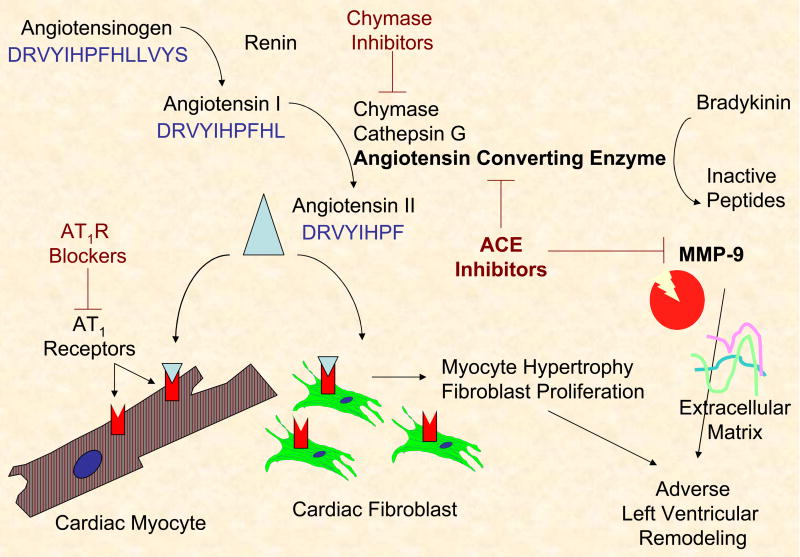
Since the early 1990’s, angiotensin converting enzyme (ACE) inhibitors have been used clinically to improve survival and reduce adverse left ventricular (LV) remodeling following myocardial infarction (MI). Seminal studies performed by the Pfeffer laboratory clearly demonstrated a survival benefit post-MI for both rats and humans treated with ACE inhibitors.[1, 2] Several avenues of research have since sprouted to better understand the underlying mechanisms behind this benefit. Patten and colleagues demonstrated that both the ACE inhibitor enalapril and the angiotensin II type I receptor inhibitor losartan prevented LV hypertrophy and decreased collagen I gene expression compared to placebo-treated controls, suggesting that these events signaled through the angiotensin II type I receptor.[3] Yoshiyama and colleagues then showed that, in mice deficient for the angiotensin type I receptor, treatment with an ACE inhibitor prevented remodeling post-MI, indicating that ACE inhibitors also block angiotensin receptor-independent remodeling pathways.[4] In this issue, Dr. Yamamoto and colleagues examined the ability of the ACE inhibitor imidapril to directly bind to the matrix metalloproteinase MMP-9.[5] The importance of these findings is that ACE inhibition may also inhibit MMP-9, which provides a common link between strategies to prevent adverse remodeling post-MI (Figure 1). Adverse remodeling post-MI leading to congestive heart failure remains a leading cause of long-term morbidity and mortality.[6]
Figure 1.
Schematic demonstrating the relationship between angiotensin II, matrix metalloproteinase-9 (MMP-9), and angiotensin converting enzyme (ACE) inhibitors in preventing adverse LV remodeling.
ACE and MMP-9 are both zinc-dependent endopeptidases, both stimulate remodeling post-MI, and both process angiotensin I to form angiotensin II.[7] Matrix metalloproteinases are a family of 25 proteolytic enzymes defined by their ability to cleave individual extracellular matrix (ECM) components. MMP-9, also known as gelatinase B, processes multiple extracellular substrates, including denatured collagen I, fibronectin, and laminin.[8] Adding another layer of complexity, MMP-9 also cleaves non-ECM components, including cytokines and growth factors, to regulate multiple cell functions.[9] MMP-9 is inhibited in the tissue by binding one of four tissue inhibitors of metalloproteinases (TIMPs). Of the 4 TIMPs identified to date, TIMP-1 binds MMP-9 with greatest affinity.[10] MMPs have assigned roles in many cardiovascular diseases, including atherosclerosis, myocardial infarction, and heart failure[11]. Ample evidence has demonstrated a direct cause and effect relationship between MMP-9 and LV remodeling: 1) MMP-9 levels increase early post-MI[12], 2) MMP inhibition improves post-MI outcomes[13], and 3) MMP-9 gene deletion reduces remodeling and stimulates post-MI angiogenesis.[14] Furthermore, Blankenberg and colleagues have shown that MMP-9 is also a novel predictor of cardiovascular mortality, as patients with coronary artery disease who had the highest MMP-9 levels at baseline showed the greatest cardiovascular mortality rates at follow-up.[15] While global non-selective MMP inhibition studies have not fared well, strategies that specifically limit MMP-9 activity may prove beneficial.
Dr. Yamamoto and colleagues have previously published their prediction that, based on modeling studies, lisinopril, another ACE inhibitor, directly binds to MMP-9.[16] The S1′ substrate recognition site on MMP-9 forms a deep hydrophobic pocket that is compatible with the hydrophobic moieties present in ACE inhibitors. For example, both lisinopril and imidapril have a phenyl ethyl group that will bind to this hydrophobic pocket. The current study builds on this past report by adding imidapril to the list of ACE inhibitors that bind MMP-9 and also be providing additional information on the predicted complex structures. Differential binding affinities were seen for the two inhibitors, indicating that different ACE inhibitors will inhibit MMP-9 to different degrees. Lisinopril was shown to be stabilized in the active site of MMP-9 by specific hydrogen bonds and hydrophobic interactions.[16] Specifically, the hydrophobic group in lisinopril interacted preferentially with the S1 site, compared to the S1′ site. The fact that imidapril binds with greater affinity than lisinopril indicates that hydrophobic interactions with the S1 site of MMP-9 may be important for enhancing the inhibitory affect of the ACE inhibitor. This detail may be useful for the design of ACE inhibitors with increased affinity for MMP-9. Extending the earlier observational binding studies, the authors also demonstrated that use of imidapril in a unique hamster MI model resulted in decreased MMP-9 levels (both pro and active) and improved function.
These results are intriguing and raise new questions to be answered in future studies. First, it will be important to determine how selective and specific ACE inhibitors are for MMP-9. We will need to evaluate whether ACE inhibitors also bind any of the other 24 MMPs, whether ACE inhibitors are able to bind members of other metalloproteinase families, and whether MMP inhibitors can block ACE activity. The MMP-9 hydrophobic pocket is smaller than the pockets of other MMPs, indicating that selectivity may be achievable.
Second, further study is needed to better understand the multiple overlapping roles of ACE in the post-MI left ventricle. This effort is complicated by the fact that chymase also increases in the infarct region, chymase generates angiotensin II, and chymase inhibition also improves post-MI outcomes.[17, 18] Chymase-dependent angiotensin II formation has been reported to account for over 90% of total myocardial levels, although chymase levels do vary among species.[19] Chymase from human, dog, and hamster all hydrolyze the Phe8-His9 bond of angiotensin I to efficiently produce angiotensin II.[20] In contrast, rodent chymase cleaves angiotensin I at the Tyr4-Ile5 bond to generate inactive angiotensin fragments.[20] Also, mast cells are the major source of chymase, and the fact that rodents have very few mast cells in the left ventricle further defines species differences.[21] Chymase has been shown to process MMP-9 to its active form[22], indicating that the connection between angiotensin II and MMP-9 is further complicated. Studies evaluating ACE and MMP-9 connections will also need to take into account chymase activity.
Third, the effects of ACE inhibitors on blocking MMP-9 function at different, longer time points post-MI need investigation. Yamamoto and colleagues only evaluated effects at day 1 post-MI, when most of the MMP-9 in the infarcted myocardium is derived from infiltrated polymorphonuclear leukocytes.[12] It would be interesting to determine effects at day 3 through 7, when fibroblast, macrophage, and endothelial cell-derived MMP-9 likely play important roles in post-MI remodeling. In addition, Yamamoto et al showed that ACE expression did not increase until day 3 and remained elevated at day 7. Therefore, we do not know whether inhibiting both ACE and MMP-9 when both are elevated would show additive benefits, or whether there would be competitive binding of the two enzymes. Evaluating effects of the ACE inhibitor when both MMP-9 and ACE levels are high would indicate the relative in vivo affinities of the inhibitor for the different enzymes.
In conclusion, the findings that ACE inhibitors are able to bind directly to MMP-9 and that the ACE inhibitor imidapril decreases MMP-9 levels in vivo following MI emphasize the potential non-specific and non-selective nature of ACE inhibitors. It is important to consider both ACE and MMP systems when evaluating LV remodeling and when designing new drugs that target either or both systems. A novel therapeutic strategy may be to use currently available ACE inhibitors as the backbone on which to design new drugs that also target MMP-9.
Acknowledgments
The authors acknowledge support from the NSF (0649172-YJ, 0602834 and CAREER award 0644646- HCH) and the NIH (R01 HL-75360- MLL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pfeffer MA, Pfeffer JM, Steinberg C, Finn P. Survival after an experimental myocardial infarction: beneficial effects of long-term therapy with captopril. Circulation. 1985;72:406–12. doi: 10.1161/01.cir.72.2.406. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–7. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 3.Patten RD, Aronovitz MJ, Einstein M, Lambert M, Pandian NG, Mendelsohn ME, et al. Effects of angiotensin II receptor blockade versus angiotensin-converting-enzyme inhibition on ventricular remodelling following myocardial infarction in the mouse. Clin Sci (Lond) 2003;104:109–18. doi: 10.1042/CS20020219. [DOI] [PubMed] [Google Scholar]
- 4.Yoshiyama M, Nakamura Y, Omura T, Izumi Y, Matsumoto R, Oda S, et al. Angiotensin converting enzyme inhibitor prevents left ventricular remodelling after myocardial infarction in angiotensin II type 1 receptor knockout mice. Heart. 2005;91:1080–5. doi: 10.1136/hrt.2004.035618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto D, Takai S, Jin D, Inagaki S, Tanaka K, Miyazaki M. Molecular mechanism of imidapril for cardiovascular protection via inhibition of MMP-9. J Mol Cell Cardiol. 2007 doi: 10.1016/j.yjmcc.2007.08.002. in press. [DOI] [PubMed] [Google Scholar]
- 6.Azevedo CF, Cheng S, Lima JA. Cardiac imaging to identify patients at risk for developing heart failure after myocardial infarction. Curr Heart Fail Rep. 2005;2:183–8. doi: 10.1007/BF02696648. [DOI] [PubMed] [Google Scholar]
- 7.Diekmann O, Tschesche H. Degradation of kinins, angiotensins and substance P by polymorphonuclear matrix metalloproteinases MMP 8 and MMP 9. Braz J Med Biol Res. 1994;27:1865–1876. [PubMed] [Google Scholar]
- 8.Lindsey ML. Novel strategies to delineate matrix metalloproteinase (MMP)-substrate relationships and identify targets to block MMP activity. Mini Rev Med Chem. 2006;6:1243–8. doi: 10.2174/138955706778742777. [DOI] [PubMed] [Google Scholar]
- 9.Lindsey ML. MMP induction and inhibition in myocardial infarction. Heart Fail Rev. 2004;9:7–19. doi: 10.1023/B:HREV.0000011390.44039.b7. [DOI] [PubMed] [Google Scholar]
- 10.Olson MW, Gervasi DC, Mobashery S, Fridman R. Kinetic Analysis of the Binding of Human Matrix Metalloproteinase-2 and -9 to Tissue Inhibitor of Metalloproteinase (TIMP)-1 and TIMP-2. J of Biological Chemistry. 1997;272:29975–29983. doi: 10.1074/jbc.272.47.29975. [DOI] [PubMed] [Google Scholar]
- 11.Lindsey ML, Mann DL, Entman ML, Spinale FG. Extracellular matrix remodeling following myocardial injury. Ann Med. 2003;35:316 – 326. doi: 10.1080/07853890310001285. [DOI] [PubMed] [Google Scholar]
- 12.Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, et al. Matrix-Dependent Mechanism of Neutrophil-Mediated Release and Activation of Matrix Metalloproteinase 9 in Myocardial Ischemia/Reperfusion. Circulation. 2001;103:2181–2187. doi: 10.1161/01.cir.103.17.2181. [DOI] [PubMed] [Google Scholar]
- 13.Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, et al. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;15:3063–3070. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 14.Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, et al. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;290:H232–239. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- 15.Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, et al. Plasma Concentrations and Genetic Variation of Matrix Metalloproteinase 9 and Prognosis of Patients With Cardiovascular Disease. Circulation. 2003;107:1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto D, Takai S, Miyazaki M. Prediction of interaction mode between a typical ACE inhibitor and MMP-9 active site. Biochemical and Biophysical Research Communications. 2007;354:981–984. doi: 10.1016/j.bbrc.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 17.Jin D, Takai S, Yamada M, Sakaguchi M, Yao Y, Miyazaki M. Possible roles of cardiac chymase after myocardial infarction in hamster hearts. Jpn J Pharmacol. 2001;86:203–14. doi: 10.1254/jjp.86.203. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto T, Wada A, Tsutamoto T, Ohnishi M, Isono T, Kinoshita M. Chymase Inhibition Prevents Cardiac Fibrosis and Improves Diastolic Dysfunction in the Progression of Heart Failure. Circulation. 2003;107:2555–2558. doi: 10.1161/01.CIR.0000074041.81728.79. [DOI] [PubMed] [Google Scholar]
- 19.Akasu M, Urata H, Kinoshita A, Sasaguri M, Ideishi M, Arakawa K. Differences in Tissue Angiotensin II-Forming Pathways by Species and Organs In Vitro. Hypertension. 1998;32:514–520. doi: 10.1161/01.hyp.32.3.514. [DOI] [PubMed] [Google Scholar]
- 20.Muller DN, Fischli W, Clozel J-P, Hilgers KF, Bohlender J, Menard J, et al. Local Angiotensin II Generation in the Rat Heart. Circ Res. 1998;82:13–20. doi: 10.1161/01.res.82.1.13. [DOI] [PubMed] [Google Scholar]
- 21.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, et al. Of Mice and Dogs: Species-Specific Differences in the Inflammatory Response Following Myocardial Infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tchougounova E, Lundequist A, Fajardo I, Winberg J-O, Abrink M, Pejler G. A Key Role for Mast Cell Chymase in the Activation of Pro-matrix Metalloprotease-9 and Pro-matrix Metalloprotease-2. J Biol Chem. 2005;280:9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]