Abstract
Takayasu's arteritis is a primary vasculitis that affects large vessels and is characterized by chronic granulomatous inflammation. Diagnosis has been primarily clinical, with verification by angiography as the gold standard. More recently, however, it has become apparent that positron emission tomography enables better evaluation of vascular inflammation.
This study presents 2 cases of Takayasu's arteritis. Magnetic resonance angiography was used to evaluate aortic anatomy by analyzing vascular wall thickness and also to quantify disease activity by measuring gadolinium enhancement. Positron emission tomography was used to evaluate active vascular inflammation by quantifying fluorodeoxyglucose F18 uptake. We conclude that both techniques support clinical diagnosis and aid in the evaluation of disease activity during and after treatment.
Key words: Arteritis/diagnosis; fluorodeoxyglucoseF18/diagnostic use; inflammation; magnetic resonance angiography; radiopharmaceuticals/diagnostic use; Takayasu's arteritis/diagnosis/radiography; tomography, emission-computed
Takayasu's arteritis (TA) is a chronic primary vasculitis that affects large vessels, predominantly the aorta,1,2 and is characterized by chronic inflammation. In the early stages, the diagnosis can be difficult because clinical manifestations (myalgias, arthralgias, fever, and malaise) are not specific to the disease. The progress of this inflammation leads to stenosis and occlusion of arteries, sometimes giving rise to late collateral circulation. In advanced stages of TA, the clinical manifestations are clearly recognized and the diagnosis easily established.
Although clinical manifestations can suggest the diagnosis, the American College of Rheumatology's 1990 criteria3 for the classification of TA proposed angiography as the gold standard for diagnosis.4 Today, newer techniques such as computed tomography, Doppler ultrasound, magnetic resonance angiography (MRA), and positron emission tomography (PET) are useful noninvasive imaging methods for the evaluation of the disease.5 Although other imaging techniques provide more specific anatomic evaluation of the vascular beds, PET enables better evaluation of vascular inflammatory activity. It is well known that inflammatory cells show an increased uptake of 18F–fluorodeoxyglucose (18F-FDG).6 In patients with TA, increased uptake of 18F-FDG by the wall of the aorta and the aortic branches may be seen, which demonstrates the disease's activity and in some cases confirms the diagnosis. Uptake evaluation by 18F-FDG may be useful in measuring response to therapy upon follow-up.7,8 There have been reports of 18F-FDG uptake in patients whose vascular inflammation arises from a variety of causes.6–10
In this paper, we present the cases of 2 young women in whom TA was suspected, although they had no cardiovascular risk factors. In both patients, MRA enabled the evaluation of the aorta and its branches by measuring the thickness of the vascular wall, and the quantification of inflammatory activity by measuring gadolinium uptake. Both patients showed positive 18F-FDG uptake on PET imaging.
Patients and Methods
The patients gave written informed consent for all procedures and were studied in the National Institute of Cardiology “Ignacio Chávez” in Mexico City. Laboratory analysis included a complete blood count, erythrocyte sedimentation rate (ESR), fibrinogen, and C-reactive protein (CRP). To evaluate disease activity, we applied a clinical test that combines simple clinical and laboratory data; a score higher than 5 points was considered to indicate active disease.11 Both of our patients received prednisone (1 mg/kg per day) and intravenous cyclophosphamide (750 mg/m2 body surface) for 3 months.
Magnetic resonance angiography was performed by use of a magnetom Sonata Maestro Class (1.5-tesla) magnet (Siemens Medical Solutions USA, Inc.; Malvern, Pa). After peripheral intravenous access was obtained, the patient was placed into the equipment in a supine position. Half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequences and cineradiographic views were acquired for thoracic anatomy study. Gadolinium was administered intravenously, and images were acquired in both arterial and venous stages. Before and after the administration of gadolinium, T1 sequences were acquired for fat-saturation study and for the evaluation of aortic wall thickness. Left ventricular dimensions, volumes, and ejection fractions were obtained.
Both patients were referred to the PET-cyclotron unit, where a whole-body scan with 18F-FDG was performed as follows. In a fasting state, they received 15 mCi of 18F-FDG intravenously, followed by 180 min of complete rest. A transmission-and-emission scan was performed with an ecat® exact™ HR+ PET camera (Siemens) in 3D mode. Images were reconstructed automatically. Two different observers, who had been blinded to the clinical diagnosis, interpreted the study. At the aorta, they quantified 18F-FDG uptake in standard uptake value (SUV) units. In the absence of disease, 18F-FDG uptake is not observed in the aorta. A study was considered positive when the observers detected regional 18F-FDG uptake in the area corresponding to the aorta.
Patient 1
A 26-year-old woman presented initially with cutaneous maculopapular lesions and vesicles in the legs, back, and buttocks. She later developed, on both pretibial areas, hard and painful erythematous nodules of 2 to 3 cm in diameter. She also had lumbar pain, fever of 40 °C, arthralgia and epigastric pain, systemic hypertension, headache, amaurosis fugax, blurred vision, dyspnea, palpitations, and left-neck carotidynia.
Two years after the initial presentation, she was referred to our institute. On her 1st visit, she presented with carotidynia, fever of 40 °C, arthralgias, systemic arterial hypertension, heart failure symptoms, a neck murmur, and an aortic regurgitation murmur. Physical examination also revealed a hard subcutaneous nodule in the thigh, different blood pressures (left arm, 130/60 mmHg; right arm, 170/90 mmHg), claudication of the extremities, and decreased pulse amplitude on the left arm. Aortic arteriography showed a pattern indicative of TA type V, in accordance with Numano's classification system.12
The laboratory tests revealed leukocytosis (14,700 cells/mm3), mild anemia (hemoglobin, 9 g/dL), accelerated ESR (55 mm/hr), and high levels of CRP (51 g/dL) and fibrinogen (7.8 g/dL). The patient's clinical activity score was 5.5.
The double-inversion, black-blood, spin-echo MRA image showed a thick aortic wall (4 mm); the left oblique projection after gadolinium administration revealed an irregular vascular wall, progressive “mouse tail” occlusions of the descending aorta and left subclavian artery, 2 pseudoaneurysms at the thoracoabdominal junction, and a small infrarenal fusiform aneurysm (Fig. 1A). Endovascular exploration also revealed pseudoaneurysm formation and irregularities and thickening of the vascular wall (Fig. 1A). Positron emission tomography showed increased uptake (2.7 SUV) of 18F-FDG in the aortic arch (Fig. 2A).
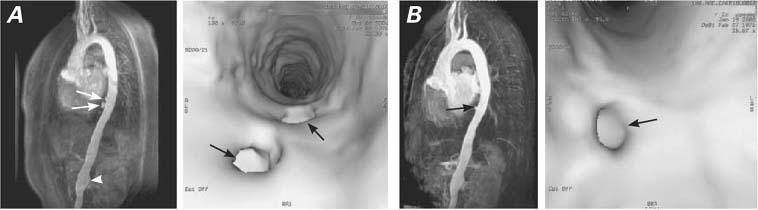
Fig. 1 Patient 1. A) Before treatment (images at left): 3D time-of-flight magnetic resonance angiography (MRA) and endovascular exploration of the thoracic aorta show 2 pseudoaneurysms (arrows) at the thoracic abdominal junction, and a small infrarenal fusiform aneurysm (arrowhead). B) After treatment (images at right): MRA image reveals the absence of 1 pseudoaneurysm (arrow), and the endovascular image at the far right also reveals the absence of that pseudoaneurysm.
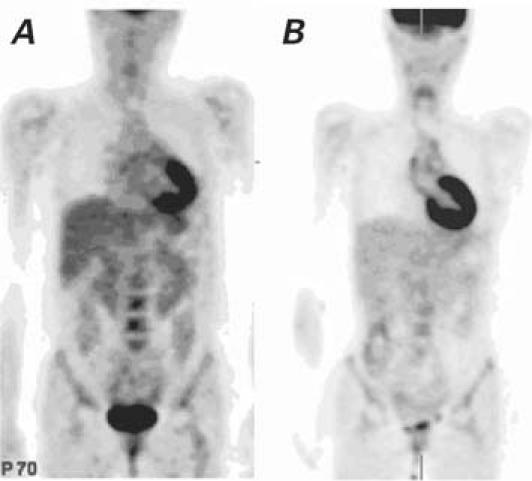
Fig. 2 Patient 1. A) Before treatment: Positron emission tomography shows increased uptake (2.7 SUV) of 18F-FDG in the aortic arch. B) Three months after treatment: Decreased uptake (1.7 SUV) of 18F-FDG in the aortic arch.
After 3 months of treatment, this patient displayed clinical activity at 0.5; there was no carotidynia, and her laboratory results were normal, except for mild anemia (hemoglobin, 12 g/dL). Magnetic resonance angiographic images after treatment revealed absence of a pseudoaneurysm, a smooth intima, and a 1-mm decreasein abdominal aortic wall thickness (Fig. 1B).
Although this patient's 18F-FDG SUV was reduced to 1.7 (Fig. 2B), 18F-FDG uptake was still abnormally high in the ascending aorta and the aortic arch.
Patient 2
A 27-year-old woman came to our institute through the emergency department, with complaints of carotidynia, high fever (39 °C), and arthralgia. She reported a history of progressive-effort dyspnea, paroxysmal nocturnal dyspnea and orthopnea, syncope, and amaurosis fugax. Physical examination revealed an arterial blood pressure of 117/50 mmHg in the right arm, no detectable systolic blood pressure in the left arm, and wide and asymmetric carotid arterial pulses. Transthoracic echocardiography recorded severe aortic regurgitation, without evidence of ventricular failure. Primary vasculitis of the large vessels was suspected. Aortic arteriography revealed lesions in each carotid artery, important stenosis of the right external carotid, and partial stenosis of the left subclavian and vertebral arteries. The left coronary artery showed an ostial stenosis estimated at 40%. Diagnosis of type-IV Takayasu's arteritis was established.
The laboratory results showed leukocytosis (11,000/mm3), mild anemia (hemoglobin, 11 g/dL), high platelet count (575,000/mm3), CRP of 94, accelerated ESR (43 mm/hr), and fibrinogen of 8.27 g/dL. Polyclonal hypergammaglobulinemia was found. The patient's clinical activity score was valued at 6.5.
Magnetic resonance angiographic imaging sequences showed occlusion of the left subclavian artery and of the aortic arch. The MRA images also revealed a 7-mm perivascular thickening of the thoracic aorta. Positron emission tomography revealed an 18F-FDG SUV of 4.5 at the aortic arch, indicating inflammation (Fig. 3A).
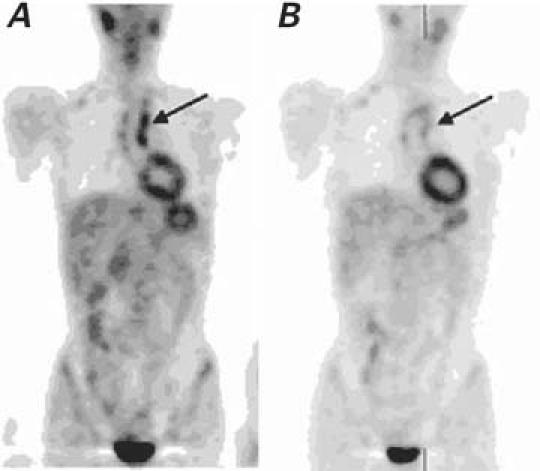
Fig. 3 Patient 2. A) Before treatment: Whole-body positron emission tomographic (PET) scan shows increased 18F-FDG uptake in the ascending aorta (arrow). B) Three months after treatment: Whole-body PET image shows decreased 18F-FDG uptake in the ascending aorta (arrow).
After 3 months of treatment, the patient had a clinical activity score of 1.5, due to mild anemia, elevated ESR, and elevated CRP.
Magnetic resonance angiographic imaging revealed a small reduction in the thickness of the aortic wall at the thoracic level (to 6 mm); PET revealed changes in 18F-FDG uptake (SUV, 3.4). Positron emission tomography (Fig. 3B) correlated with the MRA findings, which suggested persistent inflammation; however, the patient did not fulfill the clinical activity criteria.
Discussion
These cases illustrate the potential usefulness of 18F-FDG PET as an imaging tool for the diagnosis and follow-up of vascular inflammatory activity in patients with Takayasu's arteritis. Active vascular inflammation is not easily appreciated with other imaging techniques, such as computed tomography, magnetic resonance, or angiography.4 Most of the published papers8–10,13–17 that emphasize the role of 18F-FDG PET in the diagnosis of inflammation in the arterial wall in Takayasu's arteritis are case reports. Recently, the use of 18F-FDG PET has been studied in groups of patients with vasculitis, specifically to detect acute inflammatory events in association with atherosclerotic plaques in the carotid arteries.18
When a whole-body 18F-FDG PET scan is negative for aortitis, the aorta cannot be identified by its tracer uptake, and it is generally below 1.2 SUV.19 Both of our patients had well-established diagnoses of Takayasu's arteritis: they showed 18F-FDG uptake in the thoracic aorta, mainly in the ascending aorta and the arch. These are the regions most frequently described in other reports.13,14,20,21 Our suspicion of vascular inflammation, raised by the clinical and serologic data, was well corroborated through the PET scans.
The activity of Takayasu's arteritis, using current approaches, still presents a diagnostic challenge because of its uncertainty.11 In the cases presented here, we correlated clinical activity criteria with PET imaging; in both cases, the clinical criteria and PET were in agreement with a positive result. In the setting of suspected Takayasu's arteritis, PET is a promising noninvasive imaging technique that can help to determine disease activity. In one of our patients, we were able to reduce the clinical activity value, although her MRA and PET results still showed abnormally high values. According to Kerr and colleagues,22 there is ongoing vascular wall inflammation in TA patients, even when there is no clinical activity.
In conclusion, our observations provide clear examples of the advantages of modern imaging for the diagnosis and follow-up of patients with Takayasu's arteritis. Both 18F-FDG PET and MRA are effective noninvasive imaging methods that support the clinical diagnosis of active inflammation, an unresolved issue in the evaluation of TA patients. Although it can be said that each technique has advantages over the other, a prospective study would be needed to establish the comparative usefulness of 18F-FDG PET and MRA in regard to cost/benefit issues.
Acknowledgments
We would like to thank nuclear medicine technicians Isabel Porras Orta and Luis Osorio Cardiel for their collaboration in the acquisition and reconstruction of the images.
Footnotes
Address for reprints: Erick Alexanderson, MD, Instituto Nacional de Cardiología “Ignacio Chávez,” Juan Badiano No. 1 Col. Sección XVI Tlalpan, México D.F. 14080, Mexico. E-mail: alexanderick@yahoo.com
References
- 1.Lupi-Herrera E, Sanchez-Torres G, Marcushamer J, Mispireta J, Horwitz S, Vela JE. Takayasu's arteritis. Clinical study of 107 cases. Am Heart J 1977;93:94–103. [DOI] [PubMed]
- 2.Robles M, Reyes PA. Takayasu's arteritis in Mexico: a clinical review of 44 consecutive cases. Clin Exp Rheumatol 1994;12: 381–8. [PubMed]
- 3.Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990;33:1129–34. [DOI] [PubMed]
- 4.Johnston SL, Lock RJ, Gompels MM. Takayasu arteritis: a review. J Clin Pathol 2002;55:481–6. [DOI] [PMC free article] [PubMed]
- 5.Kissin EY, Merkel PA. Diagnostic imaging in Takayasu arteritis. Curr Opin Rheumatol 2004;16:31–7. [DOI] [PubMed]
- 6.Brown RS, Leung JY, Fisher SJ, Frey KA, Ethier SP, Wahl RL. Intratumoral distribution of tritiated fluorodeoxyglucose in breast carcinoma: I. Are inflammatory cells important? J Nucl Med 1995;36:1854–61. [PubMed]
- 7.Bleeker-Rovers CP, Bredie SJ, van der Meer JW, Corstens FH, Oyen WJ. Fluorine 18 fluorodeoxyglucose positron emission tomography in the diagnosis and follow-up of three patients with vasculitis. Am J Med 2004;116:50–3. [DOI] [PubMed]
- 8.Webb M, Chambers A, Al-Nahhas A, Mason JC, Maudlin L, Rahman L, Frank J. The role of 18F-FDG PET in characterising disease activity in Takayasu arteritis. Eur J Nucl Med Mol Imaging 2004;31:627–34. [DOI] [PubMed]
- 9.Meller J, Strutz F, Siefker U, Scheel A, Sahlmann CO, Lehmann K, et al. Early diagnosis and follow-up of aortitis with [(18)F]FDG PET and MRI. Eur J Nucl Med Mol Imaging 2003;30:730–6. [DOI] [PubMed]
- 10.Hara M, Goodman PC, Leder RA. FDG-PET finding in early-phase Takayasu arteritis. J Comput Assist Tomogr 1999; 23:16–8. [DOI] [PubMed]
- 11.Dabague J, Reyes PA. Takayasu arteritis in Mexico: a 38-year clinical perspective through literature review. Int J Cardiol 1996;54 Suppl:S103–9. [DOI] [PubMed]
- 12.Hata A, Noda M, Moriwaki R, Numano F. Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol 1996;54 Suppl:S155–63. [DOI] [PubMed]
- 13.Malik IS, Harare O, AL-Nahhas A, Beatt K, Mason J. Takayasu's arteritis: management of left main stem stenosis. Heart 2003;89:e9. [DOI] [PMC free article] [PubMed]
- 14.Meller J, Grabbe E, Becker W, Vosshenrich R. Value of F-18 FDG hybrid camera PET and MRI in early takayasu aortitis. Eur Radiol 2003;13:400–5. [DOI] [PubMed]
- 15.Meller J, Altenvoerde G, Munzel U, Jauho A, Behe M, Gratz S, et al. Fever of unknown origin: prospective comparison of [18F] FDG imaging with a double-head coincidence camera and gallium-67 citrate SPET. Eur J Nucl Med 2000;27:1617–25. [DOI] [PubMed]
- 16.Wiest R, Gluck T, Schonberger J, Scholmerich J, Eilles C, Muller-Ladner U. Clinical image: occult large vessel vasculitis diagnosed by PET imaging. Rheumatol Int 2001;20:250. [DOI] [PubMed]
- 17.Wenger M, Gasser R, Donnemiller E, Erler H, Glossmann H, Patsch JR, et al. Images in cardiovascular medicine. Generalized large vessel arteritis visualized by 18fluorodeoxyglucose-positron emission tomography. Circulation 2003;107:923. [DOI] [PubMed]
- 18.Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002;105:2708–11. [DOI] [PubMed]
- 19.Blockmans D, Stroobants S, Maes A, Mortelmans L. Positron emission tomography in giant cell arteritis and polymyalgia rheumatica: evidence for inflammation of the aortic arch. Am J Med 2000;108:246–9. [DOI] [PubMed]
- 20.Derdelinckx I, Maes A, Bogaert J, Mortelmans L, Blockmans D. Positron emission tomography scan in the diagnosis and follow-up of aortitis of the thoracic aorta. Acta Cardiol 2000; 55:193–5. [DOI] [PubMed]
- 21.Kobayashi Y, Ishii K, Oda K, Nariai T, Tanaka Y, Ishiwata K, Numano F. Aortic wall inflammation due to Takayasu arteritis imaged with 18F-FDG PET coregistered with enhanced CT. J Nucl Med 2005;46:917–22. [PubMed]
- 22.Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, Hoffman GS. Takayasu arteritis. Ann Intern Med 1994;120:919–29. [DOI] [PubMed]


