Abstract
In this study, long-term (90-day) hemocompatibility and end-organ effects of a centrifugal left ventricular assist device (the Heartware HVAD™) were evaluated in 6 healthy sheep. The device was implanted into the left ventricular apex on beating hearts. The outflow graft of each device was anastomosed to the descending aorta. None of the sheep received anticoagulation or antiaggregation medication during the study.
Hematologic and biochemical tests of liver and kidney function were performed pre-operatively (baseline) and throughout the study. Data associated with pump function were collected continuously until 90 ± 1 days of support, at which time the sheep were humanely killed, and the end-organs were examined macroscopically and histopathologically.
Hematologic and biochemical test results were within normal limits during the study period. There were no significant complications. Postmortem examination of the explanted organs revealed no evidence of ischemia or infarction, except in 2 sheep, in which small foci of infarction were detected in each of their left kidneys. There was no significant device failure. In all sheep, the pump's inflow and outflow conduits were free of thrombus.
During the 90-day study, the HeartWare HVAD showed exceptional hemocompatibility and reliability, both of which are crucial to the clinical success of any implantable left ventricular assist device.
Key words: Animals; assisted circulation/instrumentation; blood pump, centrifugal; equipment design; heart assist device, left ventricular; hemodynamic processes; heparin; sheep; thrombosis/prevention & control
Implantable left ventricular assist devices (LVADs) were developed because of the limitations of medical therapy and surgical interventions, and the shortage of donor hearts.1–7 Researchers have been working on this technology since the early 1960s, and LVADs have become invaluable as a bridge to heart transplantation, for myocardial recovery, and as destination therapy.3–5,8–12
Despite clinical success, the use of 1st-generation pulsatile LVAD systems has been limited by their large size and lack of reliability. In 2nd-generation LVAD systems, continuous axial-flow pumps have been incorporated; this improved technology allows assist devices to be smaller and eliminates most moving parts. Clinical trials involving the newer systems have shown encouraging results,13 with lower rates of mechanical failure and other complications.14–19 However, the drawback of these 2nd-generation axial-flow pumps is the potential for the mechanical bearings to wear out and to cause clots.20–23 Third-generation implantable LVAD designs have eliminated the bearings; instead, these devices suspend the impeller with magnetic or hydrodynamic suspension systems.
The HeartWare HVAD™ (HeartWare, Inc.; Miramar, Fla), the smallest, 3rd-generation, implantable LVAD, is a hydrodynamic centrifugal pump with a short inflow cannula that is integrated with the device. The cannula design and the device's small size facilitate intrapericardial placement, thereby eliminating the need for a concomitant abdominal approach and for device pockets. In addition, the simple, “wearless” suspension of the impeller is designed to substantially reduce the risk of mechanical failure. Most LVADs require partial or total cardiopulmonary bypass support for left ventricular (LV) apex cannulation, which substantially increases surgical time and can further compromise cardiac performance in patients who have acute heart failure.4,10,20 In contrast, assist devices such as the HeartWare HVAD can be implanted on beating hearts, which greatly shortens the surgical time and avoids the deleterious effects of cardiopulmonary bypass. We performed this study in order to evaluate the hemocompatibility and end-organ effects of the HeartWare HVAD for up to 90 days in an ovine model.
Materials and Methods
Animal Model. Six healthy sheep that weighed between 57 and 65 kg were used in the study. The sheep received humane care in compliance with the Principles of Laboratory Animal Care (National Society of Medical Research) and the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication No. 85-23, revised 1996). The study protocol was approved by our Institutional Animal Care and Use Committee.
The Device. The HeartWare HVAD is a small, wearless, centrifugal blood pump (Fig. 1). It has a displaced volume of just 45 cc, weighs 145 g, and can deliver flows up to 10 L/min (Fig. 2). When the device is placed, the short, integrated inflow cannula is inserted into the LV apex, and its preattached outflow graft is connected to the aorta. The device's fatigue-resistant percutaneous driveline (cable) is 3 mm in diameter and extends from the pump through the skin to an external controller.
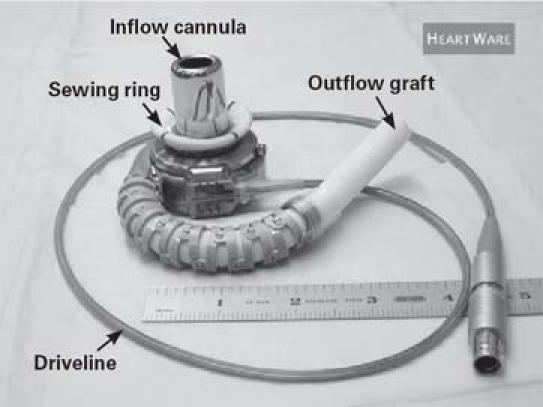
Fig. 1 The HeartWare HVAD left ventricular assist device.
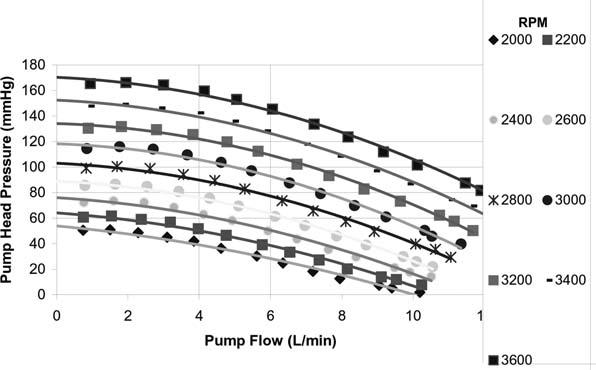
Fig. 2 The HeartWare HVAD pump flow rate as a function of speed and pump head pressure.
The HVAD uses a wide-blade impeller that is designed to achieve optimal performance and hemocompatibility, size minimization, long-term reliability, and overall system efficiency. The impeller—the device's only moving part—is suspended in place by a proprietary hybrid magnetic and hydrodynamic bearing system to avoid mechanical contact and wear. This design integrates 2 motor stators for single-motor fault protection to increase reliability. In addition, the driveline (modeled after pacemaker technology) contains cables from each stator. These features help to ensure that a partial driveline break will not disrupt device operation, which improves the durability of the system. The wearless impeller suspension system uses a passive magnetic bearing for radial stiffness. Axial magnetic preload and hydrodynamic thrust bearings on top of each impeller blade provide axial constraint. The magnetic bearing consists of a stack of rare-earth ring magnets, near the impeller's inside diameter, that repel the magnetic force of a similar stack of magnets inside the center post. The axial alignment of the center-post magnet stack is set to provide an axial force that pushes the impeller toward the forward housing (the assembly with the inflow cannula). Physical contact between the housing and the impeller is prevented by a thin blood film generated by the hydrodynamic thrust bearings. The thrust bearings feature a shrouded design that is intended to maximize the blood-film thickness and improve surface washing, in order to reduce the risk of thrombosis.
The HeartWare HVAD sewing ring comprises a titanium frame surrounded by a polyester-covered felt ring, which is used to secure the inflow cannula to the myocardium. The inflow cannula is secured to the sewing ring with a uniquely designed screw (Fig. 3).
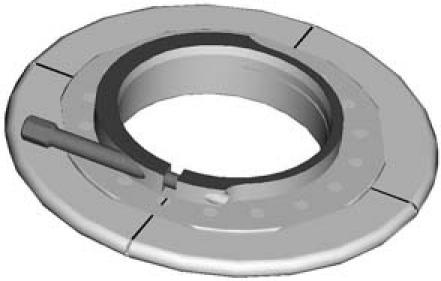
Fig. 3 The HeartWare HVAD titanium sewing ring.
The external controller is a microprocessor unit that controls the operation of the device. The controller sends power and operating signals to the blood pump and collects information from the pump. The percutaneous driveline is connected to the controller, which is connected to 2 power sources for redundancy—either 2 rechargeable batteries, or an AC or DC adapter and a rechargeable battery. An internal, nonreplaceable, rechargeable battery inside the controller powers an audible “No Power” alarm. A data port serves as an interface between the controller and the monitor.
Anesthesia and Surgical Preparation. A standard institutional anesthesia protocol was followed. Each sheep was premedicated with glycopyrrolate (0.02 mg/kg) and xylazine (0.2–0.7 mg/kg); both drugs were administered intramuscularly. Intravenous ketamine (10–20 mg/kg) was administered to induce anesthesia. General anesthesia was maintained with isoflurane (1%–3%) in oxygen (40%–100%).
Surgical Technique. Each sheep was placed in the right lateral decubitus position for a left thoracotomy and left neck cutdown. A lidocaine drip (2 mg/min) was initiated to prevent arrhythmias, and intravenous pancuronium bromide (3.7 mg) was administered as a muscle relaxant. A left thoracotomy was performed at the 5th intercostal space, and the 5th rib was removed. The left internal thoracic artery (LITA) was exposed. An arterial pressure line was then introduced. The pump's driveline and 2 flow probe lines were tunneled to exit near the left paraspinal area at the 8th intercostal space. The pericardium was incised from the apex to the pulmonary artery, and the heart was suspended in a pericardial cradle. A bolus of bovine heparin was administered (3 mg/kg).
The descending thoracic aorta was dissected for outflow graft anastomosis, and a partial occlusion clamp wasapplied. The pump's 10-mm outflow graft was sewn end-to-side to the descending aorta with 4–0 Prolene sutures. The sewing ring was attached to the LV apex with 10 to 12 interrupted 2–0 Ethibond pledgeted sutures (Ethicon, Inc., a Johnson & Johnson company; Somerville, NJ). A cruciform incision was made on the beating heart. Then a conical coring knife with obturator was advanced through the ventricular sewing ring, the LV core was removed, and the pump was inserted into the LV cavity and secured. The pump was de-aired and started at 1,800 rpm; pump speed was increased to 2,400 rpm as the occlusion clamp was removed from the outflow graft. The arterial pressure tubing line was transferred from the LITA to the left carotid artery for postoperative aortic pressure measurement. A 10-mm ultrasonic flow probe (Transonic Systems Inc.; Ithaca, NY) was attached to the outflow graft. Protamine was then administered to reverse the effects of heparin. One chest tube was inserted into the pleural cavity. After intercostal nerve block and placement of a pain-prevention device (ON-Q® Soaker Catheter™, I-Flow Corporation; Lake Forest, Calif), the chest cavity was closed with use of a standard technique.
Postoperative Care. The sheep were taken to the intensive care unit for continuous monitoring of aortic pressures and pump flows, and for detection of any sign of distress or pain. Within 4 hours of extubation, the sheep were allowed to eat. None of the sheep received anticoagulation or antiaggregation therapy during the study. A general physical examination to evaluate appetite, infection, and neurologic status was performed daily.
Preoperative and Postoperative Data Collection. Routine hematologic and biochemical tests were performed preoperatively to measure baseline levels. These tests were repeated daily for 2 weeks and then weekly until study termination. Pump operating parameters (fixed-rate rotational speed setting and pump flow) were recorded continuously with the ultrasonic 10-mm flow probe.
Macroscopic Postexplantation Analyses. Heparin (3 mg/kg) was administered—just before the sheep were humanely killed—in order to prevent postmortem clot formation in the pump. In each case, the HeartWare HVAD was explanted, opened, and photographed. Any infarcts or focal lesions in any tissue were noted during gross evaluation. The pump housing and impeller were carefully inspected for fibrin formation or thrombus. The inflow and outflow grafts, the aorta, and the ventricle at each cannulation site were also evaluated and photographed.
Histopathologic Evaluation. Routine histologic examination was performed on the heart, lungs, liver, kidneys, brain, spleen, and other major organs. Blocks of tissue were immersion-fixed in 10% neutral-buffered formalin with a tissue-to-volume fixative ratio of 1:10. After at least 72 hours of fixation (the brain requires 10 to 14 days to ensure a constant sampling pattern), an illustration of the sectioning site was made for each device. Cross-sections of the soft-tissue interface with the device were processed with standard paraffin. Two 5-micron-thick sections from each of the sampled regions were stained with either hematoxylin-eosin or Masson trichrome stain.
Results
Animals. All 6 sheep reached the study endpoint of 90 ± 1 days of support with the HeartWare HVAD system. Postoperatively, they recovered from anesthesia without complications and were extubated within the first 3 hours. None of the sheep developed anorexia, infection, or neurologic disorders.
Device. No notable device-related problems occurred after device implantation. Average pump data values ± SD for all 6 sheep included an average combined rpm of 2,836 ± 50, average flows of 3.83 ± 0.6 L/min, and average power consumption of 4.5 ± 0.5 watts.
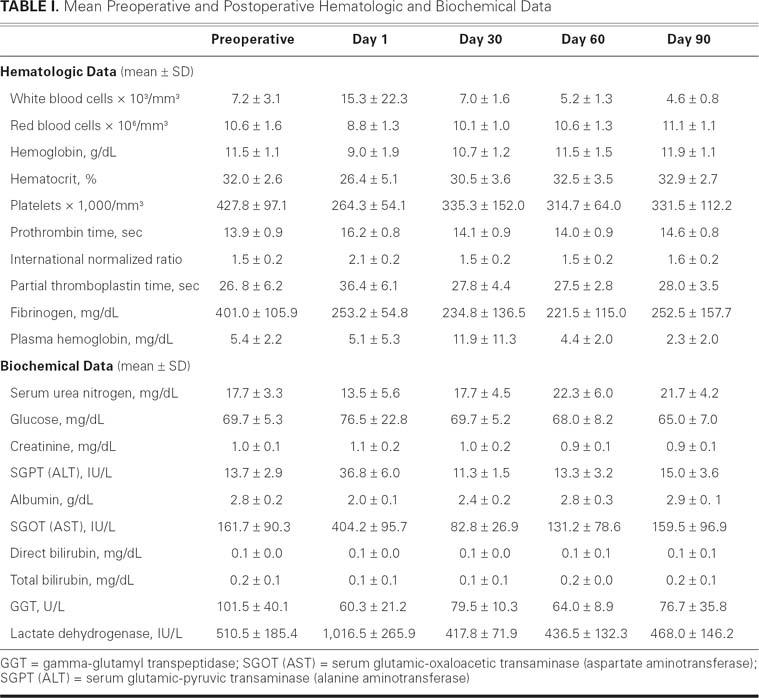
Clinical Chemistry and Hematologic Data. Values at baseline and at postoperative days 1, 30, 60, and 90 are shown in Table I. All sheep had white blood cell (WBC) counts within normal ranges during the study, except for 1 sheep that had an increased WBC count during the immediate postoperative period; this sheep's WBC returned to normal within 5 days of surgery. Expected decreases in red blood cells and hematocrit were noted; however, on average, these values increased again and remained stable throughout the study. The remaining hematologic values were within normal limits for all sheep throughout the study, as were the coagulation profiles. Clinical biochemistry values indicated normal liver and renal function; these values remained unremarkable throughout the study. Both lactate dehydrogenase and plasma-free hemoglobin levels increased transiently in 1 sheep after surgery, but these levels returned to normal ranges approximately 14 days after device implantation.
Table I. Mean Preoperative and Postoperative Hematologic and Biochemical Data
Macroscopic and Histologic Examination. Necropsy of 2 animals' explanted organs revealed mild-to-moderate fibrosis in the renal cortex; the remaining sheep showed minimal changes. One sheep had scattered intramyocardial fibrosis. Notably, the renal and myocardial findings revealed no active component in this animal and were indicative of a remote event during the perioperative or early postoperative period, or both.
Gross examination of the pump inflow conduits from all sheep showed nothing remarkable; however, 1 pump had thromboembolic fragments adjacent to the impeller housing. The thromboemboli appeared to have been dislodged from the adjacent myocardium (posteroseptal region) and not from the pump itself. Histologic analysis revealed that the thrombi consisted of spindle-shaped cells, macrophages, and loose connective tissue, which suggested the endocardium as the original point of attachment. The pump interiors were completely free of thrombus, and the impeller surface from 5 sheep showed no evidence of mural thrombosis (Fig. 4). Gross examination of the outflow grafts yielded no unusual results.
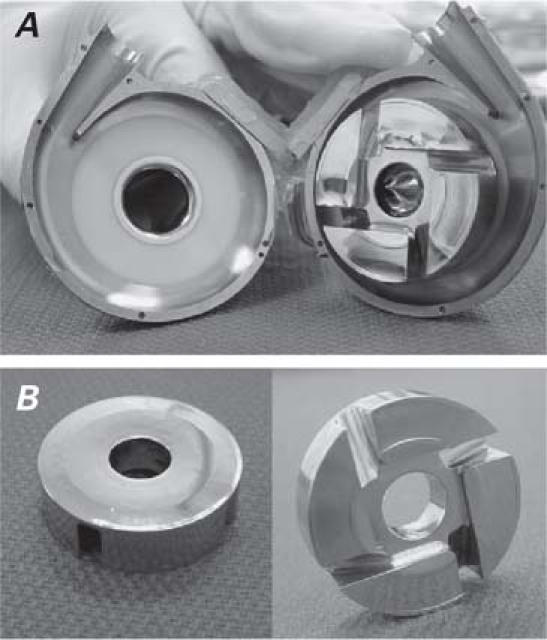
Fig. 4 A, B) The HeartWare HVAD after 90 days of support: interior surfaces (left) and impeller surfaces (right) are clean.
Discussion
The HeartWare HVAD system demonstrated appropriate blood-handling characteristics and reliability in the ovine model for 90 days. In most 2nd-generation LVADs, heat generation on the bearings is known to denaturize proteins and can lead to thromboembolism and hemolysis.8,24–28 Therefore, patients who receive these pumps require anticoagulation, which may lead to bleeding complications.24–28 Anticoagulation or antiaggregation medication was not used in our study; nevertheless, the inside surfaces of the HeartWare pumps remained completely free of thrombus. Only 1 pump contained fragments of thromboemboli adjacent to the impeller housing, and the origin of the thromboemboli was found to be the adjacent myocardium rather than the pump. In this particular sheep, the pump inlet had deviated toward the posterior wall of the left ventricle, which impaired endocardial integrity and may have resulted in the thrombus formation. In the future, low-dose or no-anticoagulation therapy after HeartWare HVAD implantation may be considered as a treatment option to decrease bleeding complications caused by anticoagulation use.
In many cases, hemolysis after LVAD implantation can be problematic and can threaten clinical success. However, the smaller size, short inlet cannula, and hydraulic, bearingless impeller mechanism of the HeartWare HVAD reduce the risk of hemolysis and thrombogenicity.29–32 In our study, none of the sheep had notable hemolysis during the 90-day follow-up period.
Insufficient perfusion or thromboembolic damage to end-organs significantly influences survival in LVAD patients, whose end-organs are already compromised from their end-stage heart failure.8 In our study of the HeartWare HVAD in a healthy ovine model, the biochemical test results for the 90-day period showed no notable impairment of renal and hepatic function. In addition, no histologic changes were observed in major end-organs.
Finally, most LVADs require partial or total cardiopulmonary bypass support for LV apex cannulation, which increases the surgical time and risk and can further compromise ventricular function in patients who have acute heart failure.4,10,20 In our study, all HeartWare pumps were implanted with the heart beating. Use of this technique shortened the surgical time and prevented the deleterious effects of cardiopulmonary bypass, as noted in recent publications from our institution.33,34
In summary, in our ovine model, the HeartWare HVAD system demonstrated excellent blood-handling characteristics and reliability for 90 days, both of which are crucial to the clinical success of any implantable LVAD.
Footnotes
Address for reprints: Egemen Tuzun, MD, Cardiovascular Surgical Research Laboratories, Texas Heart Institute, MC 1-268, P.O. Box 20345, Houston, TX 77225-0345. E-mail: etuzun@heart.thi.tmc.edu
References
- 1.Dembitsky WP, Tector AJ, Park S, Moskowitz AJ, Gelijns AC, Ronan NS, et al. Left ventricular assist device performance with long-term circulatory support: lessons from the REMATCH trial. Ann Thorac Surg 2004;78:2123–30. [DOI] [PubMed]
- 2.Digiorgi PL, Reel MS, Thornton B, Burton E, Naka Y, Oz MC. Heart transplant and left ventricular assist device costs. J Heart Lung Transplant 2005;24:200–4. [DOI] [PubMed]
- 3.Radovancevic B, Vrtovec B, Frazier OH. Left ventricular assist devices: an alternative to medical therapy for end-stage heart failure. Curr Opin Cardiol 2003;18:210–4. [DOI] [PubMed]
- 4.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med 2001; 345:1435–43. [DOI] [PubMed]
- 5.Schmid C, Welp H, Klotz S, Baba HA, Wilhelm MJ, Scheld HH. Outcome of patients surviving to heart transplantation after being mechanically bridged for more than 100 days. J Heart Lung Transplant 2003;22:1054–8. [DOI] [PubMed]
- 6.Taylor DO, Edwards LB, Boucek MM, Trulock EP, Keck BM, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty-first official adult heart transplant report–2004. J Heart Lung Transplant 2004; 23:796–803. [DOI] [PubMed]
- 7.Wohlschlaeger J, Schmitz KJ, Schmid C, Schmid KW, Keul P, Takeda A, et al. Reverse remodeling following insertion of left ventricular assist devices (LVAD): a review of the morphological and molecular changes. Cardiovasc Res 2005;68:376–86. [DOI] [PubMed]
- 8.Birks EJ, Tansley PD, Yacoub MH, Bowles CT, Hipkin M, Hardy J, et al. Incidence and clinical management of life-threatening left ventricular assist device failure. J Heart Lung Transplant 2004;23:964–9. [DOI] [PubMed]
- 9.Dang NC, Topkara VK, Kim BT, Mercando ML, Kay J, Naka Y. Clinical outcomes in patients with chronic congestive heart failure who undergo left ventricular assist device implantation. J Thorac Cardiovasc Surg 2005;130:1302–9. [DOI] [PubMed]
- 10.Haddad M, Lam K, Hendry P, Mesana T, Davies R. Left ventricular assist devices for the treatment of congestive heart failure. Curr Treat Options Cardiovasc Med 2005;7:47–54. [DOI] [PubMed]
- 11.Westaby S. Ventricular assist devices as destination therapy. Surg Clin North Am 2004;84:91–123. [DOI] [PubMed]
- 12.Westaby S, Narula J. Surgical options in heart failure. Surg Clin North Am 2004;84:xv–xix. [DOI] [PubMed]
- 13.Siegenthaler MP, Westaby S, Frazier OH, Martin J, Banning A, Robson D, et al. Advanced heart failure: feasibility study of long-term continuous axial flow pump support. Eur Heart J 2005;26:1031–8. [DOI] [PubMed]
- 14.Chinn R, Dembitsky W, Eaton L, Chillcott S, Stahovich M, Rasmusson B, Pagani F. Multicenter experience: prevention and management of left ventricular assist device infections. ASAIO J 2005;51:461–70. [DOI] [PubMed]
- 15.Eckhauser AE, Melvin WV, Sharp KW. Management of general surgical problems in patients with left ventricular assist devices. Am Surg 2006;72:158–61. [PubMed]
- 16.Frazier OH. The development of an implantable, portable, electrically powered left ventricular assist device. Semin Thorac Cardiovasc Surg 1994;6:181–7. [PubMed]
- 17.Frazier OH. Outpatient LVAD: its time has arrived. Ann Thorac Surg 1994;58:1309–10. [DOI] [PubMed]
- 18.Martin J, Friesewinkel O, Benk C, Sorg S, Schultz S, Beyersdorf F. Improved durability of the HeartMate XVE left ventricular assist device provides safe mechanical support up to 1 year but is associated with high risk of device failure in the second year. J Heart Lung Transplant 2006;25:384–90. [DOI] [PubMed]
- 19.Simon D, Fischer S, Grossman A, Downer C, Hota B, Heroux A, Trenholme G. Left ventricular assist device-related infection: treatment and outcome. Clin Infect Dis 2005;40:1108–15. [DOI] [PubMed]
- 20.Frazier OH, Myers TJ, Westaby S, Gregoric ID. Clinical experience with an implantable, intracardiac, continuous flow circulatory support device: physiologic implications and their relationship to patient selection. Ann Thorac Surg 2004;77: 133–42. [DOI] [PubMed]
- 21.Radovancevic B, Gregoric ID, Tamez D, Vrtovec B, Tuzun E, Chee HK, et al. Biventricular support with the Jarvik 2000 axial flow pump: a feasibility study. ASAIO J 2003;49:604–7. [DOI] [PubMed]
- 22.Saito S, Nishinaka T, Westaby S. Hemodynamics of chronic nonpulsatile flow: implications for LVAD development. Surg Clin North Am 2004;84:61–74. [DOI] [PubMed]
- 23.Saito S, Westaby S, Piggot D, Dudnikov S, Robson D, Catarino PA, et al. End-organ function during chronic nonpulsatile circulation. Ann Thorac Surg 2002;74:1080–5. [DOI] [PubMed]
- 24.Horton SC, Khodaverdian R, Powers A, Revenaugh J, Renlund DG, Moore SA, et al. Left ventricular assist device mal-function: a systematic approach to diagnosis. J Am Coll Cardiol 2004;43:1574–83. [DOI] [PubMed]
- 25.Kirklin JK, Holman WL. Mechanical circulatory support therapy as a bridge to transplant or recovery (new advances). Curr Opin Cardiol 2006;21:120–6. [DOI] [PubMed]
- 26.Nakata K, Yoshikawa M, Takano T, Maeda T, Nonaka K, Linneweber J, et al. Antithrombogenicity evaluation of a centrifugal blood pump. Artif Organs 2000;24:667–70. [DOI] [PubMed]
- 27.Reilly MP, Wiegers SE, Cucchiara AJ, O'Hara ML, Plappert TJ, Loh E, et al. Frequency, risk factors, and clinical outcomes of left ventricular assist device-associated ventricular thrombus. Am J Cardiol 2000;86:1156–9, A10. [DOI] [PubMed]
- 28.Rose AG, Park SJ. Pathology in patients with ventricular assist devices: a study of 21 autopsies, 24 ventricular apical core biopsies and 24 explanted hearts. Cardiovasc Pathol 2005;14: 19–23. [DOI] [PubMed]
- 29.Nakazawa T, Makinouchi K, Takami Y, Glueck J, Takatani S, Nose Y. The effect of the impeller-driver magnetic coupling distance on hemolysis in a compact centrifugal pump. Artif Organs 1996;20:252–7. [DOI] [PubMed]
- 30.Takami Y, Nakazawa T, Makinouchi K, Glueck J, Benkowski R, Nose Y. Effect of surface roughness on hemolysis in a centrifugal blood pump. ASAIO J 1996;42:M858–62. [DOI] [PubMed]
- 31.Tuzun E, Gregoric ID, Conger JL, Golden K, Jarvik R, Frazier OH, Kadipasaoglu KA. The effect of intermittent low speed mode upon aortic valve opening in calves supported with a Jarvik 2000 axial flow device. ASAIO J 2005;51:139–43. [DOI] [PubMed]
- 32.Yoshikawa M, Nakata K, Takano T, Maeda T, Glueck J, Murabayashi S, et al. Development of an implantable small right ventricular assist device. ASAIO J 2000;46:338–43. [DOI] [PubMed]
- 33.Frazier OH, Gregoric ID, Cohn WE. Initial experience with non-thoracic, extraperitoneal, off-pump insertion of the Jarvik 2000 heart in patients with previous median sternotomy. J Heart Lung Transplant 2006;25:499–503. [DOI] [PubMed]
- 34.Myers TJ, Frazier OH, Mesina HS, Radovancevic B, Gregoric ID. Hemodynamics and patient safety during pump-off studies of an axial-flow left ventricular assist device. J Heart Lung Transplant 2006;25:379–83. [DOI] [PubMed]


