Abstract
Optimization of a process for extracting astaxanthin from Phaffia rhodozyma by acidic method was investigated, regarding several extraction factors such as acids, organic solvents, temperature and time. Fractional factorial design, central composite design and response surface methodology were used to derive a statistically optimal model, which corresponded to the following optimal condition: concentration of lactic acid at 5.55 mol/L, ratio of ethanol to yeast dry weight at 20.25 ml/g, temperature for cell-disruption at 30 °C, and extraction time for 3 min. Under this condition, astaxanthin and the total carotenoids could be extracted in amounts of 1294.7 μg/g and 1516.0 μg/g, respectively. This acidic method has advantages such as high extraction efficiency, low chemical toxicity and no special requirement of instruments. Therefore, it might be a more feasible and practical method for industrial practice.
Keywords: Astaxanthin, Phaffia rhodozyma, Extracting, Optimization, Acidic method
INTRODUCTION
Carotenoids are a class of natural fat-soluble pigments generally found in plants, algae and photosynthetic bacteria, where they play a critical role in the photosynthetic process. Carotenoids also exist in some non-photosynthetic bacteria, yeasts and molds, where they may carry out a protective function against damages induced by light and oxygen (Black and Mathews-Roth, 1991; Conn et al., 1991; Kobayashi et al., 1997; Wang and Li, 1997). In addition, carotenoids were reported to serve as antioxidants and a source of vitamin A in animals (Ong and Tee, 1992; Britton, 1995; Miki, 1991). As animals by themselves cannot synthesize carotenoids, these chemicals can be supplied in the animals’ diets, and lead to bright meat colors.
Astaxanthin (3,3′-dihydroxy-β,β-carotene-4,4′-dione) is a unique carotenoid widely distributed in nature. It is one of the major pigments in the carotenoid family that is commonly used to provide coloration characteristics to some birds, crustaceans and salmons (Johnson, 1991; Verdoes et al., 1999). Additionally, other diverse biological functions of astaxanthin have attracted more and more interest due to its health benefits to human beings in light of its roles in cancer prevention, enhancement of immune response, and serving as a free radical quencher (Fraser et al., 1997; Kurashige et al., 1990; Lawlor and O′Brien, 1995; Guerin et al., 2003; Palozza and Krinsky, 1992; Bertram and Vine, 2005). As a result, astaxanthin has a high market value to both the nutraceutical and food industries.
Astaxanthin is currently chemically synthesized and added into some animal feeds for pigmentation of animals, especially for marine fishes. However, synthetic astaxanthin is expensive (approximately 2000 USD/kg). Johnson (1991) reported that synthetic astaxanthin accounted for approximately 10% of the total cost of the fish feed. Moreover, synthetic astaxanthin has not been approved as a GRAS (generally recognized as safe) chemical in the US and thus is not allowed to be used as a functional food additive or medicinal ingredient (Tangeras and Slinde, 1994). Therefore, production of astaxanthin from natural source is a potential alternative to replace synthetic astaxanthin. This market driven force has prompted considerable research for developing natural astaxanthin.
Besides the extraction of astaxanthin from green alga Haematococcus pluvialis and crustaceans, the red basidiomycetous yeast, Phaffia rhodozyma, has been identified as the best biological source of astaxanthin. Phaffia rhodozyma can accumulate total carotenoids up to a level at concentrations of 500~2000 µg/g in dry yeast, of which 45%~95% is astaxanthin (Johnson, 2003). Although the concentration of astaxanthin in Phaffia rhodozyma is lower than that in the green alga Haematococcus pluvialis, the yeast has the advantage of producing higher amount of astaxanthin through rapid self-propagation. Therefore, the yeast has been recognized as the most promising source for producing natural astaxanthin (Johnson, 2003).
Phaffia rhodozyma synthesizes astaxanthin in its cytoplasm membrane, which has a rigid cell wall that makes animals have more difficulty to efficiently absorb and digest this polyfunctional substance (Johnson, 1991; Ytrestøyl et al., 2005). Therefore, disrupting the yeast cell for extracting astaxanthin more efficiently is crucial for potentially commercial utilizations of astaxanthin.
Generally speaking, yeasts can be disrupted by biological, physical and chemical methods. For example, hot dimethyl-sulphoxide (DMSO) has been successfully used for extracting astaxanthin from Phaffia rhodozyma (Sedmak et al., 1990; Johnson, 1991), but this method is not suitable for producing astaxanthin in food-and/or pharmaceutical-grade products due to the DMSO residue. Enzymatic method has also been widely used in yeast disruption (Gentles and Haard, 1991; Johnson et al., 1977; 1980; Storebakken et al., 2004), but it is time-consuming to break the cells and may simultaneously result in serious degradation of astaxanthin. High-pressure homogenization and ball mill have been commonly used to disrupt vegetable germs and cells (Tangeras et al., 1989; Gentles and Haard, 1991), but they are not efficient for astaxanthin extraction from Phaffia rhodozyma, because the high temperature and less than 80% of cell disruption resulted in a low productivity of astaxanthin. Cell wall is susceptible to alkaline conditions, however, alkaline chemicals should as much as possible not be used during the extraction and purification steps because they make astaxanthin irreversibly changed to astacene (Johnson, 1991). Alternatively, acidic method for disrupting Phaffia rhodozyma has an overall advantage due to its rapid and efficient cell disruption capability and absence of chemical additives residues, although acids are involved in the problem of possible astaxanthin degradation (Johnson, 1991). Therefore, the purpose of this work was to optimize the process of astaxanthin extraction from Phaffia rhodozyma by acidic method.
MATERIALS AND METHODS
Reagents
Hydrochloric acid, lactic acid, acetic acid and organic solvents such as acetone, alcohol and chloroform, etc., which were used for yeast cell disruption and carotenoid extraction, were all of analytical grade. Methanol of HPLC grade and Mill-Q water were used in HPLC analysis for quantitative determination of carotenoids and astaxanthin. Chemical standards of astaxanthin and β-carotene were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Strains and cultivation conditions
The Phaffia rhodozyme Past-1 strain was generously provided by Prof. Ulf Stahl, Berlin Industrial College, Germany. The yeast was maintained on yeast/malt (YM) agar slants containing: 10.0 g/L glucose, 5.0 g/L bacto-peptone, 3.0 g/L malt extract, 3.0 g/L yeast extract, and 20.0 g/L agar. A single colony was isolated from YM plate and transferred to fresh slants every half-year, incubated for 3 d, and then kept in refrigeration.
The seed culture of Phaffia rhodozyma was prepared by inoculating the yeast from a fresh slant into a 250-ml flask containing 30 ml YM broth, and incubated for 48 h in a rotary shaker (22 °C, 190 r/min).
Yeast cultures were grown in a 5-L pH-stat fermentor containing 3 L medium including: 20.0 g/L glucose, 2.0 g/L (NH4)2SO4, 1.0 g/L KH2PO4, 0.5 g/L MgSO4·7H2O, 0.1 g/L CaCl2 and 2.0 g/L yeast extract. The fermentation condition was pre-set at temperature 22 °C, air flow rate 1.5~3.6 L/min, stirring rate 290 r/min, and pH 6.0. After being cultured for 96 h, cells of Phaffia rhodozyma were harvested by centrifugation at 3000 r/min for 10 min.
Procedures for extracting carotenoids
Wet cells of the yeast were freeze-dried and then heated at 105 °C to a stable weight. All the following quantitative determinations were based on yeast dry weight. Fifteen-fold (v/w) acid solution was added to the yeast cells with continuous stirring in order to disrupt the cell wall, then the water phase was removed by centrifugation and the fraction of disrupted cells was extracted by solvents at room temperature.
Apparatus and instruments
An LXJ-IIB centrifuge was purchased from Anting Analytical Instrument Factory (Shanghai, China). An HH-6 digital water bath purchased from Fuhua Instrument Co. Ltd. (Jingtun, Jiangsu, China) was used to control the extraction temperature. Samples were lyophilized by an SNL216V freezing-dryer purchased from Thermo Savant Co. Ltd. (Holbrook, NY, USA). HPS biochemical incubator was purchased from Harbin Donglian Electronic and Technological Development Co. Ltd. (Harbin, China). A rotary shaker purchased from Zhicheng Analytical Instrument Factory (Shanghai, China) and a 5-L pH-stat fermentor (B. Braun Biotech. International, Germany) were used to culture the yeast.
Procedures for astaxanthin determination
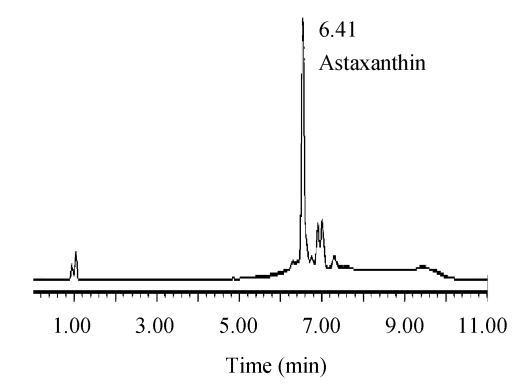
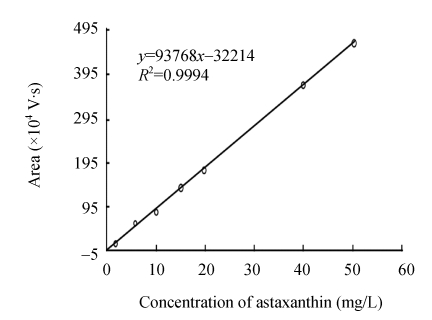
Waters HPLC system including 1525 pump, C18 reverse phase column and 2478 UV detector was used to analyze carotenoids. Carotenoids were isolated in a Nova-Pak C18 reverse phase column (3.9 mm×150 mm, 4 μm, Waters Corporation, USA) and detected at 474 nm. Under the chromatographic condition listed in Table 1, HPLC of carotenoid sample of Phaffia rhodozyma is as shown in Fig.1. Astaxanthin was quantitatively calibrated by the calibration graph shown in Fig.2.
Table 1.
HPLC condition for astaxanthin analysis
| Time (min) | Flow rate (ml/min) | Methanol (%) | Water (%) | Pressure (kPa) | Temperature (°C) |
| 0.00 | 1.20 | 80.0 | 20.0 | 0~20685 | 40.0 |
| 3.00 | 1.20 | 80.0 | 20.0 | 0~20685 | 40.0 |
| 5.00 | 1.20 | 100.0 | 0.0 | 0~20685 | 40.0 |
| 8.00 | 1.20 | 100.0 | 0.0 | 0~20685 | 40.0 |
| 9.00 | 1.20 | 80.0 | 20.0 | 0~20685 | 40.0 |
Fig. 1.
HPLC of carotenoids extract of Phaffia rhodozyma
Fig. 2.
Calibration graph of astaxanthin for HPLC analysis
Procedures for extraction optimization and statistical analysis
The acids used to disrupt cells and the organic solvents used to extract carotenoids were firstly selected by a single factor design. Fractional factorial design (FFD) was used for further process optimization, which relied on three stages of experimentation: screening, optimization and validation. Screening aims at reducing determinations as to which few variables have the greatest impacts on test performance. Optimization of experiments is designed to provide in-depth information by a few identified variables during the screening step but ensure the greatest impacts on performances. Then, hypothesized models are validated with experiments under specific optimized experimental conditions. However, if the optimal condition is not in the domain of our expected experimental scope, another statistical method called steepest ascent will be used to approach the optimal condition.
Finally, extraction condition was optimized by response surface methodology (RSM). All experiments were done in triplicates. All data presented were mean values of the triplicates. Data analyses were performed using Statistical Analysis System 6.12 software (SAS Institute Inc., Cary, NC, USA) and Excel 2000 (Microsoft Corporation, Redmont, WA, USA).
RESULTS
Effect of acids and solvents on astaxanthin extraction from Phaffia rhodozyma
Effect of acids (i.e., hydrochloric acid, lactic acid and acetic acid) on astaxanthin was investigated regarding their capacity for cell disruption and astaxanthin degradation. Different acid solutions at the same concentration of 4 mol/L were respectively added, in a liquid to solid ratio of 15 ml/g, to the dried yeast cell samples with continuous stirring for 5 min. Then the solutions were centrifuged at 3000 r/min for 5 min. After removing the upper layer liquid, 25-fold (v/w) of acetone was added to the precipitate to extract carotenoids for the subsequent HPLC analysis, whose results are listed in Table 2. Although the quantitative ratio of astaxanthin to the total amount of carotenoids in the control (84.6%) was more than that of any other experiments with added acids, which indicated the influence of acids on astaxanthin degradation, the extraction rate of astaxanthin (1118.1 μg/g) and total amount of carotenoids (1329.5 μg/g) in the treatment by lactic acid were significantly higher than those of all its counterparts, which demonstrated that lactic acid was not only more powerful for disrupting the Phaffia rhodozyma cells, but also caused the least degree of astaxanthin degradation. Therefore, in our first step of using the single factor design lactic acid was selected as the acid additive for the following tests.
Table 2.
Results of disrupting Phaffia rhodozyma by various acids
| Investigated acids | Total carotenoids (μg/g) | Astaxanthin (μg/g) | Ratio* (%) |
| Control | 1013.7c | 857.6c | 84.6a |
| Hydrochloric acid | 829.3d | 654.3d | 78.9b |
| Lactic acid | 1329.5a | 1118.1a | 84.1a |
| Acetic acid | 1259.2b | 973.4b | 77.3c |
Each digital value is expressed as a mean value of triplicate. Means with different superscript letters within the same column are significantly different (P<0.05).
Ratio of the amount of astaxanthin to the total amount of carotenoids
Yeast cells were then mixed and stirred with 15-fold (v/w) 4 mol/L lactic acid solution at 50 °C for 5 min, followed by centrifugation. After the upper layer liquid was removed, the yeast precipitates were extracted by 10 ml/g of different organic solutions for 5 min. The results are shown in Table 3. Among the tested solvents, acetone and ethanol were obviously more efficient than any of the others to extract astaxanthin from the cells. Moreover, in light of the safety concern raised by the pharmaceutical and food industries, ethanol was selected rather than acetone for the following tests.
Table 3.
Comparisons of effectiveness of organic solvents on extracting activity
| Solvents | Astaxanthin (μg/g) |
| Acetone | 967.4 |
| Ethanol | 946.2 |
| Methanol | 655.3 |
| Petroleum ether | 75.6 |
| n-hexane | 68.9 |
| Toluene | 68.1 |
| Ethyl acetate | 58.4 |
| Chloroform | 32.9 |
| Carbon tetrachloride | 24.2 |
Screening stage by fractional factorial design (FFD)
In order to further explore the effects of acid concentration (X 1), disrupting time (X 2), disrupting temperature (X 3), amount of ethanol addition (X 4) and extraction time (X 5) on extracting astaxanthin from Phaffia rhodozyma, FFD was arranged with the above mentioned factors at different levels that are listed in Table 4. The corresponding results of the experiments are shown in Table 5. When X 1, X 4 and X 5 were at the higher levels and X 2 and X 3 at the lower levels, the amount of the extracted astaxanthin was 1071.1 μg/g, which was more than that of any other experiments. However, when X 1, X 4 and X 5 were at the lower levels and X 2 and X 3 at the higher levels, extracted astaxanthin was obtained at a much lower yield.
Table 4.
Factors and levels for the fractional factorial design (FFD)
| Level | Variations |
||||
| X1 (mol/L) | X2 (min) | X3 (°C) | X4 (ml/g) | X5 (min) | |
| −1 | 0 | 1 | 10 | 2 | 1 |
| 0 | 2 | 3 | 30 | 6 | 3 |
| +1 | 4 | 5 | 50 | 10 | 5 |
X 1: Concentration of lactic acid; X 2: Time for disrupting; X 3: Temperature for disrupting; X 4: Amount of ethanol; X 5: Time for extraction
Table 5.
Experimental results of the fractional factorial design
| No. | X1 | X2 | X3 | X4 | X5 | Astaxanthin (µg/g) |
| 1 | 1 | −1 | −1 | 1 | 1 | 1071.1 |
| 2 | 1 | 1 | 1 | 1 | 1 | 908.1 |
| 3 | −1 | 1 | −1 | 1 | −1 | 417.1 |
| 4 | 1 | 1 | −1 | −1 | −1 | 269.3 |
| 5 | 1 | −1 | 1 | −1 | −1 | 271.2 |
| 6 | −1 | −1 | 1 | 1 | −1 | 527.9 |
| 7 | −1 | 1 | 1 | −1 | 1 | 180.1 |
| 8 | −1 | −1 | −1 | −1 | 1 | 205.8 |
| 9 | 0 | 0 | 0 | 0 | 0 | 633.1 |
| 10 | 0 | 0 | 0 | 0 | 0 | 615.2 |
| 11 | 0 | 0 | 0 | 0 | 0 | 621.8 |
| 12 | 0 | 0 | 0 | 0 | 0 | 627.9 |
X 1: Concentration of lactic acid; X 2: Time for disrupting; X 3: Temperature for disrupting; X 4: Amount of ethanol; X 5: Time for extraction
The ANOVA for FFD experiments shown in Table 6 (F=15.05>F (5,11,0.01)=5.32) indicates that the variables significantly affect the extraction rate of astaxanthin. Furthermore, regressive analysis of the variables shown in Table 7 demonstrates that X 1 (Prob>|T|=0.62%), X 4 (Prob>|T|=0.05%) and X 5 (Prob>|T|=2.26%) affect the extraction rate significantly, whereas effects of X 2 (Prob>|T|=33.68%) and X 3 (Prob>|T|=80.12%) could be neglected. Moreover, the deduced first-order multiple regression Eq.(1):
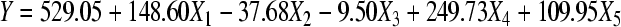 , ,
|
(1) |
including all the above mentioned variables gave R 2=0.93 and Adj. R 2=0.86, which indicated that our data were well fitted by the model whose variations caused by the variables accounted for 93% of the variation in the astaxanthin extraction. Also, it is obviously shown in Table 7 that the most important variable is X 4, followed by X 1 and X 5. Parameters of X 1, X 4 and X 5 all positively affected the extraction of astaxanthin. In other words, the higher the levels of X 1, X 4 and X 5 were set, the more astaxanthin would be extracted. On the contrary, less than zero parameters X 2 and X 3 in the model demonstrated the negative effects of these two factors on the results.
Table 6.
Analysis of variance of fractional factor design
| Source | df | Sum of squares | Mean square | F value | Prob>F |
| Model | 5 | 784345.55 | 156869.11 | 15.05 | 0.0024 |
| Error | 6 | 62521.14 | 10420.19 | ||
| C total | 11 | 846866.69 |
RMSE=102.08; R 2=0.93; Dependent mean=529.05; Adj. R 2=0.86; CV=19.29; C total: Corrected total
Table 7.
Regressive analysis of fractional factor design
| Variable | Parameter estimate | Standard error | T for H0: parameter=0 | Prob>|T| |
| Intercept | 529.05 | 29.47 | 17.95 | 0.0001 |
| X1 | 148.60 | 36.09 | 4.12 | 0.0062 |
| X2 | −37.68 | 36.09 | −1.04 | 0.3368 |
| X3 | −9.50 | 36.09 | −0.26 | 0.8012 |
| X4 | 249.73 | 36.09 | 6.92 | 0.0005 |
| X5 | 109.95 | 36.09 | 3.05 | 0.0226 |
X 1: Concentration of lactic acid; X 2: Time for disrupting; X 3: Temperature for disrupting; X 4: Amount of ethanol; X 5: Time for extraction
The steepest ascent experiment and analysis
The results shown in Table 5 and Table 7, however, also imply that the optimal region for the experimental condition is out of the current design scale. In this case, a directional search method (i.e., the steepest ascent) was subsequently used to determine the next set of experiments. The steepest ascent is a method that uses the magnitude and sign of the linear effects to determine the direction toward predictive higher response. The path begins at the center of the current design and stretches well outside the design space. A sequence of equally spaced locations along the path is then selected for a set of experiments. Thus, the path of the steepest ascent was stretched to increase the concentration of lactic acid and the addition of ethanol in order to improve the extraction rate of astaxanthin. Meanwhile, the other factors (X 2, X 3 and X 5) were fixed at the coded value of zero. The extraction rates of astaxanthin obtained from the re-designed experiments are listed in Table 8. The data clearly show that the extraction rate of astaxanthin increased until the seventh step on the path when the concentration of lactic acid and addition of ethanol increased. After that, further experimentation could not increase the extraction rate of astaxanthin. The highest extraction rate of astaxanthin was achieved in the seventh step with a yield of 1310.8 μg/g. This meant that the optimal condition was around the factor levels for the seventh step (i.e., X 1=5.6 mol/L and X 4=18 ml/g).
Table 8.
Experimental design of the steepest ascent and its corresponding responses
| No. | X1 (mol/L) | X4 (ml/g) | Astaxanthin (µg/g) |
| 1 | 2.0 | 6 | 582.0 |
| 2 | 2.6 | 8 | 798.1 |
| 3 | 3.2 | 10 | 964.6 |
| 4 | 3.8 | 12 | 1015.1 |
| 5 | 4.4 | 14 | 1275.8 |
| 6 | 5.0 | 16 | 1294.3 |
| 7 | 5.6 | 18 | 1310.8 |
| 8 | 6.2 | 20 | 1309.1 |
| 9 | 6.8 | 22 | 1305.5 |
| 10 | 7.4 | 24 | 1282.3 |
| 11 | 8.0 | 26 | 1294.9 |
| 12 | 8.6 | 28 | 1201.2 |
X 1: Concentration of lactic acid; X 4: Amount of ethanol
Central composite design (CCD) and response surface analysis
Further optimization of the running process for extracting maximum astaxanthin was carried out by using a Box-Wilson CCD with four-star points and five replicates at center point for each of the two factors of X 1 and X 4. Table 9 lists the variables and their levels in coded and real values for the CCD. Table 10 shows the CCD in coded values with the corresponding results of the astaxanthin yield. Moreover, a response surface was plotted to help determine the optimal region.
Table 9.
Factors and levels for central composite design experiments
| Levels | Factors |
||||
| X1 (mol/L) | X2 (min) | X3 (°C) | X4 (ml/g) | X5 (min) | |
| +1.414 | 9.0 | 3 | 30 | 29.3 | 3 |
| +1 | 8.0 | 3 | 30 | 26.0 | 3 |
| 0 | 5.6 | 3 | 30 | 18.0 | 3 |
| −1 | 3.2 | 3 | 30 | 10.0 | 3 |
| −1.414 | 2.2 | 3 | 30 | 6.7 | 3 |
X 1: Concentration of lactic acid; X 2: Time for disrupting; X 3: Temperature for disrupting; X 4: Amount of ethanol; X 5: Time for extraction
Table 10.
Central composite design and the corresponding responses
| No. | X1 | X4 | Astaxanthin (µg/g) |
| 1 | −1 | −1 | 1255.6 |
| 2 | +1 | −1 | 1125.4 |
| 3 | −1 | +1 | 1216.9 |
| 4 | +1 | +1 | 1249.3 |
| 5 | −1.414 | 0 | 1117.2 |
| 6 | +1.414 | 0 | 1068.4 |
| 7 | 0 | −1.414 | 1203.9 |
| 8 | 0 | +1.414 | 1232.8 |
| 9 | 0 | 0 | 1283.2 |
| 10 | 0 | 0 | 1303.7 |
| 11 | 0 | 0 | 1277.9 |
| 12 | 0 | 0 | 1274.4 |
| 13 | 0 | 0 | 1276.3 |
X 1: Concentration of lactic acid; X 4: Amount of ethanol
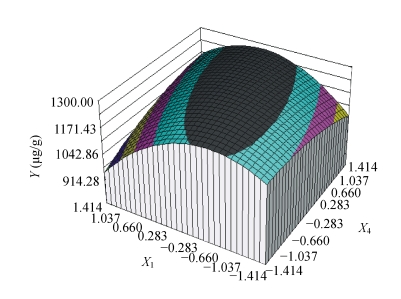
As shown in Table 10, the maximum response (1303.7 μg/g) occurred at the central point and the minimum response occurred at X 1=1.414 combined with X 4=0. The 3D graph plotted by the SAS software with the data listed in Table 10 is shown in Fig.3. The 3D response surface plotted by the concentration of lactic acid vs added amount of ethanol against the extraction rate of astaxanthin can further explain the results of the statistical and mathematical analysis. The downward bell-shaped graph obtained from RSM demonstrated that there should be a maximum in the stable range. According to the parameters obtained from the multi-regressive-analysis of the central composite experiments (Table 11), Eq.(2) in a second-order polynomial prediction model was obtained:
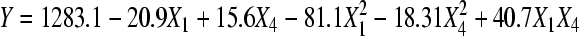 . .
|
(2) |
Fig. 3.
The response surface plot of the extraction rate of astaxanthin as a function of coded values of concentration of lactic acid (X 1) and quantity of ethanol (X 4)
Table 11.
Regressive analysis of central composite experimentation
| Parameter | Parameter estimate | Standard error | T for H0: parameter=0 | Prob>|T| |
| Intercept | 1283.10 | 14.39 | 89.180 | 0.0000 |
| X1 | −20.85 | 11.37 | −1.830 | 0.1094 |
| X4 | 15.76 | 11.37 | 1.385 | 0.2084 |
| X1×X1 | −81.09 | 12.20 | −6.648 | 0.0003 |
| X4×X1 | 40.65 | 16.09 | 2.527 | 0.0394 |
| X4×X4 | −18.32 | 12.20 | −1.502 | 0.1768 |
X 1: Concentration of lactic acid; X 4: Amount of ethanol
Table 11 exhibits the significant effects of X 1 2 (Prob>|T|=0.03%) and the interaction between the two factors X 1×X 4 (Prob>|T|=3.94%), on the contrary, unapparent effects of X 1 (Prob>|T|=10.94%), X 4 (Prob>|T|=20.84%), and X 4 2 (Prob>|T|=17.68%). It is evident from Fig.3 and Eq.(2) that extraction rate of astaxanthin reached its maximum value (1286.5 μg/g) at the combination of coded levels of X 1 at 0.020334 and X 4 at 0.281588, which corresponds to the real variable values of 5.55 mol/L lactic acid and 20.25 ml/g ethanol. The statistical significance for the second-order equation model was checked by an F-test (ANOVA) with data shown in Table 12. The fit-value, termed R 2 (determinant coefficient), of the polynomial model was calculated to be 0.898, indicating that 89.8% of the variability in the response could be explained by the second-order polynomial prediction equation given by Eq.(2). Lack-of-fit value that is as low as 1.14% also implies that the quadratic model has adequately described the trend of our experimental data. The model also suggests that the extraction rate of astaxanthin was primarily determined by quadratic product and interaction of concentrations of lactic acid and ethanol.
Table 12.
ANOVA of central composite experiment
| Sum of squares | Mean square | F-ratio | Prob>F | |
| Linear | 5465.10 | 0.0834 | 2.64 | 0.1398 |
| Quadratic | 46171.00 | 0.7050 | 22.31 | 0.0009 |
| Crossproduct | 6609.69 | 0.1009 | 6.39 | 0.0394 |
| Total regress | 58246.00 | 0.8894 | 11.26 | 0.0031 |
| Lack-of-fit |
6671.77 |
2223.9200 |
15.52 |
0.0114 |
| Pure error | 573.34 | 143.3300 | ||
| Total error | 7245.11 | 1035.0200 |
Response mean=1221.92; RMSE=32.17; R 2=0.898; CV=2.6329
Validation of the optimal experimental condition
Since the model predicted a maximum response of 1286.5 μg/g extraction of astaxanthin at the optimal condition, it is necessary to run an experiment at this point to ensure the predicted result was not biased against the practical value. Therefore, experimental rechecking was performed using the deduced optimal condition. As shown in Table 13, a mean extraction rate of astaxanthin at 1294.7 μg/g was obtained. The excellent reconfirmation by the real experiments in turn validated our models again. In addition, the extraction rate of the total carotenoids was 1516.0 μg/g and ratio of astaxanthin to total carotenoids was calculated to be 85.4% at the maximum point. These data are much better than those of the control shown in Table 2.
Table 13.
Results of confirmation experiments
| No. | Astaxanthin (μg/g) | Total carotenoids (μg/g) | Ratio* (%) |
| 1 | 1263.7 | 1495.5 | 84.5 |
| 2 | 1317.5 | 1537.3 | 85.7 |
| 3 | 1268.9 | 1485.8 | 85.4 |
| 4 | 1304.1 | 1528.8 | 85.3 |
| 5 |
1319.4 |
1532.4 |
86.1 |
| Mean value | 1294.7 | 1516.0 | 85.4 |
Ratio of the amount of astaxanthin to the total amount of carotenoids
DISCUSSION AND CONCLUSION
As shown by the above experiments and other data (Table 14) in the primary experiments, lactic acid is most suitable for disrupting the yeast for astaxanthin extraction. This phenomenon may be explained in two aspects: acidic intensity and carbon chain length. The more intense the acid is, the more efficient it is for disrupting the yeast cell and the more completely the astaxanthin decomposes. Concerning the effects of carbon chain length, the longer the acid carbon chain is, the more lipophilic it is. Thus, it was presumed that organic acid with suitable long carbon chain can help facilitate it to contact and permeate the cell membranes. Lactic acid (pKa=3.83) is weaker in acidic intensity than hydrochloric acid, but stronger than acetic acid (pKa=4.74). This property results in some advantages in the extraction of astaxanthin and carotenoids from Phaffia rhodozyma. On the one hand, it can efficiently disrupt the yeast cell walls; on the other hand, degradation of carotenoids by the lactic acid was not as severe as imagined. In addition, lactic acid contains three carbon atoms and may have strong ability to combine with cell membrane. We can make a conclusion that lactic acid is better than ether hydrochloric acid or acetic acid to be used to disrupt the yeast. Therefore, using lactic acid to disrupt Phaffia rhodozyma for astaxanthin extraction might be a good choice in the acidic method.
Table 14.
Comparison of effects of lactic acid and hydrochloric acid on astaxanthin extraction rate
| Concentration of acid (mol/L) | Astaxanthin (μg/g) |
|
| Lactic acid | Hydrochloric acid | |
| 1 | 961.94 | 752.65 |
| 2 | 1036.32 | 680.50 |
| 3 | 1065.61 | 676.16 |
| 4 | 1131.10 | 664.23 |
| 5 | 1067.58 | 654.30 |
| 6 | 1038.75 | 633.59 |
| 7 | 1000.17 | 627.53 |
Astaxanthin is a lip soluble substance and can be easily dissolved in the solvents listed in Table 3 (Johnson, 1991), but the results shown in Table 3 are dramatically away from it. The phenomena can be explained from the basic properties of cell membrane. When the cell is wet, the yeast membrane is still double lip layer structure and capsulated with a water layer. If the solvent is not water-soluble, chances are that it is difficult to permeate the water surface of the double-layer membrane and thus could seldom contact the membrane fixed astaxanthin. Therefore, the efficiency of solvents such as chloroform and hexane is very low. On the contrary, some water-soluble solvents such as acetone, ethanol and methanol can permeate the outer water cover easily and can extract astaxanthin out of the yeast efficiently. Although some measures, for example, dehydrating the cell and treating the cell fraction with ultrasonic or microwave in extraction, can help water-insoluble solvents permeate cell membrane and enhance extraction rate of astaxanthin, these operations have main drawbacks that will accelerate the carotenoids decomposing and we do not know if these drawbacks can be avoided or to what degree it can be decreased.
In addition, many factors such as concentration of lactic acid, amount of ethanol addition, and extraction time could significantly affect the extraction rate of astaxanthin. The optimal working condition was at the concentration of lactic acid at 5.55 mol/L, ratio of ethanol to yeast dry weight at 20.25 ml/g, disruption temperature at 30 °C for 3 min, and extraction time for 3 min. Under these conditions, astaxanthin and the total carotenoids could be extracted in amounts of 1294.7 μg/g and 1516.0 μg/g, respectively, in a corresponding ratio of astaxanthin to total carotenoids of 85.4%. This acidic method has an advantage in regard to its high extraction efficiency, low chemical toxicity, no special demand for instruments, lactic acid and ethanol reusable with little waste of water, and no pollution. Therefore, it might be a more feasible and practical method for industrial practice.
Acknowledgments
The authors are grateful to Prof. Cai-hua Guo, Mr. Qiu-ming Yang and Mr. Qi-biao Zhang for their technical help on yeast fermentation and astaxanthin determination.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 20702019), and the Foundation for Young Professors of Jimei University, China
References
- 1.Bertram JS, Vine AL. Cancer prevention by retinoids and carotenoids: independent action on a common target. Biochimica et Biophysica Acta. 2005;1740(2):170–178. doi: 10.1016/j.bbadis.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Black HS, Mathews-Roth MM. Protective role of butylated hydroxytoluene and certain carotenoids in photocarcinogenesis. Photochem Photobiol. 1991;53(5):707–716. doi: 10.1111/j.1751-1097.1991.tb08501.x. [DOI] [PubMed] [Google Scholar]
- 3.Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995;9(15):1551–1558. [PubMed] [Google Scholar]
- 4.Conn PF, Schalch W, Truscott TG. The singlet oxygen and carotenoid interaction. J Photochem Photobiol B Biol. 1991;11(1):41–47. doi: 10.1016/1011-1344(91)80266-K. [DOI] [PubMed] [Google Scholar]
- 5.Fraser PD, Miura Y, Misawa N. In vitro characterization of astaxanthin biosynthetic enzymes. J Biochem. 1997;272(10):6128–6135. doi: 10.1074/jbc.272.10.6128. [DOI] [PubMed] [Google Scholar]
- 6.Gentles A, Haard NF. Pigmentation of rainbow trout with enzyme-treated and spray-dried Phaffia rhodozyma . The Progressive Fish-Culturist. 1991;53(1):1–6. doi: 10.1577/1548-8640(1991)053<0001:PORTWE>2.3.CO;2. [DOI] [Google Scholar]
- 7.Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin: application for human health and nutrition. Trends Biotechnol. 2003;21(5):210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 8.Johnson EA. Astaxanthin from microbial sources. Crit Rev Biotechnol. 1991;11(4):297–326. doi: 10.3109/07388559109040622. [DOI] [Google Scholar]
- 9.Johnson EA. Phaffia rhodozyma: colorful odyssey. Int Microbiol. 2003;6(3):169–174. doi: 10.1007/s10123-003-0130-3. [DOI] [PubMed] [Google Scholar]
- 10.Johnson EA, Conklin DE, Lewis MJ. The yeast Phaffia rhodozyma as a dietary pigment source for salmonids and crustaceans. J Fish Res Board Can. 1977;34:2417–2421. [Google Scholar]
- 11.Johnson EA, Villa TG, Lewis MJ. Phaffia rhodozyma as an astaxanthin source in salmonid diets. Aquaculture. 1980;20(2):123–134. doi: 10.1016/0044-8486(80)90041-1. [DOI] [Google Scholar]
- 12.Kobayashi M, Kakizono T, Hishio N, Nagai S, Kurimura Y, Tsuji Y. Antioxidant role of astaxanthin in the green alga, Haematococcus pluvialis . Appl Microbiol Biotechnol. 1997;48(3):351–356. doi: 10.1007/s002530051061. [DOI] [Google Scholar]
- 13.Kurashige M, Okimasu E, Inoue M, Utsumi K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol Chem Phys Med NMR. 1990;22(1):27–38. [PubMed] [Google Scholar]
- 14.Lawlor SM, O′Brien NM. Astaxanthin: antioxidant effects in chicken embryo fibroblasts. Nutr Res. 1995;15(11):1695–1704. doi: 10.1016/0271-5317(95)02040-9. [DOI] [Google Scholar]
- 15.Miki W. Biological functions and activities of animal carotenoids. Pure Appl Chem. 1991;63(1):141–146. doi: 10.1351/pac199163010141. [DOI] [Google Scholar]
- 16.Ong ASH, Tee ES. Natural sources of carotenoids from plants and oils. Meth Enzymol. 1992;213:142–167. [Google Scholar]
- 17.Palozza P, Krinsky NI. Astaxanthin and canthaxanthin are potent antioxidants in a membrane model. Arch Biochem Biophys. 1992;297(2):291–295. doi: 10.1016/0003-9861(92)90675-M. [DOI] [PubMed] [Google Scholar]
- 18.Sedmak JJ, Weerasinghe DK, Jolly SO. Extraction and quantification of astaxanthin from Phaffia rhodaozyma . Biotechnol Tech. 1990;4(2):107–112. doi: 10.1007/BF00163282. [DOI] [Google Scholar]
- 19.Storebakken T, Sørensen M, Bjerkeng B, Harris J, Monahan P, Hiu S. Stability of astaxanthin from the red yeast, Xanthophyllomyces dendrorhous, during feed processing: effects of enzymatic cell wall disruption and extrusion temperature. Aquaculture. 2004;231(1-4):489–500. doi: 10.1016/j.aquaculture.2003.10.034. [DOI] [Google Scholar]
- 20.Tangeras A, Slinde E. Coloring of Salmonids in Aquaculture: The Yeast Phaffia rhodozyma as a Source of Astaxanthin. In: Martin AM, editor. Fisheries Processing: Biotechnological Application. London: Chapman & Hall; 1994. pp. 394–431. [Google Scholar]
- 21.Tangeras A, Marøy T, Wahlstrøm S, et al. Release of Astaxanthin from the Red Yeast Phaffia rhodozyma by High Pressure Homogenization. Program 1; Int. Mar. Biotechnol. Conf. (IMB ’89); Tokyo, Japan: 1989. [Google Scholar]
- 22.Verdoes JC, Misawa N, van Ooyen AJJ. Cloning and characterization of the astaxanthin biosynthetic gene encoding photogene desaturase of Xanthophyllomyces dendrorhous . Biotechnol Bioeng. 1999;63(6):750–756. doi: 10.1002/(SICI)1097-0290(19990620)63:6<750::AID-BIT13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Wang QY, Li QS. Natural Carotenoids-Advancements in Studies, Producing and Application. Beijing: Press of China Pharmaceutical Technology; 1997. (in Chinese) [Google Scholar]
- 24.Ytrestøyl T, Struksnæs G, Koppe W, Bjerkeng B. Effects of temperature and feed intake on astaxanthin digestibility and metabolism in Atlantic salmon, Salmo salar. Comp Biochem Physiol Part B Biochem Mol Biol. 2005;142(4):445–455. doi: 10.1016/j.cbpb.2005.09.004. [DOI] [PubMed] [Google Scholar]