.Patella V, Marinò I, Arbustini E, Lamparter-Schummert B, Verga L, Monika A, Marone G. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation. 1998;97:971–8. doi: 10.1161/01.cir.97.10.971. [DOI] [PubMed] [Google Scholar]
- 16.Gross RW, Sobel BE. Lysophosphatidylcholine Metabolism in the Rabbit Heart: Characterization of Metabolic Pathways and Partial Purification of Myocardial Lysophospholipase-Transacylase. J Biol Chem. 1982;257:6702–8. [PubMed] [Google Scholar]
- 17.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 18.Conde-Frieboes K, Reynolds LJ, LIO YC, Hale M, Wasserman HH, Dennis EA. Activated ketones as inhibitors of intracellular Ca2+-dependent and Ca2+-independent phospholipase A2. J Am Chem Soc. 1996;118:5519–25. [Google Scholar]
- 19.Hazen SL, Zupan LA, Weiss RH, Getman DP, Gross RW. Suicide inhibition of canine myocardial cytosolic calcium-independent phospholipase A2. Mechanism based discrimination between calcium-dependent and -independent phospholipase A2. J Biol Chem. 1991;266:7227–32. [PubMed] [Google Scholar]
- 20.Creer MH, McHowat J. Selective Hydrolysis of Plasmalogens in Endothelial Cells Following Thrombin Stimulation. Am J Physiol. 1998;275:C1498–1507. doi: 10.1152/ajpcell.1998.275.6.C1498. [DOI] [PubMed] [Google Scholar]
- 21.Meyer M, McHowat J. The role of platelet-activating factor in the adherence of circulating cells to the endothelium. In: Pandalai SG, editor. Recent Research Developments in Physiology. India: Research Signpost; 2004. pp. 129–147. [Google Scholar]
- 22.Gamble JR, Skinner MP, Berndt MC, Vadas MA. Prevention of activated neutrophil adhesion to endothelium by soluble adhesion protein GMP-140. Science. 1990;249:414–7. doi: 10.1126/science.1696029. [DOI] [PubMed] [Google Scholar]
- 23.Geng JG, Bevilacqua MP, Moore KL, McIntyre TM, Prescott SM, Kim JM, Biss GA, Zimmerman GA, McEver RP. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990;343:757–60. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- 24.Lorant DE, Patel KD, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Co-expression of GMP-140 and PF by endothelium stimulated by histamine and thrombin: a juxtacrine system for adhesion and activation for neutrophils. J Cell Biol. 1991;115:223–34. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennis EA. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem Sci. 1997;22:1–2. doi: 10.1016/s0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- 26.Balsinde J, Balboa MA, Insel PA, Dennis EA. Regulation and inhibition of phospholipase A2. Ann Rev Pharm Tox. 1999;39:175–89. doi: 10.1146/annurev.pharmtox.39.1.175. [DOI] [PubMed] [Google Scholar]
- 27.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–60. [PubMed] [Google Scholar]
- 28.McHowat J, Creer MH. Catalytic Features, Regulation and Function of Myocardial Phospholipase A2. Curr Med Chem. 2004;2:209–18. doi: 10.2174/1568016043356282. [DOI] [PubMed] [Google Scholar]
- 29.McHowat J, Creer MH. Calcium-independent phospholipase A2 in isolated rabbit ventricular myocytes. Lipids. 1998;33:1203–12. doi: 10.1007/s11745-998-0324-5. [DOI] [PubMed] [Google Scholar]
- 30.McHowat J, Creer MH. Thrombin activates a membrane-associated calcium-independent PLA2 in ventricular myocytes. Am J Physiol. 1998;274:C447–54. doi: 10.1152/ajpcell.1998.274.2.C447. [DOI] [PubMed] [Google Scholar]
- 31.McHowat J, Creer MH, Hicks KK, Jones JH, McCrory RD, Kennedy RH. Induction of Ca-independent PLA2 and conservation of plasmalogen polyunsaturated fatty acids in diabetic heart. Am J Physiol. 2000;279:E25–32. doi: 10.1152/ajpendo.2000.279.1.E25. [DOI] [PubMed] [Google Scholar]
- 32.McHowat J, Tappia PS, Liu SY, McCrory RD, Panagia V. Redistribution and abnormal activity of phospholipase A2 isoenzymes in postinfarct congestive heart failure. Am J Physiol. 2000;280:C573–80. doi: 10.1152/ajpcell.2001.280.3.C573. [DOI] [PubMed] [Google Scholar]
- 33.McHowat J, Swift LM, Arutunyan A, Sarvazyan N. Clinical concentrations of doxorubicin inhibit activity of myocardial membrane-associated, calcium-independent phospholipase A2. Cancer Res. 2001;61:4024–9. [PubMed] [Google Scholar]
- 34.McHowat J, Kell PJ, O’Neill HB, Creer MH. Endothelial Cell PAF Synthesis Following Thrombin Stimulation Utilizes Ca2+-Independent Phospholipase A2. Biochem. 2001;40:14921–31. doi: 10.1021/bi0156153. [DOI] [PubMed] [Google Scholar]
- 35.Balsinde J, Dennis EA. Bromoenol lactone inhibits magnesium-dependent phosphatidate phosphohydrolase and blocks triacylglycerol biosynthesis in mouse P388D1 macrophages. J Biol Chem. 1996;271:31937–41. doi: 10.1074/jbc.271.50.31937. [DOI] [PubMed] [Google Scholar]
- 36.Meyer MC, McHowat J. Calcium-Independent Phospholipase A2-Catalyzed Plasmalogen Hydrolysis in Hypoxic Human Coronary Artery Endothelial Cells. Am J Physiol. 2006 doi: 10.1152/ajpcell.00120.2006. in press. [DOI] [PubMed] [Google Scholar]
- 37.Prescott SM, Zimmerman GA, McIntyre TM. Human endothelial cells in culture produce platelet-activating factor when stimulated with thrombin. Proc Natl Acad Sci USA. 1984;81:3534–8. doi: 10.1073/pnas.81.11.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuijpers TW, Hakkert BC, Hart MHL, Roos D. Neutrophil Migration across Monolayers of Cytokine-prestimulated Endothelial Cells: A Role for Plateleyctivating Factor and IL-8. J Cell Biol. 1992;117:565–72. doi: 10.1083/jcb.117.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]