Abstract
Breast metastases from extramammary neoplasms are very rare. We presented a 66 year-old female with metastasis of small cell lung carcinoma to the breast. She presented with consolidation over the left upper lobe of her lung undetermined after endobronchial or video-assisted thoracoscopic surgery (VATS) biopsy, and this was treated effectively after antibiotic therapy at initial stage. The left breast lumps were noted 4 months later, and she underwent a modified radical mastectomy under the impression of primary breast carcinoma. However, the subsequent chest imaging revealed re-growing mass over the left mediastinum and hilum, and cells with the same morphological and staining features were found from specimens of transbronchial brushing and biopsy. An accurate diagnosis to distinguish a primary breast carcinoma from metastatic one is very important because the therapeutic planning and the outcome between them are different.
Keywords: Small cell carcinoma, Breast metastasis, Lung
INTRODUCTION
Breast metastases from extramammary primary neoplasms are very rare (Ribeiro-Silva et al., 2006; Chaignaud et al., 1994; Oksüzoğlu et al., 2003; Nielsen et al., 1981; Cangiarella et al., 1998), accounting for about 2% of all breast malignancies (Sadikot et al., 1997; Silverman et al., 1987; Hajdu and Urban, 1972). Reported primary tumors metastasizing to the breast include melanoma (Cangiarella et al., 1998), hematopoietic malignancies (Shukla et al., 2005), and carcinomas from the lung, genitourinary or gastrointestinal tract (Shukla et al., 2005; Ramar et al., 2003; Masmoudi et al., 2003; Santeufemia et al., 2006; Yeh et al., 2004; Mihai et al., 2005). However, metastasized breast malignancies from small cell carcinomas of the lung were very rarely reported before (Shukla et al., 2005; Inoue et al., 2006; Jakovljević et al., 2003). Here we report a female patient with breast metastasis from small cell lung carcinoma, of which the diagnosis was established from the metastatic breast lesions. Her pulmonary lesion could not be diagnosed initially after series of diagnostic procedures but was subsequently confirmed by repeated endobronchial brushing cytology and biopsy.
CASE REPORT
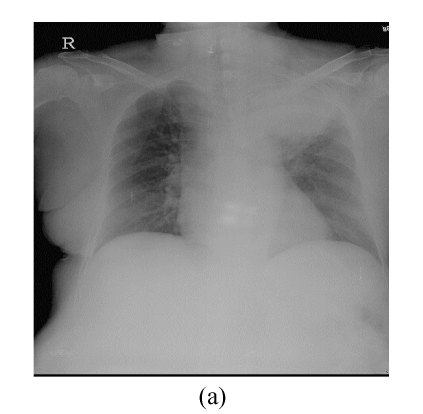
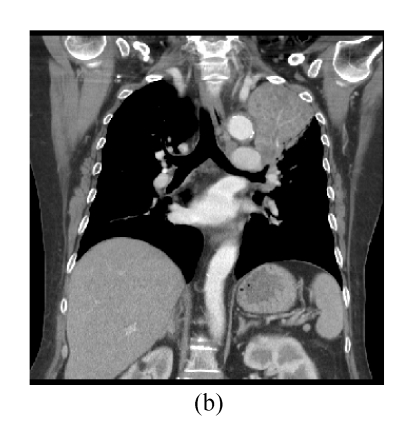
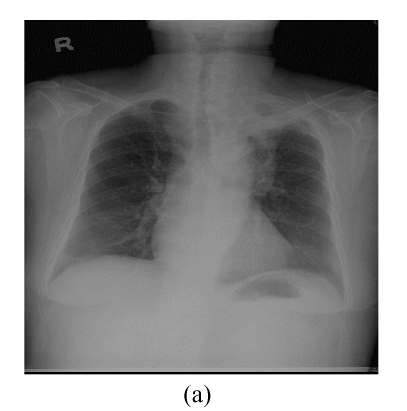
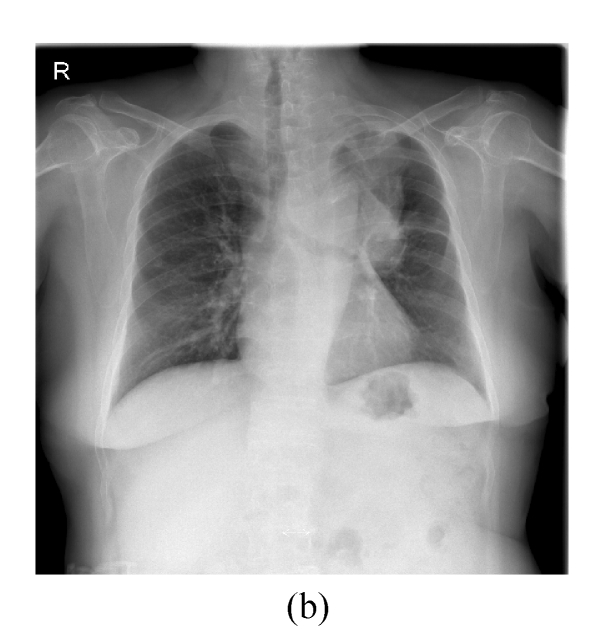
This 66 year-old female had complained of cough with intermittent fever (up to 38.5 °C) for 20 d. Chest roentgenogram revealed opacified shadow over the left upper lung field (Fig.1a). Chest computed tomography showed that this lesion was composed of pulmonary parenchymal consolidation and a suspected mass lesion over the central hilar area (Fig.1b). Bronchoscopic examination showed narrowing in the bronchial orifice of the left upper lobe. However, repeated sputum cytological examinations, and transbronchial and trans-thoracoscopic biopsies revealed no evidence of malignancy. On admission, she underwent intravenous piperacillin-tazobactam treatment because Pseudomonas aeroginosa was isolated from her sputum specimens. These symptoms and signs improved after the above treatment; chest X-ray revealed much smaller shadow on the left upper lung field (Fig.2a). Unfortunately, this lung lesion re-grew 4 months later (Fig.2b) and two lumps over her left breast appeared 3 months later.
Fig. 1.
(a) Chest roentgenogram revealed opacified shadow over the left upper lung field; (b) Chest computed tomography showed that this lesion was composed of pulmonary parenchymal consolidation and a suspected mass lesion over the central hilar area
Fig. 2.
(a) Compared with Fig.1a, the shadow regressed 1 month after treatment with antibiotics; (b) The mass shadow re-grew 4 months after treatment
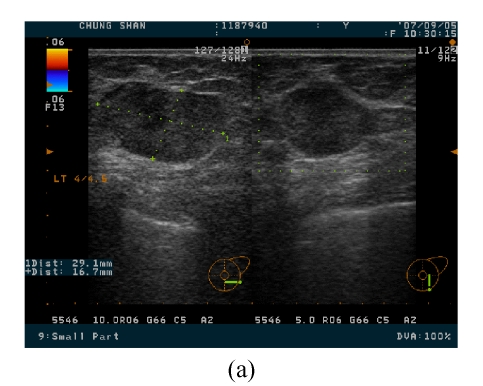
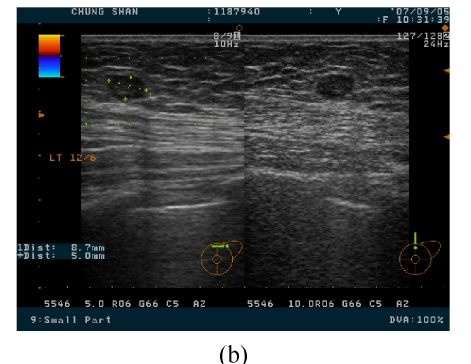
She came to our clinic 4 months after the first treatment, and the two breast masses were 3 cm and 0.8 cm in diameter over the 4 o’clock direction, 4.5 cm from the nipple and 12 o’clock direction, 4 cm from the nipple sites of the left breast, respectively (Fig.3). These two masses were elastically firm on palpation. Breast ultrasound revealed a well marginated but heterogeneous mass in the internal density. Under the suspicion of breast carcinoma, which was proved pathologically by specimen from the excised mass, she underwent left mastectomy and axillary lymph node dissection at the second admission.
Fig. 3.
Two breast masses, with 3 cm (a) and 0.8 cm (b) in diameter over the 4 o’clock direction, 4.5 cm from the nipple and 12 o’clock direction, 4 cm from the nipple sites of the left breast being noted on breast ultrasound imaging
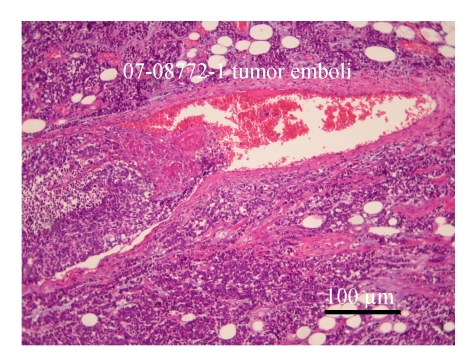
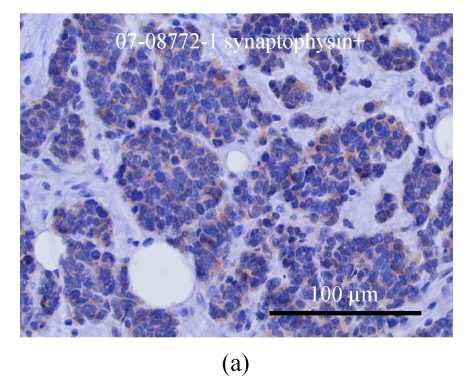
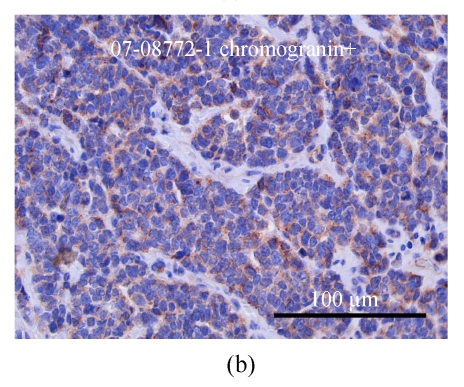
The specimen contained two elastically firm, well marginated masses, 3.l cm×2.5 cm×2.2 cm and 0.8 cm×0.7 cm×0.5 cm in size, over the 4 o’clock direction, 4.5 cm from the nipple and 12 o’clock direction, 4 cm from the nipple sites of the left breast, respectively. Microsocpically, the sections of tumor lesions revealed small hyperchromatic cells with high nucleus-to-cytoplasm (N/C) ratios. Tumor necrosis, emboli and vascular invasion were noted frequently. Non-neoplastic breast tissue showed unremarkable change. The nipple and skin are free of tumor involvement. Three out of 13 lymph nodes isolated from axillary levels 1 and 2 lymph nodes were infiltrated by tumor cells. Perinodal extension was noted. The tumor cells were negatively stained for mucin, c-erbB-2 oncoprotein, estrogen and progesterone receptor; but positively stained for synaptophysin, chromogranin and CD56 (Figs.4 and 5).
Fig. 4.
Microscopically, the sections of breast tumor lesions reveal small hyperchromatic cells with high nucleus-to-cytoplasm (N/C) ratio. Tumor necrosis, emboli and vascular invasion are noted frequently (H & E stain)
Fig. 5.
The breast tumor cells are positively stained for synaptophysin (a) and chromogranin (b)
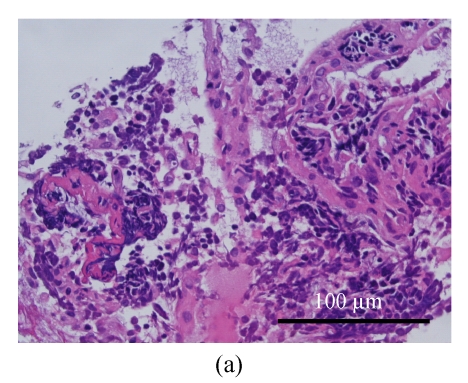
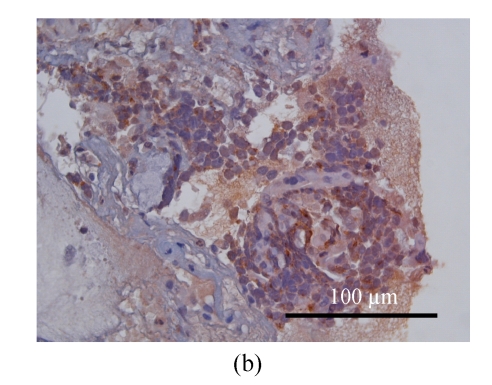
The patient underwent bronchoscopy for the second time. The endobronchial biopsy and brushing revealed similar cells and staining patterns as seen in the breast tumors (Fig.6). Small cell carcinomas over the lung and extrapulmonary site (breast) were diagnosed, but their relationship (double primary or metastatic, or the primary tumor) cannot be confirmed pathologically. However, after reviewing clinical data of this patient, primary small cell lung carcinoma with breast metastases should be the most likely diagnosis. Thus she underwent subsequent cisplatin-based chemotherapy. One episode of severe hyponatremia (serum sodium ion level <120 mol/L) was noted during the postoperative period. Syndrome of inappropriate anti-diuretic hormone (SIADH) was suspected after urine electrolyte and specific gravity were measured. This condition was improved after chemotherapy, sodium supplement and restriction of water intake.
Fig. 6.
Microscopically, the lung mass is proved as small cell carcinoma with similar morphological and staining patterns as those of the breast mass. (a) H & E stain; (b) Focal positive for synaptophysin staining
DISCUSSION
Small cell carcinoma most commonly occurs in the lung, but can also in the extrapulmonary sites such as gastrointestinal or genitourinary tract, gynecological systems, the head and neck, and the breast (Haider et al., 2006; Galanis et al., 1997; Kim et al., 2004; Adegbola et al., 2005; Shin et al., 2000). Primary extrapulmonary small cell carcinomas are rare, and their behaviors are somewhat comparable to the small cells of the lung. The survivals of extrapulmonary small cell carcinomas are related to the extent and the primary site of diseases, and usually aggressive with poor prognosis (Haider et al., 2006; Galanis et al., 1997). Smoking is regarded as an important risk factor not only for pulmonary but also for over 50% of extrapulmonary small cell carcinomas. Primary breast small cell carcinoma is extremely rare, and absence of other non-mammary primary sites as well as the presence of in situ component will be required before establishing this diagnosis (Sridhar et al., 2004). Breast small cell carcinoma, being classified as tumors with endocrine differentiation, is the rarest, but the highest, grade of malignancy with prominent vascular invasion and nodal metastasis (Ajisaka et al., 2003).
Metastatic lesions of the breast only account for 0.4% to 6.6% of all breast malignant tumors, and this difference is due to the inclusion or exclusion of hematopoietic malignancies. Besides leukemia or lymphoma, breast metastasis has been reported to be most commonly from melanoma, rhobdomyosarcoma, and the lung (Shukla et al., 2005; Ramar et al., 2003; Masmoudi et al., 2003; Santeufemia et al., 2006; Yeh et al., 2004; Mihai et al., 2005; Inoue et al., 2006; Jakovljević et al., 2003). Differentiation between the primary and metastatic breast carcinoma is important in determining treatment plans. Metastatic mammary carcinoma is usually well-defined, and in the absence of characters of primary breast carcinoma such as distortion of adjacent architecture or microcalcification (Kelly et al., 1988; Vizcaíno et al., 2001). Multiplicity of the masses and absence of hormone receptors favor the diagnosis of metastatic lesions.
In our presented case, since common features, such as cytopathological pictures and staining patterns, were shared by the tumors in these two organs, and characters of metastatic tumors as described above were noted at the breast, thus small cell lung carcinoma with left breast metastasis was the most reasonable diagnosis. Furthermore, episode of SIADH, which might be a type of paraneoplastic syndrome in small cell carcinoma, occurred after the removal of breast mass. This syndrome was alleviated and the size of the left hilar mass was reduced after chemotherapy, proving the existence of pulmonary malignancy, although the pathology only revealed “suspicious” lesion after repeated biopsy or brushing cytology. However, the route of metastases from the lung was still unclear because axillary lymph nodes metastases were noted, which rarely occurred in hematogeneous metastasis. Fine-needle aspiration, core needle biopsy, as well as surgical biopsy can make pre-mastectomy diagnosis for patients with breast lumps. In our presented case, since the pathological diagnosis from frozen section is carcinoma without more specific histological subtype, and the possible primary site (lung) was suspected after confirming the diagnosis of the breast lumps, modified radical mastectomy would be a reasonable choice of procedure under this condition. If the diagnosis of lung lesion is definite before detecting the breast lump, avoiding or limited operation could be considered as the choice of management.
Small cell carcinomas, either from pulmonary or extrapulmonary site, are aggressive in their behaviors and require combined modalities of treatments including chemotherapy. Neoadjuvant chemotherapy followed by resection has been considered for selective cases without distant metastasis (Jakovljević et al., 2003; Haider et al., 2006). In our presented case, chemotherapy protocol follows the guideline of pulmonary small cell carcinoma. It is still unknown why the pulmonary lesion reduced in size after antibiotic treatment 3 months before its re-appearance. While the improvement of obstructive pneumonia could partially explain this phenomenon, we still think this was an unusual case according to our previous experiences.
In summary, a patient of small cell carcinoma manifested as pulmonary and breast mass lesions could not be confirmed by endobronchial or transthoracic biopsies but was diagnosed subsequently from breast lesions. The initial response of the lung lesion to antibiotic treatment confused us in making the decision of treatment strategies. It is important to differentiate between primary and metastatic breast malignancies according to their clinical and image features, and to consider the possibility of metastasis or other rare primary malignancies if a breast lump was noted.
References
- 1.Adegbola T, Connolly CE, Mortimer G. Small cell neuroendocrine carcinoma of the breast: a report of three cases and review of the literature. J Clin Pathol. 2005;58(7):775–778. doi: 10.1136/jcp.2004.020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajisaka H, Maeda K, Miwa A, Yamamoto K. Breast cancer with endocrine differentiation: report of two cases showing different histologic patterns. Surg Today. 2003;33(12):909–912. doi: 10.1007/s00595-003-2612-5. [DOI] [PubMed] [Google Scholar]
- 3.Cangiarella J, Symmans WF, Cohen JM, Goldenberg A, Shapiro RL, Waisman J. Malignant melanoma metastatic to the breast: a report of seven cases diagnosed by fine-needle aspiration cytology. Cancer. 1998;84(3):160–162. doi: 10.1002/(SICI)1097-0142(19980625)84:3<160::AID-CNCR7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Chaignaud B, Hall TJ, Powers C, Subramony C, Scott-Conner CE. Diagnosis and natural history of extramammary tumors metastatic to the breast. J Am Coll Surg. 1994;179(1):49–53. [PubMed] [Google Scholar]
- 5.Galanis E, Frytak S, Lloyd RV. Extrapulmonary small cell carcinoma. Cancer. 1997;79(9):1729–1736. doi: 10.1002/(SICI)1097-0142(19970501)79:9<1729::AID-CNCR14>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Haider K, Shahid RK, Finch D, Sami A, Ahmad I, Yadav S, Alvi R, Popkin D, Ahmed S. Extrapulmonary small cell cancer: a Canadian province’s experience. Cancer. 2006;107(9):2262–2269. doi: 10.1002/cncr.22235. [DOI] [PubMed] [Google Scholar]
- 7.Hajdu SI, Urban JA. Cancers metastatic to the breast. Cancer. 1972;29(6):1691–1696. doi: 10.1002/1097-0142(197206)29:6<1691::AID-CNCR2820290637>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Inoue T, Tanaka E, Sakuramoto M, Minakuchi M, Maeda Y, Maniwa K, Terada K, Goto S, Takeda T, Okamoto M, et al. A case of small cell lung cancer with an initial symptom of breast metastasis. Nihon Kokyuki Gakkai Zasshi. 2006;44(1):39–42. [PubMed] [Google Scholar]
- 9.Jakovljević B, Stevanović O, Bacić G. Metastases to the breast from small-cell lung cancer: MR findings. A case report. Acta Radiol. 2003;44(5):485–488. doi: 10.1034/j.1600-0455.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 10.Kelly C, Henderson D, Corris P. Breast lumps. Rare presentation of oat cell carcinoma of lung. J Clin Pathol. 1988;41(2):171–172. doi: 10.1136/jcp.41.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Lee SH, Park J, Kim HY, Lee SI, Nam EM, Park JO, Kim K, Jung CW, Im YH, et al. Extrapulmonary small-cell carcinoma: a single-institution experience. Jpn J Clin Oncol. 2004;34(5):250–254. doi: 10.1093/jjco/hyh052. [DOI] [PubMed] [Google Scholar]
- 12.Masmoudi A, Mathieu MC, Soria JC. Breast metastasis from lung adenocarcinoma: a case report. Anticancer Res. 2003;23(2C):1825–1826. [PubMed] [Google Scholar]
- 13.Mihai R, Christie-Brown J, Bristol J. Breast metastases from colorectal carcinoma. Breast. 2005;14(1):80–81. doi: 10.1016/j.breast.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen M, Andersen JA, Henriksen FW, Kristensen PB, Lorentzen M, Ravn V, Schiødt T, Thorborg JV, Ornvold K. Metastases to the breast from extramammary carcinomas. Acta Pathol Microbiol Scand A. 1981;89(4):251–256. doi: 10.1111/j.1699-0463.1981.tb00218.x. [DOI] [PubMed] [Google Scholar]
- 15.Oksüzoğlu B, Abali H, Güler N, Baltali E, Ozişik Y. Metastasis to the breast from nonmammarian solid neoplasms: a report of five cases. Med Oncol. 2003;20(3):295–300. doi: 10.1385/MO:20:3:295. [DOI] [PubMed] [Google Scholar]
- 16.Ramar K, Pervez H, Potti A, Mehdi S. Breast metastasis from non-small-cell lung carcinoma. Med Oncol. 2003;20(2):181–184. doi: 10.1385/MO:20:2:181. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro-Silva A, Mendes CF, Costa IS, de Moura HB, Tiezzi DG, Andrade JM. Metastases to the breast from extramammary malignancies: a clinicopathologic study of 12 cases. Pol J Pathol. 2006;57(3):161–165. [PubMed] [Google Scholar]
- 18.Sadikot RT, Renwick DS, DaCosta P, Chalmers AG, Pearson SB. Breast metastasis from non-small cell lung cancer. South Med J. 1997;90(10):1063–1064. doi: 10.1097/00007611-199710000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Santeufemia DA, Piredda G, Fadda GM, Cossu Rocca P, Costantino S, Sanna G, Sarobba MG, Pinna MA, Putzu C, Farris A. Successful outcome after combined chemotherapeutic and surgical management in a case of esophageal cancer with breast and brain relapse. World J Gastroenterol. 2006;12(34):5565–5568. doi: 10.3748/wjg.v12.i34.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin SJ, DeLellis RA, Ying L, Rosen PP. Small cell carcinoma of the breast: a clinicopathologic and immunohistochemical study of nine patients. Am J Surg Pathol. 2000;24(9):1231–1238. doi: 10.1097/00000478-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Shukla R, Pooja B, Radhika S, Nijhawan R, Rajwanshi A. Fine-needle aspiration cytology of extramammary neoplasms metastatic to the breast. Diagn Cytopathol. 2005;32(4):193–197. doi: 10.1002/dc.20198. [DOI] [PubMed] [Google Scholar]
- 22.Silverman JF, Feldman PS, Covell JL, Frable WJ. Fine needle aspiration cytology of neoplasms metastatic to the breast. Acta Cytol. 1987;31(3):291–300. [PubMed] [Google Scholar]
- 23.Sridhar P, Matey P, Aluwihare N. Primary carcinoma of breast with small-cell differentiation. Breast. 2004;13(2):149–151. doi: 10.1016/j.breast.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Vizcaíno I, Torregrosa A, Higueras V, Morote V, Cremades A, Torres V, Olmos S, Molins C. Metastasis to the breast form extramammary malignancies. A report of four cases and a review of literature. Eur Radiol. 2001;11(9):1659–1665. doi: 10.1007/s003300000807. [DOI] [PubMed] [Google Scholar]
- 25.Yeh CN, Lin CH, Chen MF. Clinical and ultrasonographic characteristics of breast metastases from extramammary malignancies. Am Surg. 2004;70(4):287–290. [PubMed] [Google Scholar]