Abstract
Some meditation techniques reduce pain, but there have been no studies on how meditation affects the brain’s response to pain. Functional magnetic resonance imaging of the response to thermally induced pain applied outside the meditation period found that long-term practitioners of the Transcendental Meditation technique showed 40–50% fewer voxels responding to pain in the thalamus and total brain than in healthy matched controls interested in learning the technique. After the controls learned the technique and practiced it for 5 months, their response decreased by 40–50% in the thalamus, prefrontal cortex, total brain, and marginally in the anterior cingulate cortex. The results suggest that the Transcendental Meditation technique longitudinally reduces the affective/motivational dimension of the brain’s response to pain.
Keywords: anterior cingulate cortex, functional magnetic resonance imaging, neuroimaging, pain, prefrontal cortex, thalamus, Transcendental Meditation
Introduction
Since the 1990s, neuroimaging studies have provided basic knowledge of how the brain responds to pain and how treatments influence this response [1,2]. These studies have verified the hypothesis that the brain’s response to pain is complex, involving multiple brain regions often referred to as the ‘pain matrix’ [1,2]. An NIH technology assessment conference on behavioral approaches to treating chronic pain found strong evidence that some forms of meditation reduce pain [3,4], yet the effects of meditation on pain have not previously been studied using neuroimaging.
Different meditation techniques have different effects on the brain that are specific to the cognitive requirements of the techniques [5–8] and may affect pain differently. We suggest four mechanisms by which meditation could reduce pain: (1) distract attention away from it; (2) resolve the underlying physiological condition responsible for chronic pain; (3) reduce anticipatory anxiety and general stress reactivity and other factors that amplify the pain response; and (4) reduce pain-related distress, perhaps through increasing endogenous endorphins. Techniques that absorb the mind into imagination and/or into some interesting sensory experience and away from action, with their corresponding changes in the brain [5], may be expected to work by mechanism 1; distract attention from pain. A form of mindfulness meditation illustrates mechanism 2. It requires the patient to put attention on the source of pain to allow it to resolve, with the result that it reduces present-moment pain and associated pain symptoms [9].
The Transcendental Meditation technique appears to reduce pain through mechanisms 2–4. It is an effortless process of attending to a mantra as it becomes progressively more refined, until the mind transcends the subtlest level of thought to experience unbounded (transcendental) consciousness [10]. A magnetoelectroencephalography study localized the source of the widespread α-electroencephalogram seen during the technique in the prefrontal cortex and anterior cingulate [11]. A positron emission tomography study found that it increases blood flow in the prefrontal cortex, the executive control center [8], apparently associated with the voluntary (though effortless) deployment of attention on the mantra. The technique also reduces activity in the thalamus and the medial occipital lobe [8], apparently related to withdrawal of the mind from sensory processing, and it reduces hippocampal activity [8], related to reduced mental processing of short-term into long-term memory. Respiratory rate and plasma lactate decrease and basal skin resistance increases, indicating a state of psychophysiological quiescence [12] during which the endogenous sources of pain could resolve via the action of homeostatic mechanisms.
The resolution of the physiological sources of pain (mechanism 2) through the Transcendental Meditation program is indicated by reduced frequency of pain symptoms in industrial workers [13], reduced headaches and backaches [14], decreased pain during pregnancy and childbirth, [15] and reduced medical care utilization for pain-related conditions such as chest and abdominal pain [16]. Evidence for mechanism 3 is that the Transcendental Meditation technique reduces trait anxiety [17], produces lower resting baseline levels of sympathetic arousal outside the practice as well as improving stress reactivity [12].
Mechanism 4 is suggested by the finding that it decreases distress from acute pain caused by the cold-pressor test and increases the ability to withstand the test longer, but does not change sensory ratings of pain intensity [18]. This implies that the practice may impact the affective/motivational dimension of pain more than the sensory dimension. Individuals who have high sensitivity to pain show a greater response in the prefrontal and anterior cingulate cortices than less pain sensitive people, with no difference in the thalamus [19]. Moreover, studies have associated the affective dimension of pain with the anterior cingulate cortex [1,2,20].
A recent magnetic resonance imaging study demonstrated the principle of experience-dependent cortical plasticity associated with meditative practice [21]. Our specific a priori hypothesis was that the Transcendental Meditation program would have a long-term effect of reducing responses in the affective component of the pain matrix. As the anterior cingulate appears to mediate how emotions direct the focus of attention via the prefrontal cortex [22], we reasoned that reduced distress through the practice may also reduce the response of the prefrontal cortex to pain. As the thalamus is involved in the nonspecific arousal component of the attention system [1], which could be expected to relax with decreases in anticipatory anxiety, we also hypothesized that the thalamic response would decrease.
Methods
The research protocol was approved by the human subjects committees of the University of California at Irvine and Maharishi University of Management in Fairfield, Iowa. All participants signed an informed consent form. The study used a ‘partial crossover’ design. At pretest, long-term meditators were compared with nonpractitioners interested in learning the technique. The healthy control nonpractitioners then learned and practiced the technique for approximately 5 months, after which both groups were posttested on the same pain protocol.
Subjects
A total of 24 normal, right-handed, healthy, pain-free participants (12 men and 12 women) were recruited from the Transcendental Meditation Centers of Orange County and Los Angeles. The healthy controls (n = 12) were individuals who had attended an introductory lecture and were interested in learning the Transcendental Meditation technique. They were recruited from individuals who met the age criterion (45 + for men, 50 + for women). The long-term meditators (n = 12) were individuals of similar age known to the center directors and who had been practicing the technique for a mean of 31.3±2.3 years. The mean age±standard deviation for the healthy controls was 57.8±3.3, compared with 56.3±5.8 years for the long-term meditators, a nonsignificant difference. In all, there were seven men and five women long-term meditators and five men and seven women healthy controls, a nonsignificant difference.
Intervention
The Transcendental Meditation technique was taught in a standard 4-day course, and practiced for 20 min twice daily [10]. Instruction of the healthy controls in the technique was funded by the supporting grant.
Functional neuroimaging
The study was conducted at the Functional Brain Imaging Laboratory of the University of California at Irvine under the direction of Dr Z. H. Cho. The functional magnetic resonance imaging (fMRI) methodology employed single-block stimulation and dynamic regression analysis, which has previously been described [23]. The experimental protocol at both the pretest and the posttest 5 months later entailed a total imaging time of 3 min (60 volumes) for acquisition of fMRI data: 1.5 min of immersion of the left index and middle fingers in warm water (43°C) to maintain them at a standard, uniform baseline temperature, then 30 s of the pain condition of the same fingers immersed in hot water (approximately 51°C), followed by immersion of the fingers in the warm (43°C) water again for 1 min. The fingers were immersed from the distal phalanx to the proximal interphalangeal joint and were not moved. An assistant placed the participant’s hand on a structure to support their arm, moved the hot water to immerse the participant’s fingers into it, and timed the immersions.
Water temperature calibration was confirmed before every fMRI scan and immediately after the fingers were removed from the hot water. At pretest, the mean temperature of the hot water was 50.75±1.1°C for both groups for the initial comparison of the two groups. At posttest 5 months later, after the healthy controls had learned and regularly practiced the Transcendental Meditation technique, the temperature was 51.5±0.9°C for the healthy controls and 51.4±0.9°C for the long-term meditators. For data processing, the first 30 s (10 volumes) were discarded. The rest of the data, 30 s (10 volumes) during stimulation and 1 min (20 volumes) after recent stimulation, were compared with the 1 min (20 volumes) before stimulation.
The fMRI imaging yielded blood oxygen level-dependent (BOLD) signal intensity, indirectly reflecting changes in neural activity during the finger immersion in hot water. Functional measurements were conducted on a 1.5 T Philips MRI system (Philips Electronics North America Corporation, New York, New York USA) equipped with a head volume coil, using a T2*-weighted echo-planar imaging sequence. Whole volume data were acquired in contiguous 25 axial slices with 5 mm slice thickness. The voxel size of the image was 4 mm × 4 mm × 5 mm within a 256 mm × 256 mm field of view. The 60 volumes of image were acquired during each experimental session, with repetition time/echo time = 3000/35 ms.
To prevent excessive head movements, the participant’s head was fixed by placing foam pads on each side of the head within the head cage, and participants were instructed to constrain head movement as much as possible. Possible motion during the experiment was corrected using a realignment algorithm in the SPM99 routine [23,24]. A response was defined as an increase in the BOLD response equal to one voxel above a threshold T = 3.8 (i.e. P < 0.0001) during the hot water compared with the warm water. This high threshold was used to filter out artifacts in the BOLD response. The same threshold was used uniformly across participants at both the pretest and the posttest to provide valid interparticipant and intraparticipant comparisons of the significance of the signals evoked by the same intensity of the heat stimulus.
The images were transformed to the Montreal Neurological Institute space to spatially normalize them to the same coordinates. The areas studied were the left and right thalami, left and right anterior cingulate cortex, and prefrontal cortex (equivalent to Brodmann’s areas 9, 10, 11, 12), which were defined according to the Montreal Neurological Institute brain, using algorithms from routine SPM99 [24]. The central coordinates (mediolateral, anterior–posterior, and dorsoventral, in mm) for the different regions were the thalamus (±14, −18, 8), prefrontal cortex (0, 54, 16), anterior cingulate cortex (±4, 34, 26). The number of voxels subtended by each area were thalamus = 5900; prefrontal cortex = 39 951; anterior cingulate cortex = 1320; and total brain = 510 340. The data were the number and percent of voxels within each brain area studied that responded to pain. Regression was used to replace artifact or missing data in one session for three of the participants, two healthy controls and one long-term meditator. The data were analyzed by a two-way ANOVA, providing results for groups, trials, and the groups × trials interaction.
Psychophysical assessment
Immediately following each fMRI acquisition at both the pretest and the posttest, participants were given the Pain Visual Analog Scale [25]. The scale instructs the participant to ‘Please make a mark on the (10 cm) line below to indicate your experience somewhere in the range from ‘no pain’ to the ‘worst possible pain’. The score is the length of the line in centimeters from the left (low end) to the mark.
Results
The long-term practitioners of Transcendental Meditation did not significantly differ from the healthy controls on the Pain Visual Analog Scale rating of the degree of pain induced by the hot water, either at pretest, at posttest, or collapsed across trials. Both groups, however, decreased on pain ratings by 25% from pretesting to posttesting 5 months later (P’s<0.02). The groups × trials interaction for the Pain Visual Analog Scale was not significant, indicating that the groups changed in a similar way over the trial.
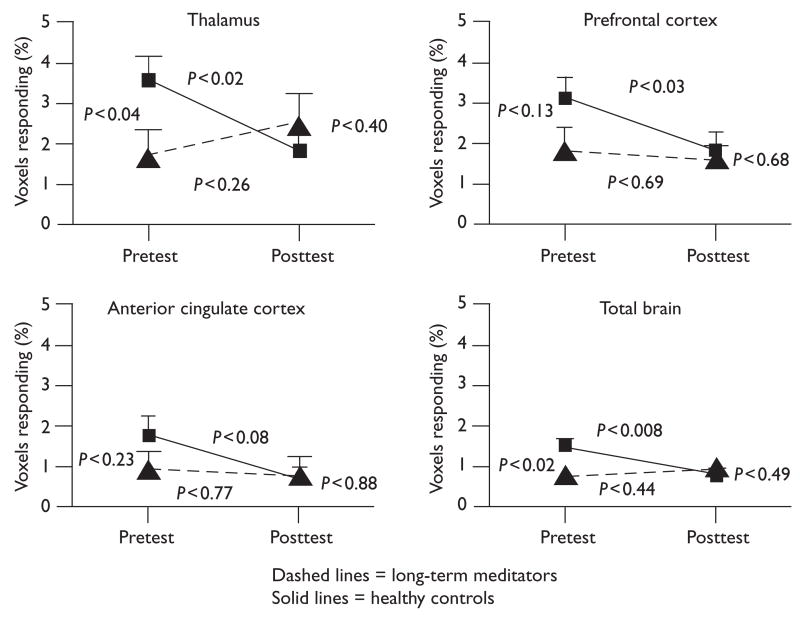
Despite the similarity in their participative ratings of the painfulness of the thermal stimulus, the two groups were quite different in their fMRI responses to it. At pretest, the long-term meditators showed a 40–50% lower response than the healthy controls in the brain regions studied. Moreover, after learning and practicing the Transcendental Meditation technique for 5 months, the brain response in the healthy controls then decreased by 40–50%, with no significant further change in the long-term meditators. The brain responses of the two groups at posttest did not statistically differ (see Fig. 1).
Fig. 1.
Group means with standard error bars of the percent functional magnetic resonance imaging voxels responding to the painful thermal stimulus in each brain region for the healthy controls and long-term meditators at pretest and 5 months later at posttest. At pretest, before the healthy controls had learned the Transcendental Meditation technique, the groups differed significantly in the thalamus and total brain, with a trend for the prefrontal cortex, but not for the anterior cingulate cortex. At posttest there was no significant difference between the groups on any variable. After the healthy controls learned to meditate and practiced it for 5 months, they decreased in the thalamus, prefrontal cortex, total brain, and anterior cingulate cortex (trend), whereas the long-term meditators did not change significantly on any variable.
No significant main effects exist for groups or trials. The group × trials interactions indicated that the healthy controls decreased more from pretest to posttest than the long-term meditators for the thalamus (P < 0.02) and total brain (P < 0.02), but with no significant difference for the prefrontal cortex (P < 0.2) or anterior cingulate cortex (P < 0.28).
Discussion
We hypothesized that the Transcendental Meditation program would reduce the brain’s response to pain because neuroimaging and autonomic studies indicate that it produces a state of psychophysiological quiescence [12], which over time resolves the physiological conditions underlying various kinds pain [13–16]. In time, it reduces trait anxiety [17], improves stress reactivity [12], and decreases distress from acute pain [18]. On the basis of the literature [1,2], these factors could be expected to reduce the response of the affective component of the pain matrix to acute pain, seen as reductions in the anterior cingulate cortex, prefrontal cortex, and thalamus.
All tests in this study were conducted outside the meditation period, not during it, and therefore distraction from pain by meditation (mechanism 1) is not relevant here. As the study was on acute laboratory pain rather than on chronic endogenous pain, resolution of the physiological sources of the pain (mechanism 2) is also not relevant. The study results are relevant to mechanism 3, reduced general arousal and anticipatory anxiety that may attenuate the pain response; and mechanism 4, decreased distress caused by pain.
We found that long-term meditators showed 40–50% less cerebral blood flow response to the painful thermal stimulus in the brain area studied than did healthy controls, but did not differ on their participative ratings of pain. Moreover, after learning the technique and practicing it for 5 months, the response of healthy controls then decreased by 40–50% compared with no significant change in the long-term participants, and the two groups did not differ significantly at posttest.
The above discrepancy between subjective reports of pain intensity and neural correlates of the pain response seems unusual, given that they usually covary [19]. It, however, accords with previous research on the Transcendental Meditation technique showing that practitioners’ sensory experience of pain is just as intense as controls, but that they are less distressed by it [18].
As the anterior cingulate cortex is involved in the affective/motivational dimension of the pain response [1,2,20], we were somewhat surprised that this area showed the least significant effects of meditation. It should be noted, however, that after the controls learned to meditate, their largest pretest to posttest change was for the anterior cingulate cortex (−59.5%). The large mean percent decrease, together with low statistical significance, indicates high variability among participants arising from considerable individual differences in how they responded. As individuals with different sensitivities to pain respond differently in the anterior cingulate cortex [19], future studies that use pain sensitivity as a grouping variable might help clarify the effect of meditation on this area.
Although self-selection of participants is a possible limitation of the study, replication with the longitudinal, partial crossover design, along with matching for age, sex, and interest in meditation, provides strong internal validity. In addition, it seems unlikely that the results can be explained by expectation because there is no suggestion in the Transcendental Meditation course that it would reduce pain. It also seems unlikely that the reduction in the total cerebral blood flow response could be attributed to a reduction in general cardiovascular response to pain [6], because previous research has shown that meditators do not show less of the heart rate response to noxious stimuli than controls [18]. We suggest that the reduced total brain response to pain can also be attributed to a general reduction in expectation anxiety and distress.
Conclusion
The Transcendental Meditation program appears to longitudinally reduce the brain’s response to acute pain along major sectors of the affective dimension of the pain matrix, apparently related to reduced distress, but with no reduction in the sensory experience of pain intensity [18]. This may help explain the reduction in stress reactivity and improvements in cardiovascular disease found to result from practice of this program. Future research could focus on its concomitant effects on endogenous endorphins, on other areas of the pain matrix, as well as cardiovascular and autonomic responses.
Acknowledgments
Sponsorship: This study was funded by NIH: National Center for Complementary and Alternative Medicine award #5-IP50-AT00082-05-Developmental Research component (CFDA #93.213).
The authors wish to thank Cindy and Barry Katz and Sibongile West of the Orange County and Los Angeles Transcendental Meditation Centers, and Drs Vernon Barnes, Ken Walton, and Laura Zambreanu for their fine editorial suggestions.
References
- 1.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin. 2000;5:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 2.Derbyshire SW. Exploring the pain ‘neuromatrix’. Curr Rev Pain. 2000;4:467–477. doi: 10.1007/s11916-000-0071-x. [DOI] [PubMed] [Google Scholar]
- 3.Orme-Johnson DW, Walton K, Lonsdorf N. Meditation in the treatment of chronic pain and insomnia, National Institutes of Health Technology Assessment Conference on Integration of Behavioral and Relaxation Approaches into the Treatment of Chronic Pain and Insomnia: Programs and Abstracts; National Institutes of Health, Bethesda, Maryland. 1995. pp. 27–32. [Google Scholar]
- 4.NIH Technology Assessment Panel. Report: integration of behavioral and relaxation approaches into the treatment of chronic pain and insomnia. JAMA. 1996;276:313–318. doi: 10.1001/jama.1996.03540040057033. [DOI] [PubMed] [Google Scholar]
- 5.Lou HC, Kjaer TW, Friberg L, Wildschiodtz G, Holmn S, Nowalk A. 15O-H2O PET study of meditation and the resting state of normal consciousness. Human Brain Map. 1999;7:98–105. doi: 10.1002/(SICI)1097-0193(1999)7:2<98::AID-HBM3>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11:1581–1585. [PubMed] [Google Scholar]
- 7.Newberg AB, Alavi A, Baime M, Pourdehnad M, Santnana J, D’Aquili EG. The measurement of regional cerebral blood flow during the complex cognitive task of meditation: a preliminary SPECT study. Psychiatr Res Neuroimaging. 2001;106:113–122. doi: 10.1016/s0925-4927(01)00074-9. [DOI] [PubMed] [Google Scholar]
- 8.Newberg AB, Travis F, Wintering TN, Nidich S, Alavi A, Schneider R. Cerebral glucose metabolic changes associated with a meditation based relaxation technique. Soc Nucl Med. 2006;47:314. [Google Scholar]
- 9.Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8:163–190. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- 10.Travis FT. Transcendental Meditation technique. In: Craighead WE, Nemeroff CB, editors. The Corsini encyclopedia of psychology and behavioral science. 3. New York: John Wiley & Sons; 2001. pp. 1705–1706. [Google Scholar]
- 11.Yamamoto S, Kitamura Y, Yamada N, Nakashima Y, Kuroda S. Medial prefrontal cortex and anterior cingulate cortex in the generation of alpha activity induced by Transcendental Meditation: a magneto-encephalographic study. Acta Med Okayama. 2006;60:51–58. doi: 10.18926/AMO/30752. [DOI] [PubMed] [Google Scholar]
- 12.Dillbeck MC, Orme-Johnson DW. Physiological difference between Transcendental Meditation and rest. Am Psychol. 1987;42:879–881. [Google Scholar]
- 13.Haratani T, Henmi T. Effects of Transcendental Meditation on health behavior of industrial workers. Jpn J Public Health. 1990;37:729. [Google Scholar]
- 14.Alexander CN, Swanson GC, Rainforth MV, Carlisle TW, Todd CC, Oates RM. Effects of the Transcendental Meditation program on stress reduction, health, and employee development: a prospective study in two occupational settings. Anxiety, Stress and Coping: Intern J. 1993;6:245–262. [Google Scholar]
- 15.Heidelberg R. Transzendentale meditation in der geburtshilflichen psychoprophylaxe: MD thesis, Medical Faculty, Free University of Berlin, 1979. In: Chalmers RA, Clements G, Schenkluhn H, Weinless M, editors. Scientific research on Maharishi’s Transcendental Meditation and TM-Sidhi program: Collected papers. Vol. 3. Vlodrop, The Netherlands: Maharishi Vedic University Press; 1989. pp. 1792–1814. [Google Scholar]
- 16.Orme-Johnson DW, Herron RE. An innovative approach to reducing medical care utilization and expenditures. Am J Manag Care. 1997;3:135–144. [PubMed] [Google Scholar]
- 17.Eppley KR, Abrams AI, Shear J. Differential effects of relaxation techniques on trait anxiety: a meta-analysis. J Clin Psychol. 1989;45:957–974. doi: 10.1002/1097-4679(198911)45:6<957::aid-jclp2270450622>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Mills WW, Farrow JT. The Transcendental Meditation technique and acute experimental pain. Psychosom Med. 1981;43:157–164. doi: 10.1097/00006842-198104000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Coghill RC, McHaffle JG, Yen Y-F. Neural correlates of individual differences in the subjective experience of pain. Proc Natl Acad Sci USA. 2003;100:8538–8542. doi: 10.1073/pnas.1430684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 21.Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 23.Cho ZH, Son YD, Kang C, Han J, Wong E, Bai S. Pain dynamics observed by functional magnetic resonance imaging: differential regression analysis technique. J Magn Reson Imaging. 2003;18:273–283. doi: 10.1002/jmri.10368. [DOI] [PubMed] [Google Scholar]
- 24.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labelling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single subject brain. Neuroimage. 2002;1:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 25.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56:217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]