Abstract
Partitioning-defective 1 (PAR1) and atypical protein kinase C (aPKC) are conserved serine/threonine protein kinases implicated in the establishment of cell polarity in many species from yeast to humans. Here we investigate the roles of these protein kinases in cell fate determination in Xenopus epidermis. Early asymmetric cell divisions at blastula and gastrula stages give rise to the superficial (apical) and the deep (basal) cell layers of epidermal ectoderm. These two layers consist of cells with different intrinsic developmental potential, including superficial epidermal cells and deep ciliated cells. Our gain- and loss-of-function studies demonstrate that aPKC inhibits ciliated cell differentiation in Xenopus ectoderm and promotes superficial cell fates. We find that the crucial molecular substrate for aPKC is PAR1, which is localized in a complementary domain in superficial ectoderm cells. We show that PAR1 acts downstream of aPKC and is sufficient to stimulate ciliated cell differentiation and inhibit superficial epidermal cell fates. Our results suggest that aPKC and PAR1 function sequentially in a conserved molecular pathway that links apical-basal cell polarity to Notch signaling and cell fate determination. The observed patterning mechanism may operate in a wide range of epithelial tissues in many species.
Keywords: PAR1 (MARK), aPKC, Ciliated cell, Apical-basal polarity, Xenopus, Epidermis, Notch, Ectoderm, XDelta-1 (DII1)
INTRODUCTION
Polarity is a universal cell property that is essential for the maintenance of cell shape and tissue architecture in multicellular organisms. Segregation of cellular constituents to opposite poles of a polarized cell often results in an asymmetric cell division. Asymmetric divisions are known to play important roles in cell fate determination in C. elegans blastomeres (Guo and Kemphues, 1996), Drosophila neuroblasts and sensory organ precursors (Betschinger and Knoblich, 2004; Roegiers and Jan, 2004) and have been implicated in mammalian skin stratification and central nervous system development (Gotz and Huttner, 2005; Kosodo et al., 2004; Lechler and Fuchs, 2005; Sanada and Tsai, 2005; Wodarz and Huttner, 2003). Although cell polarity has been proposed to generate cell fate diversity through asymmetric cell division (Chalmers et al., 2002; Gotz and Huttner, 2005; Muller and Hausen, 1995), the molecular components of polarized cells that are crucial in converting polarity information into cell fate determination are largely unknown (Betschinger and Knoblich, 2004; Cappello et al., 2006; Imai et al., 2006; Kosodo et al., 2004; Ohno, 2001; Wodarz and Huttner, 2003).
Xenopus ectoderm consists of the superficial (apical) and the inner (basal) cell layers, which are produced as a result of asymmetric cell divisions at blastula and gastrula stages (Chalmers et al., 2003) resulting in cells with different intrinsic developmental potential. The superficial layer of non-neural ectoderm expresses several cytokeratins and the Notch target ESR6e (Chalmers et al., 2006; Deblandre et al., 1999), whereas the inner layer is known to contain ciliated cells marked by the α-tubulin gene (Deblandre et al., 1999; Drysdale and Elinson, 1992). Since frog ectoderm shows pronounced epithelial polarity with the apical and basolateral membrane domains marked by atypical protein kinase C (aPKC) and lethal giant larvae (LGL) (Chalmers et al., 2005; Chalmers et al., 2003; Dollar et al., 2005), we used this system to assess the role of polarity proteins on ectodermal cell fates.
Apical complex proteins, including PAR3, PAR6 and aPKC, function in the polarization of Drosophila oocytes and mammalian epithelial cells and may control asymmetric divisions and developmental patterning (Betschinger and Knoblich, 2004; Ohno, 2001; Plusa et al., 2005; Rolls et al., 2003; Wodarz and Huttner, 2003; Wodarz et al., 2000). aPKC in particular is enriched in the zygote cortex (Nakaya et al., 2000), which is inherited as the apical cortex of superficial cells in the Xenopus blastula, and aPKC overexpression enhances apical character in these cells, suppressing basolateral polarity markers, such as occludin, β1-integrin and LGL (Chalmers et al., 2005; Dollar et al., 2005). A number of biochemical (i.e. phosphorylation) targets of aPKC have been elucidated, but it is not known which of these, if any, is crucial for subsequent fate determination. The serine/threonine protein kinase PAR1 [also known as MARK (MAP/microtubule affinity-regulating kinase)] is one such target, which has been implicated in cell polarization (Bayraktar et al., 2006; Benton and St Johnston, 2003; Cohen et al., 2004; Doerflinger et al., 2003; Pellettieri and Seydoux, 2002; Tomancak et al., 2000). In mammalian epithelial cells PAR1 is localized basolaterally (Bohm et al., 1997), unlike aPKC, PAR3 and PAR6 (sometimes referred to as the apical PAR complex). Segregation of aPKC and PAR1 to opposite poles of epithelial cells and the regulation of PAR1 by aPKC (Hurov et al., 2004; Suzuki et al., 2004; Vaccari et al., 2005) suggest, (1) that aPKC may influence cell fates by establishing apicobasal polarity in the ectoderm and (2) that its mechanism of action in this regard is via local regulation of PAR1. This study provides experimental evidence demonstrating that aPKC indeed functions to specify cell fates in the superficial and the deep ectoderm layers and that PAR1 as a critical molecular target of aPKC in this differential cell fate determination. Our gain- and loss-of function data show that aPKC promotes superficial cell fates, presumably by phosphorylating and locally inactivating PAR1, which may modulate cell differentiation by influencing Notch signaling.
MATERIALS AND METHODS
DNA constructs for microinjection, RNA synthesis and morpholino oligonucleotides
Xenopus PAR1 T560A, PAR1KD, Flag-tagged rat aPKC-CAAX and rat aPKC-N constructs in pCS2 were generated by polymerase chain reaction (PCR) or via single-primer-based mutagenesis essentially as described previously (Itoh et al., 2005). The dominant negative (DN) RBP/J construct (R218>H) was generated by subcloning a RBP/J cDNA obtained from the Ricken Bioresource Center (RDB 3022) into the pXT7-NotI vector. Details of plasmid construction are available upon request. Capped synthetic RNA for microinjection was generated using mMessage mMachine kit (Ambion) and the following DNA templates: pCS2-Myc-PAR1A, pCS2-nucβGal (Ossipova et al., 2005), pcDNA3.1-Myc-rPKCζ and pcDNA3.1-Myc-maPKC-N (Parkinson et al., 2004), pCS2-Delta1-Myc (Deblandre et al., 2001), pCS2-Delta1-HA (Itoh et al., 2003), β-catenin, GFP-LGL1 (Dollar et al., 2005). PAR1 MOs have been previously described (Ossipova et al., 2005), control MO had the following sequence 5′-CCTCTTACCTCAGTTACAATTTATA-3′.
Embryo culture and microinjections, in situ hybridization and lineage tracing
Xenopus fertilization and embryo culture were performed as described previously (Itoh et al., 2005). Embryos were microinjected in 1/3× MMR, 3% Ficoll-400 (Pharmacia) in the animal pole with 5 nl of a solution containing 12.5 pg-1.5 ng of RNA per blastomere at the four- to eight-cell stage, and cultured in 0.1× MMR until desired stages. In loss-of-function experiments, PAR1BX MO and PAR1B MO [referred to as PAR1BY MO in Ossipova et al. (Ossipova et al., 2005)], or control MO were injected at 5-40 ng per blastomere.
In situ hybridization and X-gal staining were carried out using standard techniques (Harland, 1991) with the following anti-sense probes: α-tubulin and ESR6e (Deblandre et al., 1999); epidermal type I keratin (XK70) (Winkles et al., 1985), MyoD (Hopwood et al., 1989). For 10 μm sections, embryos were embedded in coldwater fish gelatin-sucrose mixture as described previously (Fagotto and Gumbiner, 1994) and cryosectioned using the Leica cryostat CM3050. Images were digitally acquired on a Zeiss Axiophot microscope. Quantification of results is presented as penetrance (PN), the percentage of embryos with a conspicuous phenotypic change. RNA-injected embryos usually had a range of phenotype severity. For ciliated cell quantification, embryos were scored as positively affected if the number of ciliated cells per injected area was altered by at least 50%. Cell numbers were determined per section of three to five representative embryos and given as means±s.d. Results are representative of at least three different experiments.
Immunocytochemistry
For cryosections, RNAs encoding Myc- or GFP-tagged proteins were injected into the animal region of four-cell albino embryos. Embryos were manually devitellinized at stage 10.5 and fixed in Dent’s fixative for 2 hours. Indirect immunofluorescence analysis on cryosections was performed essentially as described previously (Fagotto and Gumbiner, 1994). The following antibodies were used: anti-aPKC (Santa Cruz, sc-216, 1:200), anti-GFP (Santa Cruz, 1:200), anti-Myc (9E10, 1:50), anti-phospho-histone 3 (Cell Signaling, 1:200), anti-acetylated tubulin (Sigma, 1:100). When necessary, cryosections were double-stained using a combination of monoclonal and polyclonal primary antibodies. Secondary antibodies were Cy3-conjugated anti-rabbit (Jackson ImmunoResearch, 1:200) and Alexa Fluor 488 (Molecular Probes, 1:200). Imaging was performed on a Zeiss Axiophot microscope with the Apotome attachment at 400× magnification. A representative section of an experimental group of 10-15 embryos is shown. For hydroxyurea treatment, embryos were placed in 0.1× MMR containing 30 mM hydroxyurea (Sigma) 1 hour after fertilization until control embryos reached stages 10-10.5, fixed in Dent’s fixative, sectioned and subjected to immunostaining with anti-phospho-H3 antibody. The number of phospho-H3-positive nuclei per section was determined after the analysis of ten midsagittal sections (Saka and Smith, 2001).
Ectodermal layer separation and RT-PCR
Isolation of superficial and deep ectodermal layers was performed essentially as described in (Chalmers et al., 2002). Two-cell embryos were injected with PAR1 T560A (300 pg) or aPKC-CAAX (30 pg) mRNA, allowed to develop to stage 9, and animal pole explants were dissected, dissociated in 0.7× Normal Amphibian Medium (NAM) without calcium and magnesium (Peng et al., 1991) and allowed to reaggregate in 1× Danilchik’s buffer with BSA (Peng, 1991). Cell aggregates were allowed to mature until stage 14 or 19, harvested, and RNA was prepared using an RNA purification kit (Qiagen) according to manufacturer’s protocol. First-strand cDNA synthesis was carried out using SuperScript II reverse transcriptase (Invitrogen) according to manufacturer’s instructions using 1.5 μg of RNA in a 20 μl reaction, and assayed by semi-quantitative RT-PCR. For PCR, 2 μl of cDNA was used in a 40 μl PCR reaction. To ensure linearity of PCR amplification, the number of PCR cycles was optimized separately for each genetic marker in preliminary experiments. PCR primers for ornithine decarboxylase (odc) and ESR6e were reported previously (Chalmers et al., 2002). Other primers were annealed at 56°C and included: for grhl3, 5′-CGATGGAAGCACTGGCACTC-3′ and 5′-CCACATCTTTGAAGATTGG-3′ amplify a 520 bp fragment, 28 cycles (based on Xenopus laevis cDNA clone xl235j16); for inca B, 5′-CCTCTCCTCAGGCGGGTTCC-3′ and 5′-TAAGAATCCAGCCCCTTCG-3′ amplify a 507 bp fragment, 30 cycles; for delta-like 1 (dll1) (also known as XDelta-1), 5′-GCCTGCCGTGGTGAGTCC-3′ and 5′-CACCTCTGTTGCAATGATG-3′, amplify a 416 bp fragment, 30 cycles; for XK70, 5′-CGCAGTATCTCTCAGTCG-3′ and 5′-CGTCATTGATCTGGGAGCGC-3′, amplify a 396 bp fragment, 21 cycles; for α-tubulin 84b, 5′-GTGGTGGAACCCTACAACGC-3′ and 5′-GAGAGCTGCTCATGATAAGC-3′, amplify a 319 bp fragment, 27 cycles.
RESULTS
aPKC suppresses ciliated cell development
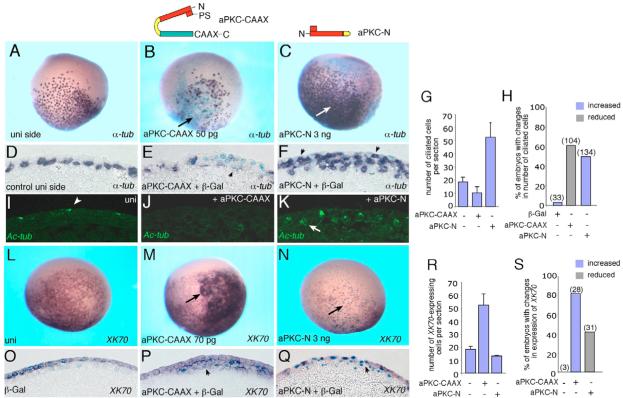
As aPKC has been implicated in the control of epithelial polarity in many species including Xenopus (Chalmers et al., 2005; Ohno, 2001; Wodarz et al., 2000) and is known to localize to the apical cortex of the early embryo, we hypothesized that it may regulate cell fate decisions resulting from asymmetric divisions of epithelial progenitors. Therefore, we examined its role in cell specification in epidermal ectoderm, since aPKC is specifically enriched in the superficial cell layer (Chalmers et al., 2003). We constructed and expressed a membrane-targeted aPKC-CAAX fusion protein, which has been described to promote self-renewal of Drosophila neuroblasts (Lee et al., 2006). We also studied effects of the N-terminal regulatory domain of aPKC, which contains a pseudosubstrate site and has been shown to function as a dominant-negative mutant (Chalmers et al., 2005; Parkinson et al., 2004). Using in situ hybridization, we assessed α-tubulin, a marker of ciliated cell differentiation in the basal (inner) ectoderm layer (Deblandre et al., 1999) in early neurulae (Fig. 1). aPKC-CAAX RNA produced half the number of ciliated cell progenitors on the injected side (penetrance or percentage of conspicuously affected embryos, PN=60%, n=104), whereas aPKC-N had the opposite effect, leading to densely packed ciliated cell precursors on the injected side (PN=50%, n=134; Fig. 1A-H). Embryo sections showed that ciliated cell development was inhibited in aPKC-CAAX-expressing tissue (Fig. 1D,E). By contrast, aPKC-N-injected embryos contained multiple layers of ciliated cell progenitors, with some α-tubulin-positive cells positioned in the outer ectoderm layer (Fig. 1F).
Fig. 1. A role of aPKC in epidermal ectoderm development.
Four- to eight-cell embryos were unilaterally coinjected with aPKC-CAAX or aPKC-N RNAs and lacZ RNA as a lineage tracer (light blue staining). Injected embryos were cultured until stages 14-16, fixed and subjected to whole mount in situ hybridization with anti-sense probes to α-tubulin (A-H) and XK70 (L-S). aPKC constructs employed are shown above panels B and C. (A-K) Changes in ciliated cell differentiation. aPKC-CAAX decreases (B,E), whereas aPKC-N increases (C,F) ciliated cell differentiation (arrows) on the injected side as compared with the uninjected side (A,D). The same embryo is shown in A and B. (D-F) Sections corresponding to embryos in A-C. (D) A single row of α-tubulin-positive cells in the deep layer of uninjected ectoderm. (F) α-Tubulin-positive cells are found in the superficial layer (arrows). (G) Quantification of the results showing mean numbers of α-tubulin-positive cells per section±s.d. Sections of at least three representative embryos per group were analyzed. (H) Frequency of embryos with altered numbers of ciliated cells (data pooled from several experiments). Embryos were scored positive if the number of ciliated cells per injected area was increased by at least 50%. The data are representative of three independent experiments. (I-K) Cilia differentiation assessed by immunostaining with antibodies to acetylated tubulin in stage 18 embryos. (I) Uninjected control embryos with regular pattern of superficially located ciliated cells, (J) aPKC-CAAX injection inhibits cilia formation, (K) aPKC-N injection results in multiple cells with many differentiated cilia. (L-S) aPKC promotes superficial cell fate. (L,O) Control XK70 staining. (M,P) aPKC-CAAX expands XK70 (arrow) on the injected side. (N,Q) aPKC-N downregulates XK70 (arrow). (O-Q) Sections corresponding to embryos in L-N. Superficial expression of XK70 (O) is extended into the deep cell layer in aPKC-CAAX RNA-injected embryos (P, arrowhead). (Q) Inhibition of XK70 by aPKC-N (arrowhead). In all panels, arrows indicate altered staining as a result of injection. (R,S) Quantification of the effects, shown as average numbers of XK70-expressing cells in affected embryos (R), and frequency of embryos with visible changes in XK70 expression (S). Numbers of embryos per group are given above the bars. The data are from three representative experiments.
By mid to late neurula stages, ciliated cells are known to migrate into the outer ectodermal layer and differentiate by forming multiple cilia (Drysdale and Elinson, 1992). We used an antibody to acetylated tubulin to assess cilia differentiation in epidermal cells with altered aPKC expression. In stage 18 embryos, many respecified ciliated cells formed cilia, indicating that cell differentiation is relatively complete (Fig. 1I-K). We noted, however, that many of these cells failed to reach the surface of the embryo, suggesting that cell migratory properties are inhibited. Together these findings indicate that aPKC functions as an inhibitor of ciliated cell differentiation in epidermal ectoderm.
aPKC promotes superficial cell fates
As aPKC inhibited ciliated cell differentiation, we hypothesized that its normal function is to maintain the superficial ectoderm layer. To prove that aPKC causes a switch in cell fates as opposed to a simple inhibitory effect on ciliated cell differentiation, we analyzed the expression of the epidermal keratin gene XK70 (Winkles et al., 1985), which we detected exclusively in the superficial cell layer of epidermal ectoderm (Fig. 1L,O). XK70 was upregulated by aPKC-CAAX (PN=82%, n=28; Fig. 1M,P,R,S) and inhibited by aPKC-N (PN=40%, n=31; Fig. 1N,Q-S), revealing changes complementary to the observed changes in ciliated cell differentiation. Note that less penetrant effects of PKC-N might be due to the incomplete inhibition of several vertebrate aPKC homologues with potentially redundant function. Lineage tracing with coinjected lacZ RNA indicated that the effects are cell-autonomous. Sectioning demonstrated an expansion of XK70-positive cells into inner ectoderm layers of aPKC-CAAX RNA-injected embryos, as compared with the superficial expression of this gene in control embryos or reduced expression in aPKC-N RNA-injected embryos (Fig. 1O-Q). These observations indicate that aPKC promotes superficial layer markers at the expense of basal layer differentiation.
aPKC regulates PAR1 localization in Xenopus ectoderm
Having established a role for aPKC in the specification of the superficial cell layer, we wanted to identify a potential molecular target that may be responsible for aPKC effects on ectodermal cell fates. Given that the PAR1 kinase has been shown to localize in a domain complementary to that of aPKC in different systems (Benton and St Johnston, 2003; Bohm et al., 1997; Ohno, 2001), we wanted to know how PAR1 is distributed in Xenopus ectoderm and whether this distribution is affected by aPKC. Overexpressed tagged PAR1 exhibited mostly basolateral localization in superficial ectoderm cells (Fig. 2A). Both PAR1A and PAR1B proteins with different epitope tags (GFP and Myc) had this localization (data not shown). This distribution was altered by coinjected aPKC-CAAX (Fig. 2B), resulting in a mostly cytoplasmic staining for PAR1, consistent with the idea that aPKC regulates PAR1 localization by phosphorylation (Kusakabe and Nishida, 2004).
Fig. 2. aPKC regulates subcellular localization of PAR1 in Xenopus ectoderm.
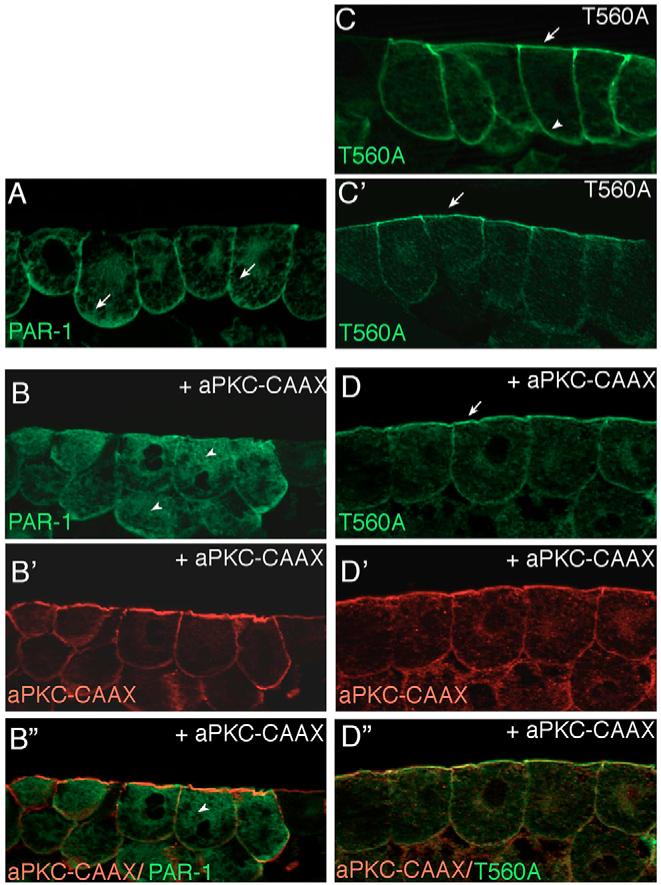
(A,B) aPKC inhibits cortical localization of PAR1. (A) PAR1 is predominantly localized to the basolateral cortex of superficial ectoderm cells (arrows). (B) Overexpression of aPKC-CAAX mislocalizes PAR1 to the cytoplasm (arrowheads). (C,D) The apical localization of T560A, the non-phosphorylatable PAR1 mutant (C’) is not affected by aPKC-CAAX (D). At higher doses of injected RNA, T560A was distributed all around the cell cortex, both apically and basolaterally (C). The distribution of coinjected membrane-associated aPKC-CAAX is shown in B’ and D’, the increased apical staining is due to high amounts of endogenous aPKC detected by anti-aPKC antibody. B” and D”, merged images. Embryo injections were as described in Fig. 1. Frozen sections of stage 10.5-11 embryonic ectoderm were stained with anti-Myc (green) to detect PAR1 (B,D) and anti-aPKC antibodies (B’ and D’, red). At least 15 embryos per group were examined and representative sections of three independent experiments are shown.
We next introduced a single mutation in the conserved threonine at position 560 in the PAR1 linker region that is known to be phosphorylated by aPKC (Hurov et al., 2004; Suzuki et al., 2004). The mutated T560A construct was predominantly localized at the apical surface, although also present at the basolateral surface when introduced into early embryos at higher levels, presumably because of saturation of the endogenous machinery that is responsible for PAR1 localization (Fig. 2C,C’). This finding indicates that endogenous aPKC not only inhibits apical localization of PAR1, in agreement with published data (Hurov et al., 2004; Suzuki et al., 2004), but may also actively promote PAR1 basolateral localization. By contrast, the cortical localization of T560A was unaffected by aPKC (Fig. 2D), confirming that this construct is insensitive to aPKC regulation. The overall levels of PAR1 and T560A were not significantly affected by aPKC-CAAX in embryo lysates (data not shown), suggesting that the observed regulation is not due to altered protein stability. Whereas PAR1 localization was modulated by aPKC, neither overexpressed T560A, nor previously characterized PAR1 MOs (Kusakabe and Nishida, 2004; Ossipova et al., 2005) influenced aPKC distribution (data not shown). These data confirm the view that the apical PAR protein complex functions upstream of PAR1 to regulate its localization and, possibly, function in embryonic ectoderm (Etemad-Moghadam et al., 1995).
PAR1 regulates cell fates in epidermal ectoderm
Given that PAR1 and aPKC are distributed in complementary domains in different systems (Benton and St Johnston, 2003; Bohm et al., 1997; Ohno, 2001) (and this study), we wanted to evaluate the hypothesis that PAR1, similar to aPKC, regulates cell fates in epidermal ectoderm. Since the deep and the superficial cells arise by asymmetric divisions (Chalmers et al., 2003), we expected that PAR1 might influence cell fates in a manner opposite to that of aPKC.
We analyzed ciliated cell differentiation in the basal cell layer in embryos, in which RNA injections were targeted to animal ventral ectoderm and would not be expected to affect mesoderm development. RNA encoding the nonphosphorylatable form of PAR1 (T560A) caused a dramatic increase in the number of α-tubulin-positive cells (PN=61%, n=64), whereas β-gal RNA (n=33) and kinase-dead PAR1 RNA (n=26) did not have this effect (Fig. 3A-K). Of note, a similar enhancing effect on ciliated cell development was observed for both T560A and wild-type PAR1 RNA, indicating that overexpression of this kinase is sufficient to influence cell fate determination (Fig. 3B,J). In cross-sections of PAR1 RNA-injected embryos, α-tubulin-positive cells frequently formed a double layer instead of a single cell layer (Fig. 3C,D and data not shown). These ectopic α-tubulin-positive cells differentiated multiple cilia, based on immunostaining with antibodies to acetylated tubulin (Fig. 3E,F), indicating that the process of cell fate respecification is relatively complete. As with aPKC-N-expressing cells many ectopic ciliated cells, formed in response to T560A RNA injection, remained in the inner layer, in contrast to control embryos in which ciliated cells intercalated into the superficial layer by stage 18.
Fig. 3. PAR1 promotes ciliated cell differentiation.
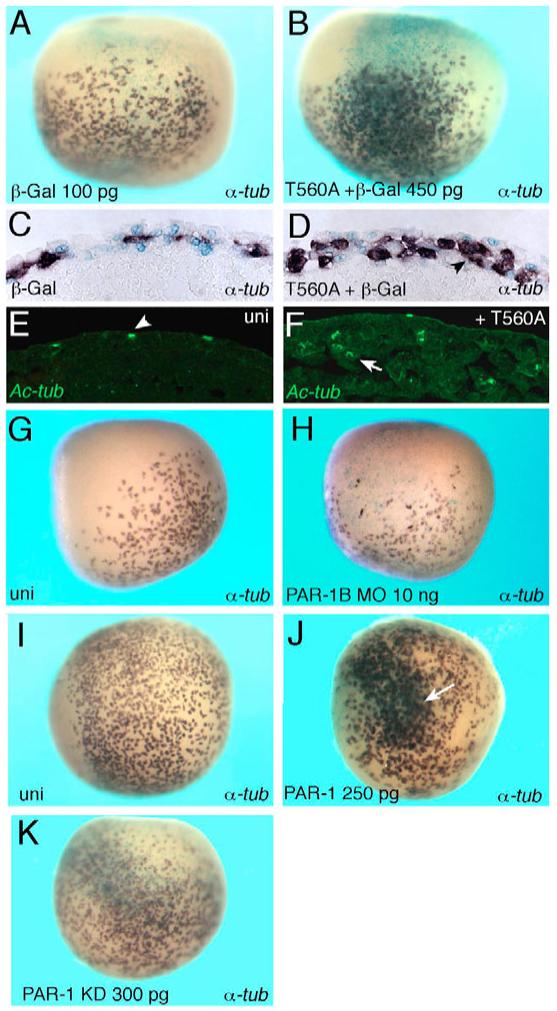
Four- to eight-cell embryos were unilaterally injected with lacZ RNA (light blue staining) or PAR1 RNAs or MO as indicated and subjected to in situ hybridization for α-tubulin expression at stages 13-14. (A-D) T560A increases the number of α-tubulin-expressing cells in epidermal ectoderm. (C,D) Cross-sections of embryos shown in A,B. A single layer of α-tubulin-positive cells in control ectoderm (C) expanded to a double layer of positive cells in T560A-expressing ectoderm (D, arrowhead). (E,F) Enhanced cilia differentiation in T560A RNA-injected embryos at stage 18 (F), when compared with uninjected controls (E), revealed by immunostaining for acetylated tubulin. Arrowhead in E demarcates ciliated cells that migrated to the surface. Arrow in F indicates ectopic ciliated cells remaining in the inner ectoderm layer. (G,H) PAR1B MO decreases the number of α-tubulin-expressing cells (H) as compared with the uninjected side (G). (I) Uninjected embryo; (J) PAR1 RNA increases ciliated cell number (white arrow); (K) PAR1 KD RNA has no significant effect on ciliated cells. Lateral view is shown in all panels, except I-K (ventral view).
By contrast, depletion of PAR1 in ventral ectoderm with the previously characterized PAR1B morpholino antisense oligonucleotide (MO) (Kusakabe and Nishida, 2004; Ossipova et al., 2005) downregulated the α-tubulin-positive cell population (PN=41%, n=18), indicating that PAR1 is required for ciliated cell differentiation (Fig. 3G,H). The same effect was observed with two different PAR1 MOs, but not a control MO, and could be rescued by PAR1 RNA, supporting specificity of these reagents (Ossipova et al., 2005). By contrast, ectoderm-targeted PAR1 MOs did not inhibit the mesodermal marker MyoD (see Fig. S1D-G in the supplementary material), confirming that the observed effects on ectoderm specification are independent of the PAR1 function in organizer formation and mesoderm development (Ossipova et al., 2005). Thus, the effects of aPKC and PAR1 on cell fates in the two ectoderm layers correlate with the corresponding apical and basolateral distribution of these proteins in embryonic cells.
The observed cell fate changes were highly specific for PAR1, since LGL1, another basolateral polarity determinant (Ohshiro et al., 2000; Peng et al., 2000; Plant et al., 2003) phosphorylated by aPKC, did not influence ciliated cell development (Fig. 4A-D). This difference between PAR1 and LGL1 may be crucial because it provides an apparent distinction between a polarity protein that affects cellular architecture and shape (LGL) (e.g. Dollar et al., 2005), from one that links polarity with cell fate (PAR1). Whereas PAR1 has been shown to positively regulate Wnt-β-catenin signaling (Ossipova et al., 2005; Sun et al., 2001), ciliated cell development was not significantly affected by β-catenin RNA (Fig. 4C,D), suggesting that the PAR1 effect on ciliated cells in not likely to be mediated by the Wnt pathway. Both LGL and β-catenin RNAs retained high functional activity in these experiments, as measured, respectively, by ectoderm pigmentation (Dollar et al., 2005) and secondary axis induction assays (Fig. 4E-J).
Fig. 4. Lack of effect of β-catenin and LGL on ciliated cell development.
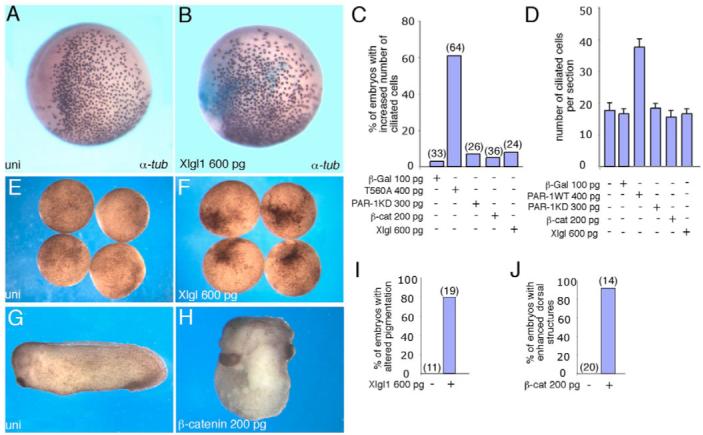
In situ hybridization with α-tubulin probe is shown. For experimental details, see Fig. 1 legend. (A) Uninjected embryo. (B) LGL1 (Xlgl1) RNA has no effect on α-tubulin-expressing cells. (C,D) Quantification of the effects of T560A, PAR1, PAR1-KD, β-catenin and LGL1 RNAs on ciliated cell development, presented as frequencies of affected embryos (C) and numbers of ciliated cells per section (D). In C, numbers of embryos per group are shown above bars. Data are representative of four different experiments. (E,F,I) LGL1 RNA, used in B, altered ectoderm pigmentation in 79% of injected embryos (n=19; F) as compared with uninjected controls (E). (I) Quantification of the results in E and F. (G,H,J) Marginal zone-injected β-catenin RNA dorsalized 92% of injected embryos (n=14), characterized by enlarged head and cement gland and truncated or missing tail (H), as compared with uninjected siblings (G). (J) Quantification of the results in G,H.
aPKC acts upstream of PAR1 to specify ectodermal cell fates
The opposite effects and non-overlapping distribution of aPKC and PAR1 in epithelial cells raise a question of the epistatic relationship between the two proteins in the pathway leading to ciliated cell differentiation. To assess whether PAR1 acts downstream of aPKC in cell fate specification, we co-expressed aPKC-CAAX and T560A, and analyzed the number of ciliated cells. We observed that aPKC-dependent suppression of ciliated cell differentiation was rescued by T560A and by wild-type PAR1 (Fig. 5A-C,G,H, and data not shown). aPKC-CAAX inhibited α-tubulin in 75% of injected embryos (n=36), whereas this number was reduced to 20% (n=68) in the presence of T560A (Fig. 5G). In a complementary loss-of-function approach, aPKC-N increased ciliated cell differentiation in 66% of injected embryos (n=56), whereas this number was reduced to 32% (n=34) in the presence of PAR1B MO (Fig. 5D-F,I,J). Together, these results indicate that aPKC functions upstream of PAR1 in cell fate determination.
Fig. 5. aPKC functions upstream of PAR1 to specify ectodermal cell fates.
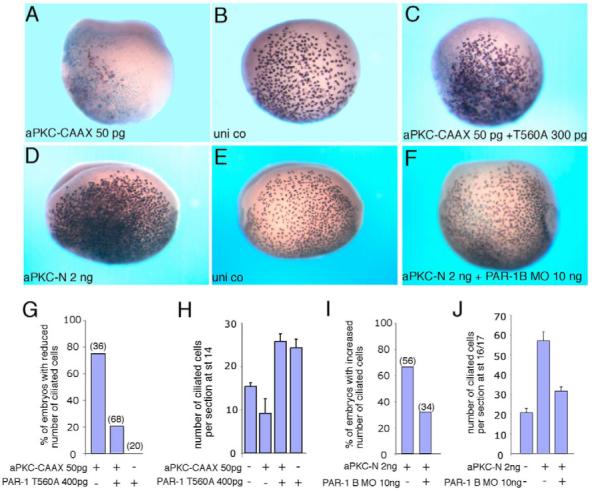
Embryos were injected with RNAs or MO as described in Fig. 1. Ciliated cells were detected at stages 14-16 by in situ hybridization with the α-tubulin probe. (A-C) T560A reverses the inhibitory effect of aPKC-CAAX on ciliated cell differentiation. Two sides of the same embryo are shown in B and C. (D-F) PAR1B MO (F) suppresses aPKC-N-mediated expansion of ciliated cells. The injected and uninjected sides of the same embryo are shown in D and E, respectively. (G-J) Quantification of the data shown in A-C (G,H) and D-F (I,J). Numbers of embryos per group are shown above bars. (G,I) Frequencies of embryos showing visible phenotypic changes. (H,J) Mean numbers of α-tubulin-positive cells per section±s.d. are shown. Sections of at least three representative embryos per group were analyzed.
Lack of PAR1 and aPKC effects on cell cycle
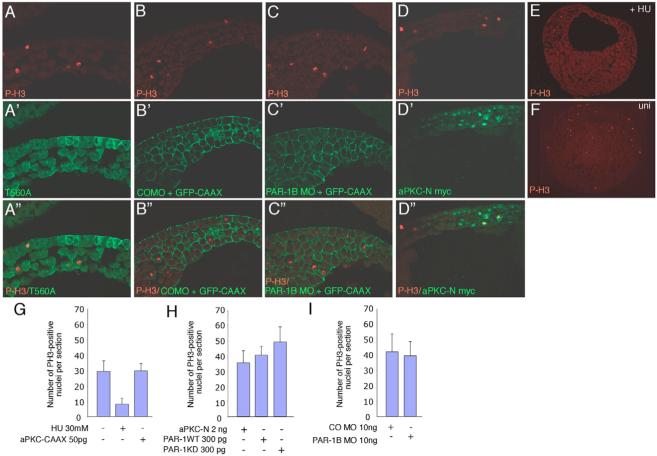
Our lineage tracing experiments suggest that aPKC and PAR1 directly modulate cell fates in the superficial and deep ectoderm layers. The alternative explanation is that the polarity proteins affect the number of progenitor cell divisions, resulting in the corresponding increase (or decrease) in the number of superficial or deep ectodermal cells, as aPKC has been shown to affect cell proliferation in Drosophila epithelial cells (Rolls et al., 2003). We tested this possibility by immunostaining injected embryos with anti-phosho-H3 antibodies that mark mitotic nuclei in many species including Xenopus (Saka and Smith, 2001). Our analyses carried out for gastrula and neurula stages showed that the expanded number of ciliated cells in PAR1 or aPKC-N-injected embryos was not due to increased number of mitoses (Fig. 6 and data not shown).
Fig. 6. PAR1 and aPKC do not significantly alter cell proliferation.
(A-F) Embryos injected at the four- to eight-cell stage with the indicated MO and RNAs (green, A’-D’). GFP-CAAX was used as a lineage tracer for MO injections (B’,C’). Embryos were cultured to gastrula stages, fixed, cryosectioned and stained with anti-phospho H3 (P-H3) antibodies (red, A-F; merged images A”-D”). No significant differences were observed in embryos with altered levels of PAR1 or aPKC (compare control MO B with A,C,D), but the number of positive cells was strongly reduced in hydroxyurea (HU)-treated embryos (E) compared with the control embryo (F). No differences in the number of mitotic nuclei were detected between injected and uninjected tissues (marked by lineage tracing) within the same embryo or in different embryos (data not shown). (G-I) Quantification of changes in P-H3-positive cells. Average numbers of P-H3-positive cells per section±s.d. are shown. At least ten embryos were examined for each group.
Opposite effects of aPKC and PAR1 on gene expression in layer separation experiments
The results of embryo injections are consistent with a model, in which aPKC and PAR1 function to establish the difference between superficial and inner cell layers acting effectively as cell layer determinants. This model predicts that aPKC and PAR1 should each be capable of switching one layer-specific cell fate to another. Alternatively, PAR1 and aPKC-CAAX may expand the corresponding pools of the inner or superficial cells without transdifferentiation. To address these possibilities, we studied how overexpressed aPKC and PAR1 alter layer-specific gene expression in separated ectodermal layers (Fig. 7A,B). We observed that T560A upregulated inner cell layer genes α-tubulin and inca B (Luo et al., 2007) and decreased the expression of the superficial layer genes ESR6e and grhl3 (Chalmers et al., 2006) in both ectodermal layers at stage 14 (Fig. 7C). Reciprocally, aPKC-CAAX inhibited α-tubulin and inca B, while inducing ESR6e and grhl3 (Fig. 7C) in the inner layer. At stage 19, we observed that T560A inhibited the expression of XK70 and increased levels of XDelta-1 and inca B in the superficial layer, whereas aPKC-CAAX upregulated XK70 together with ESR6e and grhl3 in the inner layer (Fig. 7C). These results are consistent with the idea that PAR1 and aPKC govern layer-specific gene expression. The PAR1-dependent enhancement of α-tubulin expression in inner layer cells additionally suggests that PAR1 can modulate or override the regulation of ciliated cell precursors by lateral inhibition in that layer (see below).
Fig. 7. Opposite effects of PAR1 and aPKC on gene expression in separated ectoderm cell layers.
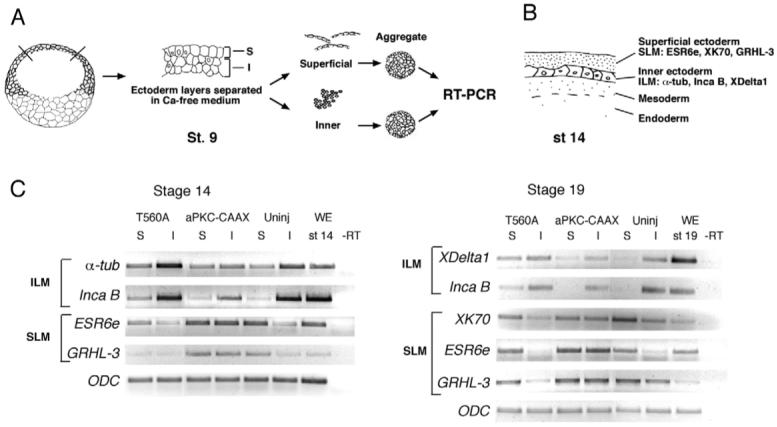
(A) Experimental scheme for the layer separation assay. Two-cell embryos were injected with T560A or aPKC-CAAX mRNA. Animal pole explants were dissected from injected or uninjected embryos at stage 9, superficial (S) and inner (I) cell layers were separated based on their different abilities to dissociate in a calcium- and magnesium-free buffer and allowed to reaggregate. Cell aggregates were cultured until sibling embryos reached stage 14 or stage 19. RNA was prepared from aggregate lysates and analyzed by semi-quantitative RT-PCR. (B) A schematic section of the frog embryo at stage 14 shows superficial and inner layers of non-neural ectoderm with distinct sets of molecular markers. SLM, superficial layer markers; ILM, inner layer markers. (C) Stage 14 aggregates: T560A RNA upregulates the inner layer markers α-tubulin and inca B and downregulates the superficial layer markers ESR6e and grhl3 in both inner and outer layer explants. aPKC-CAAX has a complementary effect. Stage 19 aggregates: T560A RNA upregulates the inner layer markers XDelta-1 and inca B and downregulates XK70 in the superficial layer. aPKC-CAAX downregulates XDelta-1 and inca B in the superficial layer and upregulates XK70, ESR6e and grhl3 in inner cell aggregates. ODC is a control for loading. Uninj, no RNA injection; WE, whole embryo; −RT, no reverse transcriptase control. The analysis of two representative sets of cDNAs from several independent experiments is shown.
PAR1 cooperates with XDelta-1 in ciliated cell differentiation
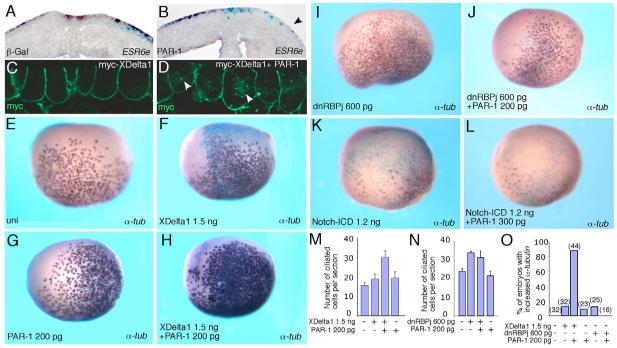
Our layer separation experiments revealed opposite effects of aPKC and PAR1 on ESR6e, a Notch target gene that is specifically expressed in superficial ectoderm (Deblandre et al., 1999). To confirm these results, we examined whether PAR1 has an effect on ESR6e expression using in situ hybridization. PAR1 RNA downregulated ESR6e in superficial cells (Fig. 8A,B), suggesting that PAR1 controls cell fates through the Notch pathway previously implicated in epidermal and ciliated cell differentiation (Alonso and Fuchs, 2003; Blanpain et al., 2006; Deblandre et al., 1999). Consistent with this interpretation, we noted that in superficial cells T560A upregulated the expression of XDelta-1 (Fig. 7C), a marker for suppressed Notch signaling (Deblandre et al., 1999).
Fig. 8. PAR1 synergizes with XDelta-1 to induce ciliated cell differentiation and inhibits the Notch target ESR6e.
Four- to eight-cell embryos were unilaterally injected with the indicated RNAs and lacZ RNA as a lineage tracer (light blue staining) and subjected to in situ hybridization with the ESR6e (A,B) or α-tubulin (E-L) probes. (A,B) Superficial staining for ESR6e is unaffected in cross-sections of lacZ RNA-injected control embryos (A, 100 pg), but is inhibited in PAR1 RNA-injected embryos (B, right side, arrowhead, 250 pg). (C) Basolateral localization of XDelta-1 in Xenopus ectoderm. (D) XDelta-1 is detected in multiple cytoplasmic vesicles (arrowheads) in the presence of PAR1. (E) Uninjected embryo. (F,G) XDelta-1 RNA alone (F) or low dose of PAR1 RNA (G) do not significantly alter the number of ciliated cells. (H) The synergistic effect of coinjected PAR1 and XDelta-1 RNAs on ciliated cell development. (I,J) PAR1 does not influence the activity of a dominant intracellular inhibitor of the Notch pathway, dnRBP/j, which can stimulate ciliated cell development. (K) Notch-ICD suppresses ciliated cell differentiation. (L) PAR1 does not alter Notch-ICD activity. (M-O) Quantification of the effects shown in E-J, presented as numbers of ciliated cells per section (M,N) and frequency of embryos with increased α-tubulin staining (O). Numbers of examined embryos are shown above bars. The data are representative of three independent experiments.
We next evaluated whether PAR1 can influence the localization and function of XDelta-1, a Notch ligand. In the absence of PAR1, we observed basolateral distribution of XDelta-1 in Xenopus ectoderm (Fig. 8C). Lack of apical localization indicates that XDelta-1 is less abundant in superficial ectoderm as compared with deep ectoderm cells. Considering that Delta and Notch are known to negatively affect each other’s activity, lower levels of Delta would correspond to higher levels of Notch receptor signaling, as evidenced by higher ESR6e expression in superficial cells. In the presence of PAR1 RNA, XDelta-1 was distributed in multiple cytoplasmic vesicles (Fig. 8D). This observation suggests that PAR1 might influence XDelta-1 endocytosis and recycling, which is expected to result in altered Notch signaling (Itoh et al., 2003).
To further investigate the relationship between PAR1 and the Notch pathway, we studied the functional interaction of PAR1 and the Notch ligand XDelta-1. Overexpressed XDelta-1 was previously reported to induce ciliated cell differentiation, an effect that has been attributed to the cell-autonomous or ‘cis-inhibitory’ activity of Delta in Notch-expressing cells (Deblandre et al., 1999). At lower doses, XDelta-1 or PAR1 RNAs did not significantly affect the number of ciliated cells. However, the coinjection of both RNAs resulted in a synergistic increase in ciliated cell number in the majority of injected embryos (Fig. 8E-H), indicating that PAR1 enhanced the inhibitory effect of XDelta-1 on Notch signaling. By contrast, PAR1 did not influence the activity of dnRBP/j (Fig. 8I,J,N,O), a dominant intracellular inhibitor of the Notch pathway, or the Notch intracellular domain (Notch-ICD), a constitutively active form of the Notch receptor (Fig. 8K,L). The simplest interpretation of these findings is that PAR1 inhibits signaling at the level of Delta, rather than downstream of Notch. Together, our results support the hypothesis that PAR1 functions to specify inner cell fates by downregulating Notch signaling in the superficial ectoderm layer.
DISCUSSION
This study supports the view that ectoderm layer-specific cell fates are coupled to intracellularly partitioned apicobasal polarity proteins that may segregate differentially in asymmetrically dividing cells (Chalmers et al., 2002; Gotz and Huttner, 2005). Additionally, our findings provide molecular identities for the postulated cell fate determinants, because interference with either apical (aPKC) or basolateral (PAR1) polarity proteins changes the balance between the two cell layers of the epidermis and results in altered cell fates. We expect that the same pathway may control asymmetric divisions of stem/progenitor cells in other epithelial tissues and organs.
Despite growing experimental evidence related to the establishment of the apical-basal polarity, how cell polarity proteins influence developmental fates of polarized progenitor cells is still poorly understood. Based on the phenotype of the zebrafish ‘heart-and-soul’ mutation, aPKC has been implicated in the formation of multiple organs, including the heart, the eye and the gut (Horne-Badovinac et al., 2001), however, the molecular mechanism underlying its function has remained unknown. We demonstrate that aPKC specifies the apical domain and superficial ectodermal cell fates and suppresses inner (basal) cell fates. Furthermore, our experiments reveal that a crucial molecular substrate for aPKC is the PAR1 kinase, which has a complementary localization in epithelial cells. Additionally, we show a bona fide role for PAR1 in the establishment of the basolateral cortical domain and the corresponding cell fates. PAR1 appears to be distinct from other basolateral determinants such as LGL, which regulate epithelial architecture but have no effect on ciliated cell specification. Thus, our study places PAR1 mechanistically downstream of or parallel to other proteins, operating to specify cell polarity and cell fate following asymmetric cell division.
At the next step, PAR1 targeted to the basolateral cortical domain by aPKC-dependent phosphorylation may influence cell fates by modulating one or more signaling pathways that are known to operate in early embryos. The Wnt pathway is unlikely to play a significant role in ectodermal layer fate determination, since β-catenin RNA does not influence ciliated cell differentiation. Moreover, different PAR1 MOs, which have distinct effects on Wnt signaling (Ossipova et al., 2005) produce similar changes in ciliated cell fate, further supporting the idea that this process is independent of Wnt signaling. By contrast, the Notch pathway does appear to be involved, since PAR1 modulates XDelta-1 localization and activity, upregulates XDelta-1 expression and inhibits the Notch target ESR6e, consistent with a recent study in Drosophila embryos (Bayraktar et al., 2006).
Notably, Notch signaling is known to repress ciliated cell differentiation in Xenopus ectoderm (Chalmers et al., 2002; Chitnis et al., 1995; Deblandre et al., 1999). The observation that PAR1 stimulates α-tubulin gene expression in the superficial as well as the inner ectodermal layers demonstrates that it can override or modulate this Notch-Delta-dependent mechanism. Although XDelta-1 is a Notch ligand and can stimulate Notch signaling in some contexts, in Xenopus epidermis, XDelta-1 overexpression has the opposite effect from that of Notch, and stimulates ciliated cell differentiation in embryos (Deblandre et al., 1999). This activity was attributed to the cis-inhibitory function of XDelta-1 in Notch-expressing cells, also observed in other systems (Itoh et al., 2003). PAR1 enhances the effect of XDelta-1, yet fails to modulate signaling stimulated by the dominant negative RBP/J or Notch intracellular domain. These findings suggest that PAR1 acts at the level of the XDelta-1 ligand, upstream of the Notch receptor. Thus, the observed functional interaction of aPKC and PAR1 may create unequal Delta activity in the superficial and the deep ectodermal cells, leading to diversification of cell fates. However, since ESR6e and other Notch target genes may have additional regulators besides Notch, whether XDelta-1 is the primary target of PAR1 in fate determination or whether other relevant PAR1 targets are also crucial for this process remains to be established.
Supplementary Material
Acknowledgments
We thank C. Kintner, A. Chitnis, T. Sargent, R. Harland, V. Joukov, E. Fuchs and Q. Ma for plasmids, D. Weinstein and members of the Sokol laboratory for useful discussions. We acknowledge support of the Department of Cancer Biology, DFCI, to O.O. This work was supported by the National Science Foundation (J.B.G.) and National Institutes of Health (S.S.).
Footnotes
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/134/23/4297/DC1
References
- Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–1200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- Bayraktar J, Zygmunt D, Carthew RW. Par-1 kinase establishes cell polarity and functions in Notch signaling in the Drosophila embryo. J. Cell Sci. 2006;119:711–721. doi: 10.1242/jcs.02789. [DOI] [PubMed] [Google Scholar]
- Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- Betschinger J, Knoblich JA. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 2004;14:R674–R685. doi: 10.1016/j.cub.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Pasolli HA, Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20:3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm H, Brinkmann V, Drab M, Henske A, Kurzchalia TV. Mammalian homologues of C. elegans PAR-1 are asymmetrically localized in epithelial cells and may influence their polarity. Curr. Biol. 1997;7:603–606. doi: 10.1016/s0960-9822(06)00260-0. [DOI] [PubMed] [Google Scholar]
- Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, Wilsch-Brauninger M, Eilken HM, Rieger MA, Schroeder TT, Huttner WB, et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat. Neurosci. 2006;9:1099–1107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- Chalmers AD, Welchman D, Papalopulu N. Intrinsic differences between the superficial and deep layers of the Xenopus ectoderm control primary neuronal differentiation. Dev. Cell. 2002;2:171–182. doi: 10.1016/s1534-5807(02)00113-2. [DOI] [PubMed] [Google Scholar]
- Chalmers AD, Strauss B, Papalopulu N. Oriented cell divisions asymmetrically segregate aPKC and generate cell fate diversity in the early Xenopus embryo. Development. 2003;130:2657–2668. doi: 10.1242/dev.00490. [DOI] [PubMed] [Google Scholar]
- Chalmers AD, Pambos M, Mason J, Lang S, Wylie C, Papalopulu N. aPKC, Crumbs3 and Lgl2 control apicobasal polarity in early vertebrate development. Development. 2005;132:977–986. doi: 10.1242/dev.01645. [DOI] [PubMed] [Google Scholar]
- Chalmers AD, Lachani K, Shin Y, Sherwood V, Cho KW, Papalopulu N. Grainyhead-like 3, a transcription factor identified in a microarray screen, promotes the specification of the superficial layer of the embryonic epidermis. Mech. Dev. 2006;123:702–718. doi: 10.1016/j.mod.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Chitnis A, Henrique D, Lewis J, Ish-Horowicz D, Kintner C. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature. 1995;375:761–766. doi: 10.1038/375761a0. [DOI] [PubMed] [Google Scholar]
- Cohen D, Brennwald PJ, Rodriguez-Boulan E, Musch A. Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. J. Cell Biol. 2004;164:717–727. doi: 10.1083/jcb.200308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblandre GA, Wettstein DA, Koyano-Nakagawa N, Kintner C. A two-step mechanism generates the spacing pattern of the ciliated cells in the skin of Xenopus embryos. Development. 1999;126:4715–4728. doi: 10.1242/dev.126.21.4715. [DOI] [PubMed] [Google Scholar]
- Deblandre GA, Lai EC, Kintner C. Xenopus neuralized is a ubiquitin ligase that interacts with XDelta1 and regulates Notch signaling. Dev. Cell. 2001;6:795–806. doi: 10.1016/s1534-5807(01)00091-0. [DOI] [PubMed] [Google Scholar]
- Doerflinger H, Benton R, Shulman JM, St Johnston D. The role of PAR-1 in regulating the polarised microtubule cytoskeleton in the Drosophila follicular epithelium. Development. 2003;130:3965–3975. doi: 10.1242/dev.00616. [DOI] [PubMed] [Google Scholar]
- Dollar GL, Weber U, Mlodzik M, Sokol SY. Regulation of Lethal giant larvae by Dishevelled. Nature. 2005;437:1376–1380. doi: 10.1038/nature04116. [DOI] [PubMed] [Google Scholar]
- Drysdale TA, Elinson RP. Cell migration and induction in the development of the surface ectodermal pattern of the Xenopus laevis tadpole. Dev. Growth Differ. 1992;34:51–59. doi: 10.1111/j.1440-169X.1992.00051.x. [DOI] [PubMed] [Google Scholar]
- Etemad-Moghadam B, Guo S, Kemphues KJ. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell. 1995;83:743–752. doi: 10.1016/0092-8674(95)90187-6. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Gumbiner BM. Beta-catenin localization during Xenopus embryogenesis: accumulation at tissue and somite boundaries. Development. 1994;120:3667–3679. doi: 10.1242/dev.120.12.3667. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Guo S, Kemphues KJ. Molecular genetics of asymmetric cleavage in the early Caenorhabditis elegans embryo. Curr. Opin. Genet. Dev. 1996;6:408–415. doi: 10.1016/s0959-437x(96)80061-x. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. In: Kay BK, Peng HB, editors. Methods Cell Biol. Vol. 36. San Diego: Academic Press; 1991. pp. 685–695. [DOI] [PubMed] [Google Scholar]
- Hopwood ND, Pluck A, Gurdon JB. MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. EMBO J. 1989;8:3409–3417. doi: 10.1002/j.1460-2075.1989.tb08505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne-Badovinac S, Lin D, Waldron S, Schwarz M, Mbamalu G, Pawson T, Jan Y, Stainier DY, Abdelilah-Seyfried S. Positional cloning of heart and soul reveals multiple roles for PKC lambda in zebrafish organogenesis. Curr. Biol. 2001;11:1492–1502. doi: 10.1016/s0960-9822(01)00458-4. [DOI] [PubMed] [Google Scholar]
- Hurov JB, Watkins JL, Piwnica-Worms H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr. Biol. 2004;14:736–741. doi: 10.1016/j.cub.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Imai F, Hirai S, Akimoto K, Koyama H, Miyata T, Ogawa M, Noguchi S, Sasaoka T, Noda T, Ohno S. Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133:1735–1744. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J. Biol. 2005;4:3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–2324. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakabe M, Nishida E. The polarity-inducing kinase Par-1 controls Xenopus gastrulation in cooperation with 14-3-3 and aPKC. EMBO J. 2004;23:4190–4201. doi: 10.1038/sj.emboj.7600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Robinson KJ, Doe CQ. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006;439:594–598. doi: 10.1038/nature04299. [DOI] [PubMed] [Google Scholar]
- Luo T, Xu Y, Hoffman TL, Zhang T, Schilling T, Sargent TD. Inca: a novel p21-activated kinase-associated protein required for cranial neural crest development. Development. 2007;134:1279–1289. doi: 10.1242/dev.02813. [DOI] [PubMed] [Google Scholar]
- Muller HA, Hausen P. Epithelial cell polarity in early Xenopus development. Dev. Dyn. 1995;202:405–420. doi: 10.1002/aja.1002020410. [DOI] [PubMed] [Google Scholar]
- Nakaya M, Fukui A, Izumi Y, Akimoto K, Asashima M, Ohno S. Meiotic maturation induces animal-vegetal asymmetric distribution of aPKC and ASIP/PAR-3 in Xenopus oocytes. Development. 2000;127:5021–5031. doi: 10.1242/dev.127.23.5021. [DOI] [PubMed] [Google Scholar]
- Ohno S. Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr. Opin. Cell Biol. 2001;13:641–648. doi: 10.1016/s0955-0674(00)00264-7. [DOI] [PubMed] [Google Scholar]
- Ohshiro T, Yagami T, Zhang C, Matsuzaki F. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature. 2000;408:593–596. doi: 10.1038/35046087. [DOI] [PubMed] [Google Scholar]
- Ossipova O, Dhawan S, Sokol S, Green JB. Distinct PAR-1 proteins function in different branches of Wnt signaling during vertebrate development. Dev. Cell. 2005;8:829–841. doi: 10.1016/j.devcel.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Parkinson SJ, Le Good JA, Whelan RD, Whitehead P, Parker PJ. Identification of PKCzetaII: an endogenous inhibitor of cell polarity. EMBO J. 2004;23:77–88. doi: 10.1038/sj.emboj.7600023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellettieri J, Seydoux G. Anterior-posterior polarity in C. elegans and Drosophila - PARallels and differences. Science. 2002;298:1946–1950. doi: 10.1126/science.1072162. [DOI] [PubMed] [Google Scholar]
- Peng CY, Manning L, Albertson R, Doe CQ. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 2000;408:596–600. doi: 10.1038/35046094. [DOI] [PubMed] [Google Scholar]
- Peng HB. Xenopus laevis: practical uses in cell and molecular biology. Solutions and protocols. Methods Cell Biol. 1991;36:657–662. [PubMed] [Google Scholar]
- Plant PJ, Fawcett JP, Lin DC, Holdorf AD, Binns K, Kulkarni S, Pawson T. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat. Cell Biol. 2003;5:301–308. doi: 10.1038/ncb948. [DOI] [PubMed] [Google Scholar]
- Plusa B, Frankenberg S, Chalmers A, Hadjantonakis AK, Moore CA, Papalopulu N, Papaioannou VE, Glover DM, Zernicka-Goetz M. Downregulation of Par3 and aPKC function directs cells towards the ICM in the preimplantation mouse embryo. J. Cell Sci. 2005;118:505–515. doi: 10.1242/jcs.01666. [DOI] [PubMed] [Google Scholar]
- Roegiers F, Jan YN. Asymmetric cell division. Curr. Opin. Cell Biol. 2004;16:195–205. doi: 10.1016/j.ceb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Rolls MM, Albertson R, Shih HP, Lee CY, Doe CQ. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J. Cell Biol. 2003;163:1089–1098. doi: 10.1083/jcb.200306079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka Y, Smith JC. Spatial and temporal patterns of cell division during early Xenopus embryogenesis. Dev. Biol. 2001;229:307–318. doi: 10.1006/dbio.2000.0101. [DOI] [PubMed] [Google Scholar]
- Sanada K, Tsai LH. G protein betagamma subunits and AGS3 control spindle orientation and asymmetric cell fate of cerebral cortical progenitors. Cell. 2005;122:119–131. doi: 10.1016/j.cell.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Sun TQ, Lu B, Feng JJ, Reinhard C, Jan YN, Fantl WJ, Williams LT. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat. Cell Biol. 2001;3:628–636. doi: 10.1038/35083016. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Kishikawa M, Hirose H, Amano Y, Izumi N, et al. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr. Biol. 2004;14:1425–1435. doi: 10.1016/j.cub.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Tomancak P, Piano F, Riechmann V, Gunsalus KC, Kemphues KJ, Ephrussi A. A Drosophila melanogaster homologue of Caenorhabditis elegans par-1 acts at an early step in embryonic-axis formation. Nat. Cell Biol. 2000;2:458–460. doi: 10.1038/35017101. [DOI] [PubMed] [Google Scholar]
- Vaccari T, Rabouille C, Ephrussi A. The Drosophila PAR-1 spacer domain is required for lateral membrane association and for polarization of follicular epithelial cells. Curr. Biol. 2005;15:255–261. doi: 10.1016/j.cub.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Winkles JA, Sargent TD, Parry DA, Jonas E, Dawid IB. Developmentally regulated cytokeratin gene in Xenopus laevis. Mol. Cell. Biol. 1985;5:2575–2581. doi: 10.1128/mcb.5.10.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Huttner WB. Asymmetric cell division during neurogenesis in Drosophila and vertebrates. Mech. Dev. 2003;120:1297–1309. doi: 10.1016/j.mod.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Grimm A, Knust E. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J. Cell Biol. 2000;150:1361–1374. doi: 10.1083/jcb.150.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.