Abstract
The aim of the study was to investigate, in adult patients after successful repair of aortic coarctation, potential relationships between B-type natriuretic peptide levels and exercise capacity and the following factors: arterial hypertension, residual stenosis of the ascending aorta, and age at the time of surgery.
The study group comprised 74 patients (45 men) aged 19 to 61 years (mean, 31.2 ± 9.8 yr), who had undergone surgery at the age of 0.5 to 34 years (mean, 10.4 ± 6.8 yr). The surgery was performed between 5 and 34 years earlier (mean, 21.4 ± 6.2 yr). A subgroup with residual aortic stenosis (significant when ≥25 mmHg) comprised 32 patients; a subgroup without residual stenosis comprised 42 patients. Patients were also divided into subgroups without arterial hypertension (n=32), with exercise-induced arterial hypertension (n=10), and with persistent arterial hypertension (n=32). All patients were in New York Heart Association functional class I. The control group comprised 30 healthy subjects (18 men) aged 26 to 46 years (mean, 32.2 ± 6.6 yr).
After testing exercise capacity in accordance with a modified Bruce protocol, we concluded that the exercise capacity of adults is reduced after surgical repair of aortic coarctation. This reduction is more pronounced in patients who have arterial hypertension, but it is unaffected by residual stenosis of the descending aorta. Serum B-natriuretic peptide concentrations, as determined by immunoradiometric assay, are significantly elevated, which may result from pressure overload of the left ventricle or from residual myocardial lesions due to coarctation repair at an older age.
Key words: Adult; age factors; aortic coarctation/complications; blood pressure/physiology; exercise/physiology; exercise test; follow-up studies; heart defects, congenital; hypertension/etiology; natriuretic peptide, brain/blood; postoperative complications
Surgical repair of coarctation of the aorta has been performed with satisfactory results for over half a century.1–4 Long-term follow-up data show that the results of the surgery are better in patients who undergo operation in early childhood,2,3,5 although neonatal patients experience a higher incidence of residual stenosis of the ascending aorta.6–11 Life expectancy is reduced, even after successful repair.4,8,10 After repair of coarctation of the aorta, a considerable number of adults present with arterial hypertension and premature coronary artery disease, and hence develop heart failure that is one of the causes of early death in this population.2,3 Although most post-coarctation-repair patients find their exercise capacity satisfactory, subjective appraisal of exercise performance does not necessarily reflect the actual clinical condition of the patient.2,3,11 Therefore, it may be essential to evaluate oxygen uptake (peak Vo2) with the maximum stress test combined with spirometry–cardiopulmonary exercise testing, which is the objective tool for measuring exercise tolerance. The results of cardiopulmonary stress testing are recognized predictors of prognosis in patients with heart failure.12 B-Type natriuretic peptide (BNP) concentration is another acknowledged diagnostic and prognostic factor in heart failure.13–16 The value of this hormone has been documented in evaluating acute, chronic, and subclinical left ventricular heart failure.13,16 Indeed, the elevation of BNP level is a risk factor for sudden cardiac death in heart failure patients.17 The importance of BNP has also been shown in evaluating selected populations of patients who have congenital heart diseases,14,18–23arterial hypertension,24,25 and left ventricular outflow tract obstruction.26–29 To our knowledge, there are no studies to date concerning BNP levels in patients who have undergone successful repair of coarctation of the aorta.
The aim of the study was to investigate, in adult patients after successful repair of coarctation of the aorta, any potential relationships between BNP levels and exercise capacity (this last measured by cardiopulmonary stress testing) and the following factors: arterial hypertension, residual stenosis of the ascending aorta, and age at the time of surgery.
Patients and Methods
The study population was selected from 107 successful coarctation-repair patients of our congenital heart disease outpatient clinic. To be eligible for inclusion, patients had to display normal left ventricular systolic function, to be able to complete a cardiopulmonary exercise test, and to show no evidence of ischemic heart disease, respiratory disorder, or significant aortic regurgitation. Ultimately, the study enrolled 74 patients (29 women and 45 men) aged 19 to 61 years (mean, 31.2 ± 9.8 yr), who had undergone operation at the age of 0.5 to 34 years (mean, 10.4 ± 6.8 yr). The control group consisted of 30 healthy individuals (12 women, 18 men) aged 26 to 46 years (mean, 32.2 ± 6.6 yr). Most patients in the study group (n=65, 88%) had undergone synthetic patch repair by the same cardiac surgeon; 3 (4%) had undergone subclavian flap aortoplasty, 6 (8%) had undergone end-to-end anastomosis, and 1 had received a St. Jude 24-mm mechanical prosthetic valve (St. Jude Medical; St. Paul, Minn). Eight study patients had undergone reoperation due to stenosis of the ascending aorta; 1 patient had received noninvasive treatment of recoarctation; and another had undergone stent-graft implantation in the ascending aorta, due to aneurysm. The following congenital heart diseases were additionally diagnosed: ventricular septal defect, surgically repaired (n=6); occluded persistent arterial duct (n=5); and trivial mitral insufficiency (n=19). All participants in the study and control groups were classified in New York Heart Association functional class I, and all displayed sinus rhythm. Creatinine serum concentrations did not exceed 140 μmol/L, and aspartate aminotransferase levels did not exceed double the normal values.
Echocardiographic examination was performed using Vivid 7 Dimension '06 (GE Healthcare; Chalfont-on-Giles, UK) with a 2.5-MHz probe in 2-D, M, and Doppler modes. Our investigator evaluated cardiac anatomy and left ventricular systolic function by means of the Simpson biplane method (normal systolic function was a criterion for inclusion in the study). The peak aortic valve pressure gradient was determined by means of continuous-wave Doppler echocardiography. The severity of residual stenosis of the ascending aorta was determined by evaluating the suprasternal notch. Restenosis was diagnosed when the ascending aorta pressure gradient was equal to or greater than 25 mmHg.2 This measurement was used to divide the study population into subgroups with (AoD+) and without (AoD–) residual stenosis of the descending aorta. All echocardiography was performed by a single investigator.
All patients in the study group underwent maximum, symptom-limited (fatigue, dyspnea, or both) treadmill exercise testing in accordance with a modified Bruce protocol (added to the standard protocol was a 0–3-min stage at 1.7 km/hr and 5% inclination). The control subjects, however, followed the standard Bruce protocol. Patients were encouraged to continue the test for as long as their respiratory quotients (RQ) exceeded 1. The maximum oxygen uptake (peak Vo2), carbon dioxide production (Vco2), and respiratory minute volume (Ve) were measured using breath-to-breath gas analysis (Vmax29®, SensorMedics Corporation, part of viasys Respiratory Care, a unit of Cardinal Health; Dublin, Ohio). The system was calibrated with a standard gas mixture of known concentration before each test. A standard 12-lead electrocardiogram was continuously recorded. Blood pressure was recorded every 2nd minute using a cuff sphygmomanometer. Peak Vo2 was determined as an average value within the last 20 sec of exercise, and was expressed both as mL/(kg·min) and as the percentage of predicted peak oxygen uptake. The VE/Vco2 slope—which indicates the increase of ventilation relative to carbon dioxide production—was calculated automatically by the Vmax29 software. Spirometry, including the measurement of forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1), was performed in all subjects before the cardiopulmonary exercise test, and the resulting values were then calculated as percentages of predicted values, taking into account age, sex, and body weight. For analysis, we selected the best achieved results from 3 to 4 spirometry cycles. The evaluation of the cardiopulmonary exercise test was performed by investigators who were blinded to the results of BNP levels.
In all patients, the blood samples were drawn from an antecubital vein after 15 minutes of rest in the supine position, before exercise testing. The concentrations of BNP in human plasma were determined with the use of an immunoradiometric assay kit (Shionoria BNP; CIS Bio International Diagnostic, part of Bayer Schering Pharma AG; Berlin, Germany). The radioactivity was measured for 1 minute with an NZ 335 gamma scintillation counter.
A cuff sphygmomanometer was used to measure blood pressure in all participants, both at rest and immediately after exercise. Exercise-induced arterial hypertension was diagnosed if systolic blood pressure at peak exercise exceeded 200 mmHg in patients whose baseline blood pressure was normal.9 Blood pressure exceeding 140/90 mmHg was called arterial hypertension in accordance with guidelines established in 2003 by the European Society of Hypertension and the European Society of Cardiology.30 The study involved both hypotensive-agent naïve patients and patients from whom antihypertensive therapy was withdrawn for at least 2 weeks before the treadmill test.
For variables following normal distribution, statistical analysis was performed with the Student t test for unpaired samples. For variables not following normal distribution, the Mann-Whitney U test was used. To determine the relationships between variables, the Spearman rank-order correlations were analyzed. Results of P <0.05 were considered statistically significant.
Informed consent was obtained from each patient, and our study protocol, as approved a priori by our institution's human research committee, conformed to the ethical guidelines set forth by the 1975 Declaration of Helsinki.
Results
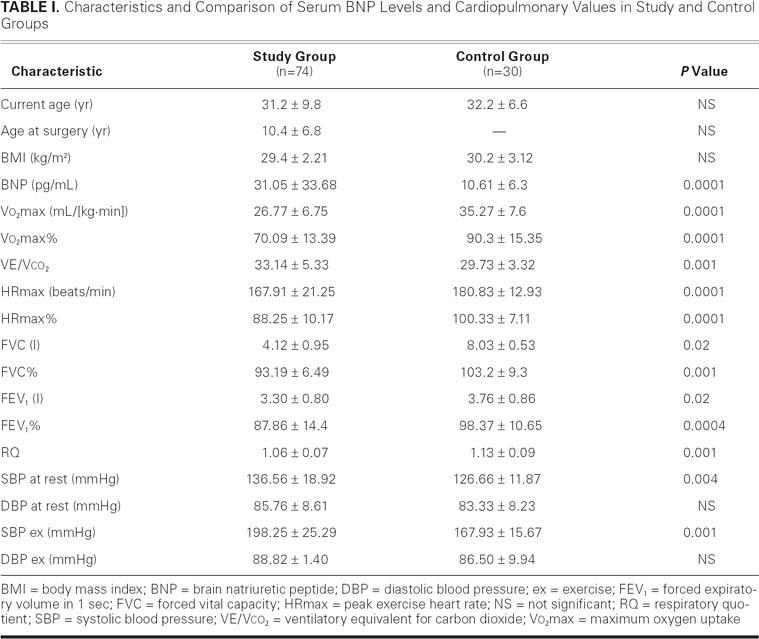
Table I shows the results of cardiopulmonary testing, which revealed that the exercise performance of adults after the repair of coarctation was significantly reduced: peak oxygen uptake (Vo2max) in study patients was lower than in healthy individuals (26.77 ± 6.75 vs 35.27 ± 7.6 mL/([kg·min]); P=0.0001), and similarly when expressed as percentages (Vo2max%, 70.09 ± 13.39 vs 90.3 ± 15.35; P=0.0001). The ventilation/carbon dioxide slope (VE/Vco2) was higher in the study group than in the control (33.14 ± 5.33 vs 29.73 ± 3.32; P=0.001). The heart rate at maximum workload in the study group was reduced in comparison with the control (167.91 ± 21.25 vs 180.83 ± 12.93 beats/min; P=0.0001); and the same was observed for HRmax% (88.25 ± 10.17 vs 100.33 ± 7.11; P=0.0001). The RQ in patients after surgery was lower than in members of the control group (1.06 ± 0.07 vs 1.13 ± 0.09; P=0.001). Diastolic blood pressure at rest and during physical exertion did not differ between the groups, whereas systolic blood pressures, both resting (136.58 ± 18.92 vs 126.66 ± 11.87 mmHg; P=0.004) and during exercise (198.25 ± 25.29 vs 167.94 ± 15.67 mmHg; P=0.001), were higher in the study patients. The measurements of pulmonary elasticity, including FVC and FVC% (percent of forced vital capacity), were lower in the study patients than in the controls: 4.12 ± 0.95 L vs 8.03 ± 0.53 L (P=0.02) and 93.19% ± 16.49% vs 103.2% ± 9.3%, respectively (P=0.001). Moreover, the parameters used to identify airway obstruction, including a FEV1 of 3.30 ± 0.80 L and FEV1% of 87.86 ± 14.41, were significantly lower in postsurgical patients: FEV1, 3.76 ± 0.86 L and FEV1%, 98.37 ± 10.65 (P=0.02 and P=0.0004, respectively).
TABLE I. Characteristics and Comparison of Serum BNP Levels and Cardiopulmonary Values in Study and Control Groups
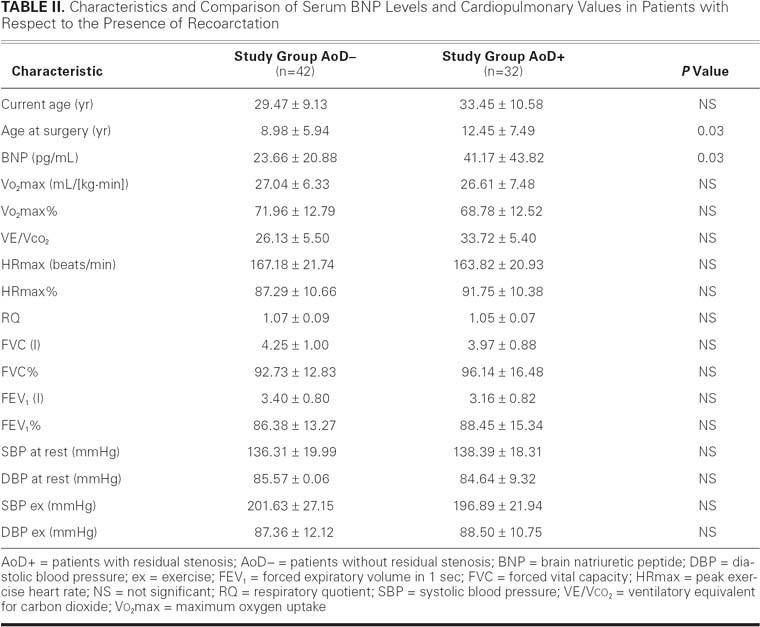
Maximum aortic valve pressure gradients ranged from 4.11 to 28.53 mmHg (mean, 11.75 ± 6.19 mmHg). In 32 patients, in whom residual stenosis of the descending aorta was found (AoD+), the pressure gradient ranged from 25.0 to 60.2 mmHg (mean, 36.66 ± 9.81 mmHg); in the remaining 42 patients, in whom no residual stenosis of the descending aorta was found (AoD–), the pressure gradient ranged from 5.51 to 24.0 mmHg (mean, 15.91 ± 5.08 mmHg). The comparison of cardiopulmonary parameters between AoD+ and AoD– groups set forth in Table II reveals no significant differences. It should be observed that AoD+ patients were operated on at an older age (12.45 ± 7.49 yr) than were AoD– patients (8.98 ± 5.94 yr; P=0.03).
TABLE II. Characteristics and Comparison of Serum BNP Levels and Cardiopulmonary Values in Patients with Respect to the Presence of Recoarctation
Thirty-three patients had normal resting blood pressure (AH–), and 10 had arterial hypertension induced by exercise (AHex); the remaining group of 31 patients (AH+) had persistent arterial hypertension (Table III). Study of AH– and AHex showed that patients with exertional arterial hypertension had higher ventilation-to-perfusion ratios—VE/Vco2 (28.31 ± 4.56 vs 33.89 ± 5.02; P=0.01)—but reached lower heart rates at peak exercise (181.00 ± 13.05 vs 171.24 ± 15.76 beats/min; P=0.001). The comparison between the AH– and AH+ subgroups showed that persistently hypertonic patients had the following values reduced: Vo2max (26.30 ± 15.08 vs 28.05 ± 7.9 mL/[kg·min]; P=0.01), Vo2max% (66.17 ± 14.58 vs 74.75 ± 12.16; P=0.02), HRmax (162.46 ± 21.76 vs 181.00 ± 13.05 beats/min; P=0.0001), HR% (84.46 ± 10.58 vs 94.31 ± 7.91; P=0.0004), and RQ (1.04 ± 0.5 vs 1.08 ± 0.09; P=0.03). However, VE/Vco2 was higher (28.31 ± 4.56 vs 34.06 ± 4.78; P=0.003). The comparison between the AHex and AH+ subgroups proved that patients with persistent arterial hypertension had lower HRmax (162.42 ± 21.13 vs 171.24 ± 15.76 beats/min; P=0.001), HR% (92.30 ± 6.11 vs 84.46 ± 10.58; P=0.01), and RQ (1.09 ± 0.07 vs 1.04 ± 0.05; P=0.02). Between all groups, we observed no significant differences in spirometric measurements.
TABLE III. Characteristics and Comparison of Serum BNP Levels and Cardiopulmonary Values in Patients with Respect to Arterial Hypertension Presence
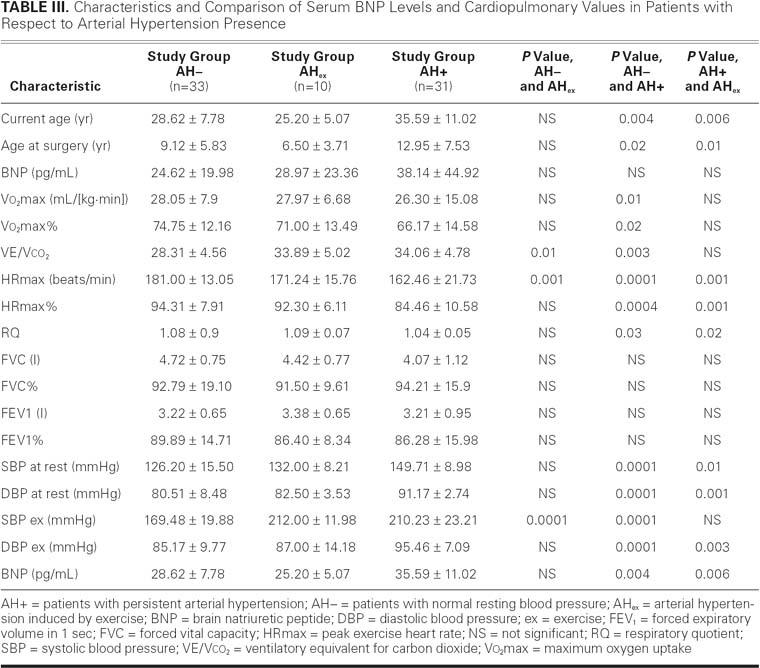
Patients with AH+ were older than those with AH– (35.59 ± 11.02 vs 28.62 ± 7.78 yr; P=0.004) and those with AHex (35.59 ± 11.02 vs 25.20 ± 5.07 yr; P=0.006). The age of AH+ patients at the time of the surgery was higher than the age of both the AH– (12.95 ± 7.53 vs 9.12 ± 5.83 yr; P=0.02) and AHex subgroups (12.95 ± 7.53 vs 6.50 ± 3.71 yr; P=0.01). At the same time, the mean pressure gradient through the descending aorta did not differ among groups (HT–, 25.52 ± 14.02 mmHg; AHex, 30.69 ± 15.3 mmHg; and AH+, 24.68 ± 13.4 mmHg).
Linear correlation analysis of results for the entire study group—including current age, age at operation, time after operation, blood pressure, and cardiopulmonary values—showed significant negative correlation between current age of patient and Vo2max (r= −0.0432, P=0.0003) and significant positive correlation between current age and VE/Vco2 (r=0.342, P=0.004); significant negative correlations were found between the age at surgical repair and Vo2max (r= −0.360, P=0.003) and between the age at surgical repair and VE/Vco2 (r=0.369, P=0.002). No significant correlations were seen between exercise-capacity parameters and blood pressure.
Serum BNP levels in the study group were higher than in the control group (31.06 ± 33.68 vs 10.61 ± 6.3 pg/mL, respectively; P=0.0001); and serum BNP levels were higher in the subgroup with residual stenosis of the descending aorta (AoD+) than in the gradient-negative (AoD–) group (41.17 ± 43.82 vs 23.66 ± 20.84; P=0.03) but did not reach statistical significance. Differences in serum BNP concentrations with respect to the diagnosis of arterial hypertension were insignificant. It was observed that BNP level positively correlated with age at surgery (r=0.275, P=0.02) (Fig. 1) and with aortic valve pressure gradients (r=0.233, P=0.04) (Fig. 2). The BNP level did not correlate significantly with the descending aorta pressure gradient or with any of the cardiopulmonary parameters tested.
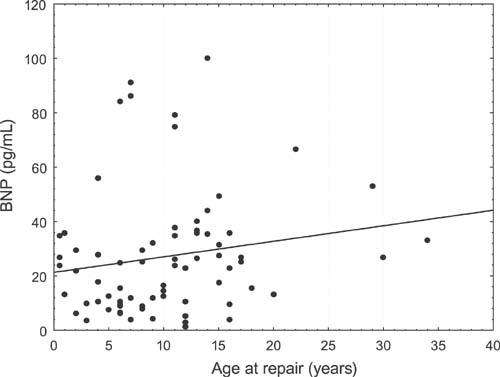
Fig. 1 B-Type natriuretic peptide levels (BNP) and age at repair.
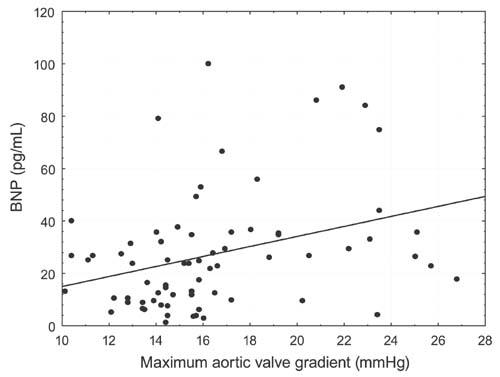
Fig. 2 B-Type natriuretic peptide levels (BNP) and maximum aortic valve gradient.
Discussion
According to a large number of investigators,2,3,11 adult patients, after repair of coarctation of the aorta, tend to think that their exercise capacity is satisfactory; however, our objective evaluation by means of cardiopulmonary exercise testing showed its considerable impairment. In children and adolescents, diverse observations have been made, respectively, by Balderston and colleagues31 and Markham and associates.8 Our findings are consistent with those of Rhodes and coworkers32 and with the results of a comprehensive analysis of a population of adults with congenital heart disease (including patients who had undergone surgical repair of coarctation of the aorta) that was carried out by investigators from Royal Brompton Hospital in London.11 These authors showed that Vo2max ≥15.5 mL/(kg·min) was an independent predictor of hospitalization and death in patients with congenital heart disease. All of the patients in our study group reached at least this low point in peak oxygen uptake. Chronotropic reaction was compromised in our study patients, as others have observed in adults with various congenital heart diseases.8,11,33 Swan6 and Balderston31 and their associates did not make such an observation, but their populations included only children.
Our study patients exhibited restrictive and obstructive respiratory disorders. Because the pathomechanisms that lead to these abnormalities reduce pulmonary compliance, physicians should investigate the possibility of heart enlargement, pulmonary fibrosis secondary to chronic fluid retention in the pulmonary circulation, and respiratory myopathy.11,34 The degree of impairment of pulmonary function did not differ, however, between subgroups of the study group in regard to recoarctation, persistent arterial hypertension, or exercise-induced arterial hypertension.
Clearly, exercise-capacity impairment worsened as the age of the patient increased,12 but at the same time it was inversely related to age at the time of coarctation repair (improving with younger age). A similar relationship between exercise capacity and age at operation was observed by the Royal Brompton Hospital investigators in 335 adults with congenital heart diseases.11 This finding supports early repair of this anatomical defect.2–4,9
Exercise-capacity impairment was similar in patients with recoarctation and those without residual stenosis of the descending aorta. However, it is worth emphasizing that our patients with residual stenosis of the descending aorta did not manifest higher blood pressures than did our patients who were free of recoarctation; this finding is in accord with the results of some studies,35,36 but not others.1,6–9 Isolated arterial hypertension was associated with exercise capacity. Significantly worse cardiopulmonary values (peak oxygen uptake and ventilation–perfusion ratio) were observed in hypertensive than in normotensive patients. In addition, patients with exercise-induced arterial hypertension showed higher ventilation–perfusion ratios than did normotensive patients.
The severity of chronotropic impairment was also significantly different among subgroups of our study group. Among patients with persistent arterial hypertension (in comparison with normotensive patients), our findings might well have been affected by the patients' older age, both at present and at the time of repair. Normotensive patients did not differ from patients with exercise-induced arterial hypertension in regard to current age and age at operation; however, the exercise capacity of the hypertensive group was worse. This suggests that—contrary to the theory of Swan6 and Leandro37 and their colleagues, who challenge the need for identifying patients with “latent hypertension” after repair of coarctation of the aorta—patients with exercise-induced arterial hypertension should receive special clinical surveillance1–3,7 and, if we follow the judgment of some investigators,4 earlier implementation of antihypertensive therapy.
We found that the level of B-type natriuretic hormone was significantly increased in adults after surgical repair of coarctation of the aorta. As we have said above, the elevation of BNP is also observed in adults with other congenital heart diseases.18–23 Mair and coworkers24 and Bolger's group14 have noted that an increase of BNP level in these populations depends specifically upon cardiac dysfunction, rather than the variant anatomic substrate. Law and colleagues18 have largely associated the elevation of serum BNP levels in this anatomically heterogeneous population with increased strain upon the left ventricular walls. In common with Westerlind and associates,38 we observed that B-type natriuretic hormone levels correlated with left ventricular outflow tract pressure gradients, after surgical repair of coarctation. Our calculations showed that BNP concentrations were significantly higher in subjects who had substantial pressure gradients through the descending aorta. Therefore, we infer that BNP secretion is activated by increased strain on the left ventricular walls.19,26,27,29 Holmgren's group20 documented that left ventricular volume overload (rather than pressure overload) was responsible for stimulating the production of BNP, but their observation was restricted to children. Analysis of the pathogenesis of the decreased exercise capacity of adult coarctation-repair survivors should take into consideration the fact that arteries arising from the aorta proximal to the stenosis are characterized by increased stiffness. Although early coarctation repair appears to help preserve the elastic properties of these arteries, their vascular reactivity remains reduced.10 Coronary artery lesions have been observed in 25% to 37% of this population.1,2,4,9 Because BNP levels are known to increase in coronary artery disease,24,40 such a cause of natriuretic hormone level elevation cannot be excluded. Moreover, an association has been shown between BNP concentration and age at the time of repair, which may indirectly indicate the severity of myocardial damage before surgery and (once again) weigh in favor of early repair.2–4,9
Conclusions
Our most important conclusions are these:
The exercise capacity of adults, after surgical repair of coarctation of the aorta, is reduced.
This reduction is more pronounced in patients who have arterial hypertension, and it is unaffected by residual stenosis of the descending aorta.
Serum BNP concentration is significantly increased in patients who have substantial residual pressure gradients through the descending aorta, which may result from pressure overload of the left ventricle or from residual myocardial lesions due to repair at an older age.
Footnotes
Address for reprints: Olga Trojnarska, MD, 1st Department of Cardiology, ul. Dluga ½, 61-848 Poznan, Poland. E-mail: olgatroj@wp.pl
References
- 1.Corno AF, Botta U, Hurni M, Payot M, Sekarski N, Tozzi P, von Segesser LK. Surgery for aortic coarctation: a 30 years experience. Eur J Cardiothorac Surg 2001;20:1202–6. [DOI] [PubMed]
- 2.Cohen M, Fuster V, Steele PM, Driscoll D, McGoon DC. Coarctation of the aorta. Long-term follow-up and prediction of outcome after surgical correction. Circulation 1989;80:840–5. [DOI] [PubMed]
- 3.Toro-Salazar OH, Steinberger J, Thomas W, Rocchini AP, Carpenter B, Moller JH. Long-term follow-up of patients after coarctation of the aorta repair. Am J Cardiol 2002;89:541–7. [DOI] [PubMed]
- 4.Celermajer DS, Greaves K. Survivors of coarctation repair: fixed but not cured. Heart 2002;88:113–4. [DOI] [PMC free article] [PubMed]
- 5.O'Sullivan JJ, Derrick G, Darnell R. Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24 hour blood pressure measurement. Heart 2002;88:163–6. [DOI] [PMC free article] [PubMed]
- 6.Swan L, Goyal S, Hsia C, Hechter S, Webb G, Gatzoulis MA. Exercise systolic blood pressures are of questionable value in the assessment of the adult with a previous coarctation repair. Heart 2003;89:189–92. [DOI] [PMC free article] [PubMed]
- 7.Vriend JW, van Montfrans GA, Romkes HH, Vliegen HW, Veen G, Tijssen JG, Mulder BJ. Relation between exercise-induced hypertension and sustained hypertension in adult patients after successful repair of aortic coarctation. J Hypertens 2004;22:501–9. [DOI] [PubMed]
- 8.Markham LW, Knecht SK, Daniels SR, Mays WA, Khoury PR, Knilans TK. Development of exercise-induced arm-leg blood pressure gradient and abnormal arterial compliance in patients with repaired coarctation of the aorta. Am J Cardiol 2004;94:1200–2. [DOI] [PubMed]
- 9.Vriend JW, Zwinderman AH, de Groot E, Kastelein JJ, Bouma BJ, Mulder BJ. Predictive value of mild, residual descending aortic narrowing for blood pressure and vascular damage in patients after repair of aortic coarctation. Eur Heart J 2005;26:84–90. [DOI] [PubMed]
- 10.de Divitiis M, Pilla C, Kattenhorn M, Zadinello M, Donald A, Leeson P, et al. Vascular dysfunction after repair of coarctation of the aorta: impact of early surgery. Circulation 2001; 104(12 Suppl 1):I165–70. [DOI] [PubMed]
- 11.Diller GP, Dimopoulos K, Okonko D, Li W, Babu-Narayan SV, Broberg CS, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation 2005;112:828–35. [DOI] [PubMed]
- 12.Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, Coats AJ. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2) slope and peak VO(2). Eur Heart J 2000;21:154–61. [DOI] [PubMed]
- 13.Remme WJ, Swedberg K; Task Force for the Diagnosis and Treatment of Chronic Heart Failure, European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure [published erratum appears in Eur Heart J 2001;22:2217–8]. Eur Heart J 2001;22:1527–60. [DOI] [PubMed]
- 14.Bolger AP, Sharma R, Li W, Leenarts M, Kalra PR, Kemp M, et al. Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation 2002;106:92–9. [DOI] [PubMed]
- 15.Packer M. Should B-type natriuretic peptide be measured routinely to guide the diagnosis and management of chronic heart failure? Circulation 2003;108:2950–3. [DOI] [PubMed]
- 16.Cleland JC, Goode K. Natriuretic peptides for heart failure. Fashionable? Useful? Necessary? Eur J Heart Fail 2004;6:253–5. [DOI] [PubMed]
- 17.Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, Pacher R. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 2002;105: 2392–7. [DOI] [PubMed]
- 18.Law YM, Keller BB, Feingold BM, Boyle GJ. Usefulness of plasma B-type natriuretic peptide to identify ventricular dysfunction in pediatric and adult patients with congenital heart disease. Am J Cardiol 2005;95:474–8. [DOI] [PubMed]
- 19.Cowley CG, Bradley JD, Shaddy RE. B-type natriuretic peptide levels in congenital heart disease. Pediatr Cardiol 2004; 25:336–40. [DOI] [PubMed]
- 20.Holmgren D, Westerlind A, Lundberg PA, Wahlander H. Increased plasma levels of natriuretic peptide type B and A in children with congenital heart defects with left compared with right ventricular volume overload or pressure overload. Clin Physiol Funct Imaging 2005;25:263–9. [DOI] [PubMed]
- 21.Hopkins WE, Chen Z, Fukagawa NK, Hall C, Knot HJ, LeWinter MM. Increased atrial and brain natriuretic peptides in adults with cyanotic congenital heart disease: enhanced understanding of the relationship between hypoxia and natriuretic peptide secretion. Circulation 2004;109:2872–7. [DOI] [PubMed]
- 22.Iivainen TE, Groundstroem KW, Lahtela JT, Talvensaari TJ, Pasternack A, Uusitalo A. Serum N-terminal atrial natriuretic peptide in adult patients late after surgical repair of atrial septal defect. Eur J Heart Fail 2000;2:161–5. [DOI] [PubMed]
- 23.Ohuchi H, Takasugi H, Ohashi H, Yamada O, Watanabe K, Yagihara T, Echigo S. Abnormalities of neurohormonal and cardiac autonomic nervous activities relate poorly to functional status in Fontan patients. Circulation 2004;110:2601–8. [DOI] [PubMed]
- 24.Mair J, Hammerer-Lercher A, Puschendorf B. The impact of cardiac natriuretic peptide determination on the diagnosis and management of heart failure. Clin Chem Lab Med 2001; 39:571–88. [DOI] [PubMed]
- 25.Cheung BM. Plasma concentration of brain natriuretic peptide is related to diastolic function in hypertension. Clin Exp Pharmacol Physiol 1997;24:966–8. [DOI] [PubMed]
- 26.Gerber IL, Legget ME, West TM, Richards AM, Stewart RA. Usefulness of serial measurement of N-terminal pro-brain natriuretic peptide plasma levels in asymptomatic patients with aortic stenosis to predict symptomatic deterioration. Am J Cardiol 2005;95:898–901. [DOI] [PubMed]
- 27.Fukuda N, Shinohara H, Sakabe K, Nada T, Tamura Y. Plasma levels of brain natriuretic peptide in various forms of obstruction to the left ventricular outflow tract. J Heart Valve Dis 2003;12:333–40. [PubMed]
- 28.Weber M, Arnold R, Rau M, Brandt R, Berkovitsch A, Mitrovic V, Hamm C. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004;94:740–5. [DOI] [PubMed]
- 29.Patel DN, Bailey SR. Role of BNP in patients with severe asymptomatic aortic stenosis. Eur Heart J 2004;25:1972–3. [DOI] [PubMed]
- 30.European Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension [published errata appear in J Hypertens 2003;21:2203–4 and J Hypertens 2004; 22:435]. J Hypertens 2003;21:1011–53. [DOI] [PubMed]
- 31.Balderston SM, Daberkow E, Clarke DR, Wolfe RR. Maximal voluntary exercise variables in children with postoperative coarctation of the aorta. J Am Coll Cardiol 1992;19:154–8. [DOI] [PubMed]
- 32.Rhodes J, Geggel RL, Marx GR, Bevilacqua L, Dambach YB, Hijazi ZM. Excessive anaerobic metabolism during exercise after repair of aortic coarctation. J Pediatr 1997;131:210–4. [DOI] [PubMed]
- 33.Fredriksen PM, Veldtman G, Hechter S, Therrien J, Chen A, Warsi MA, et al. Aerobic capacity in adults with various congenital heart diseases. Am J Cardiol 2001;87:310–4. [DOI] [PubMed]
- 34.Dimopoulou I, Daganou M, Tsintzas OK, Tzelepis GE. Effects of severity of long-standing congestive heart failure on pulmonary function. Respir Med 1998;92:1321–5. [DOI] [PubMed]
- 35.Kaemmerer H, Oelert F, Bahlmann J, Blucher S, Meyer GP, Mugge A. Arterial hypertension in adults after surgical treatment of aortic coarctation. Thorac Cardiovasc Surg 1998;46: 121–5. [DOI] [PubMed]
- 36.Tantengco MV, Ross RD, Humes RA, Sullivan NM, Joshi VM, Clapp SK, Epstein ML. Enhanced resting left ventricular filling in patients with successful coarctation repair and exercise-induced hypertension. Am Heart J 1997;134:1082–8. [DOI] [PubMed]
- 37.Leandro J, Smallhorn JF, Benson L, Musewe N, Balfe JW, Dyck JD, et al. Ambulatory blood pressure monitoring and left ventricular mass and function after successful surgical repair of coarctation of the aorta. J Am Coll Cardiol 1992; 20:197–204. [DOI] [PubMed]
- 38.Westerlind A, Wahlander H, Lindstedt G, Lundberg PA, Holmgren D. Clinical signs of heart failure are associated with increased levels of natriuretic peptide types B and A in children with congenital heart defects or cardiomyopathy. Acta Paediatr 2004;93:340–5. [DOI] [PubMed]
- 39.Mir TS, Flato M, Falkenberg J, Haddad M, Budden R, Weil J, et al. Plasma concentrations of N-terminal brain natriuretic peptide in healthy children, adolescents, and young adults: effect of age and gender. Pediatr Cardiol 2006;27:73–7. [DOI] [PubMed]
- 40.Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, et al. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation 2003;107:2786–92. [DOI] [PubMed]


