Abstract
Normal auditory perception relies on accurate judgments about the temporal relationships between sounds. Previously, we used a perceptual-learning paradigm to investigate the neural substrates of two such relative-timing judgments made at sound onset: detecting stimulus asynchrony and discriminating stimulus order. Here, we conducted parallel experiments at sound offset. Human adults practiced ∼1 h/d for 6–8 d on either asynchrony detection or order discrimination at sound offset with tones at 0.25 and 4.0 kHz. As at sound onset, learning on order-offset discrimination did not generalize to the other task (asynchrony), an untrained temporal position (onset), or untrained frequency pairs, indicating that this training affected a quite specialized neural circuit. In contrast, learning on asynchrony-offset detection generalized to the other task (order) and temporal position (onset), though not to untrained frequency pairs, implying that the training on this condition influenced a less specialized, or more interdependent, circuit. Finally, the learning patterns induced by single-session exposure to asynchrony and order tasks differed depending on whether these tasks were performed primarily at sound onset or offset, suggesting that this exposure modified circuitry specialized to separately process relative-timing tasks at these two temporal positions. Overall, it appears that the neural processes underlying relative-timing judgments are malleable, and that the nature of the affected circuitry depends on the duration of exposure (multihour or single-session) and the parameters of the judgment(s) made during that exposure.
Accurately determining the temporal relationships between sounds is critical for normal auditory perception. Two auditory tasks that rely on such relative-timing judgments are asynchrony detection and order discrimination. In an asynchrony-detection task, the listener determines whether a sound’s frequency components are synchronous or asynchronous, and in an order-discrimination task, the listener distinguishes the order of the component frequencies. Asynchrony judgments aid in the separation of sound sources (Bregman et al. 1994), while order judgments are used in the processing of speech (“mats” vs. “mast”) and music (ascending vs. descending scales). In the present investigation, we use a behavioral perceptual-learning paradigm to gain insight into the neural circuitry underlying performance on auditory asynchrony and order tasks at sound offset, for comparison with the results of a previous examination of learning on the same tasks at sound onset.
We previously reported that training on auditory relative-timing tasks at sound onset resulted in learning that did not generalize to any of a set of untrained conditions, suggesting that the underlying circuitry is highly specialized (Mossbridge et al. 2006). In that investigation, we trained one group of listeners on an asynchrony task (Fig. 1A) and one group on an order task (Fig. 1B), both at sound onset with the same frequency pair (0.25 and 4.0 kHz). Multihour training on either condition induced learning that did not generalize to the untrained task (order or asynchrony), an untrained temporal position (offset), or untrained frequency pairs. Following the reasoning used in previous perceptual-learning experiments, we interpreted this lack of generalization to indicate that the circuitry modified during training did not contribute to performance on the untrained conditions we tested (Karni 1996; Karni and Bertini 1997; Wright et al. 1997; Wright and Fitzgerald 2001; Karmarkar and Buonomano 2003). Thus, we concluded that training on auditory relative-timing tasks at sound onset affected neural circuitry that was specialized to separately process different tasks, temporal positions, and frequency pairs. Behavioral and physiological evidence, when considered as a whole, largely supports this idea (see Mossbridge et al. 2006).
Figure 1.
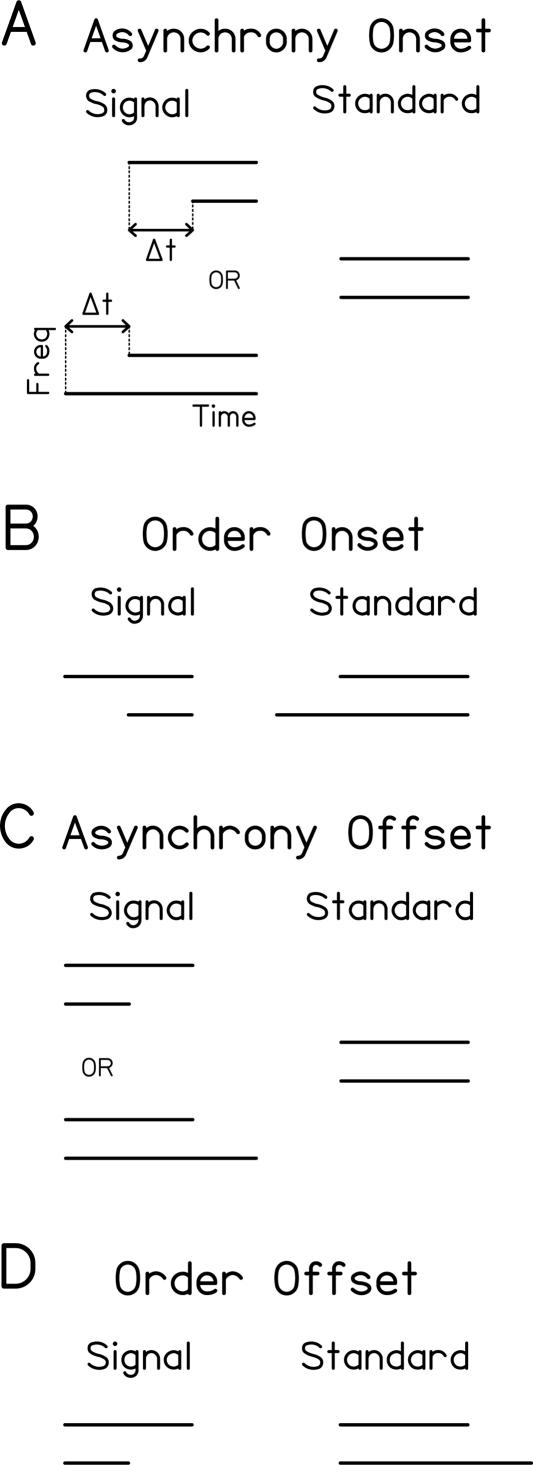
Relative-timing conditions. Schematic diagrams of the signal and standard observation periods in each two-interval forced-choice trial for four relative-timing conditions: asynchrony onset (A), order onset (B), asynchrony offset (C), order offset (D). All stimuli consisted of two-tone complexes in which the duration of the higher frequency tone was always 500 msec. Frequencies of the tones depended on the condition parameters (see Fig. 3; Materials and Methods). [Reprinted with permission from the Society for Neuroscience © 2006, Mossbridge et al. 2006.]
Here we test the possibility that there may be, as at sound onset, two separate, frequency-pair specific circuits underlying performance of asynchrony and order at sound offset. To test this idea, we conducted two parallel experiments examining the learning induced by training on auditory asynchrony or order tasks performed at sound offset, with special attention to the potential generalization of this learning to untrained conditions. We are aware of no previous investigations of learning on asynchrony-offset judgments, and of only two addressing training-induced learning, but not the generalization of that learning, on order-offset judgments. Of these two, in one, a single session of exposure yielded no statistically significant learning on an order-offset condition (Bregman et al. 1994). In the other, in a passing comment, extensive training was reported to have led to improvement on order-discrimination conditions at sound offset, but no learning data were shown (Pastore 1983).
Each of the two current experiments consisted of a pre-test, training phase, and post-test. Every listener was tested on both a pre-test and a post-test consisting of six relative-timing conditions. Between these tests, a subset of trained listeners practiced a single condition (either asynchrony, Fig. 1C, or order, Fig. 1D) at sound offset with tones at 0.25 and 4.0 kHz for ∼1 h per day for 8–9 d, while the remaining control listeners did not receive multihour training. For each group of trained listeners, we evaluated learning on the trained condition, as well as on untrained conditions differing from the trained condition in the task, temporal position, or frequency pair. The conclusions about the influence of multihour training were based on comparisons between the performance of the trained listeners and that of the controls.
The present results suggest that different neural circuits are influenced by training on the asynchrony- and order-offset conditions. Multihour training on the order-offset condition appears to have affected specialized circuitry reminiscent of that suggested by the results previously obtained at sound onset (Mossbridge et al. 2006). In contrast, training on the asynchrony-offset condition appears to have engaged a less specialized, or perhaps more interdependent, circuit. Finally, single-session exposure to relative-timing conditions seems to have modified different neural processes depending on whether that exposure consisted of conditions primarily at sound onset or offset.
Results
Improvement over training sessions
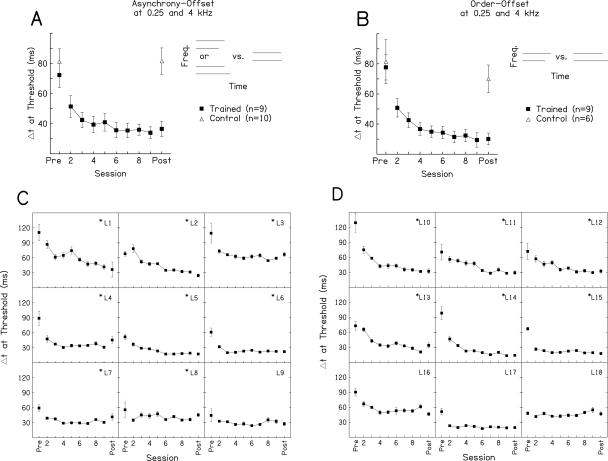
Multihour training on asynchrony and order judgments at sound offset resulted in significant improvement over the training sessions on both group and individual measures of performance (Fig. 2). On average, each of the two groups of trained listeners learned significantly on the condition on which they were trained, as indicated by one-way ANOVAs on mean thresholds with repeated measures over the training sessions (asynchrony-offset trained: F(7,56) = 6.41, P < 0.001; order-offset trained: F(7,56) = 8.67, P < 0.001) (Fig. 2). Individually, nearly all of the trained listeners improved significantly during these sessions. The thresholds of eight of the nine asynchrony-offset trained listeners (Fig. 2C, L1-L8) and of six of the nine order-offset trained listeners (Fig. 2D, L10-L15) changed significantly across training sessions according to one-way ANOVAs and yielded significant negative slopes when fitted with regression lines (α = 0.05 for both analyses). We performed all analyses both with and without the listeners who did not learn during the training phase and reached the same statistical conclusions; therefore, the results presented are based on the data from all listeners.
Figure 2.
Improvement over training sessions. (A,B) Mean learning curves for listeners trained on asynchrony-offset detection (A) or order-offset discrimination (B). Schematics of the trained conditions are at the right of each panel. Each learning curve shows the average thresholds of trained listeners on the trained condition on the pre-test, each training session, and the post-test (filled squares); the pre- and post-test thresholds of controls are also shown (open triangles). Error bars indicate ±1 SEM across listeners. (C,D) Individual learning curves for listeners trained on asynchrony-offset detection (C) and order-offset discrimination (D). Error bars indicate ±1 SEM within each listener. Asterisks indicate listeners who showed significant learning over the training phase.
Improvement on pre- and post-test conditions
In the majority of cases, controls did not show improvement between the pre- and post-tests (Fig. 3, triangles). Across the two experiments, paired t-tests on the pre- and post-test thresholds of controls revealed significant learning or a trend toward improvement on only two of the 12 conditions (asynchrony offset at 0.5 and 1.5 kHz: t9 = 3.02, P = 0.015, and asynchrony offset at 0.75 and 1.25 kHz: t9 = 1.96, P = 0.082; all other P-values ≥ 0.107).
Figure 3.
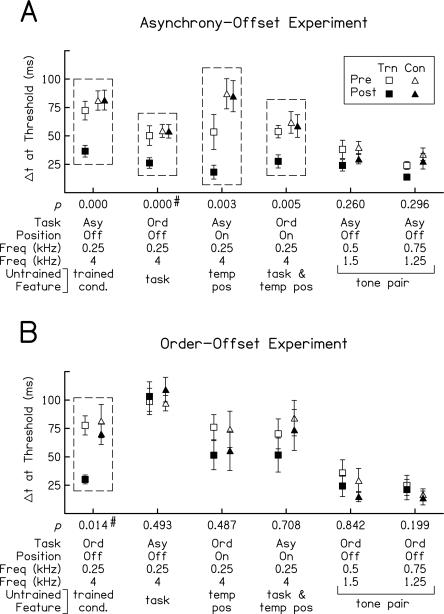
Improvement on pre- and post-test conditions. Mean thresholds on the pre-tests (open symbols) and post-tests (filled symbols) for trained listeners (squares) and controls (triangles) on each condition tested. Results are shown for nine trained listeners and 10–16 controls in the asynchrony-offset experiment (A) and for nine trained listeners and six to 16 controls in the order-offset experiment (B). Error bars indicate ±1 SEM across listeners. Condition parameters and the untrained features are listed along the abscissa. P-values are given for ANCOVAs or, in two cases, an ANOVA (marked with a # next to the P-values). Boxes indicate conditions on which trained listeners learned significantly more than controls.
Each of the two groups of trained listeners improved more than controls on the trained conditions (Fig. 3A,B, leftmost condition). For each condition on the pre- and post-tests, we determined whether multihour training resulted in learning by comparing post-test thresholds between trained listeners and controls with an ANCOVA in which pre-test threshold was the covariate. These analyses revealed that both groups of trained listeners improved significantly more than their respective controls between the pre- and post-tests on the condition that they practiced during multihour training (asynchrony-offset trained vs. controls: F(1,16) = 21.66, P < 0.001; order-offset trained vs. controls, ANOVA: F(1,13) = 8.12, P = 0.014, an ANCOVA was precluded because a test of the homogeneity of regression was significant) (Hays 1994).
Interestingly, the two groups of trained listeners differed in the generalization of this training-induced learning to untrained conditions. Listeners trained on order-offset discrimination did not learn significantly more than controls on any untrained condition (all ANCOVA P-values ≥ 0.199). In contrast, listeners trained on asynchrony-offset detection improved more than controls on the three untrained conditions that shared the same frequency pair with the trained condition (order offset ANOVA: F(1,17) = 22.96, P < 0.001, an ANCOVA was precluded because a test of the homogeneity of regression was significant [Hays 1994]; asynchrony onset: F(1,16) = 12.79, P = 0.003; order onset: F(1,16) = 10.50, P = 0.005) (Fig. 3A) but not on the two asynchrony-offset conditions with untrained frequency pairs (both ANCOVA P-values ≥ 0.260). Thus, multihour training on order-offset discrimination induced significant learning that was specific to the trained task, temporal position, and frequency pair, while multihour training on asynchrony-offset detection resulted in significant learning that generalized to the untrained task and temporal position, but was specific to the trained frequency pair.
Pre-test thresholds vs. improvement
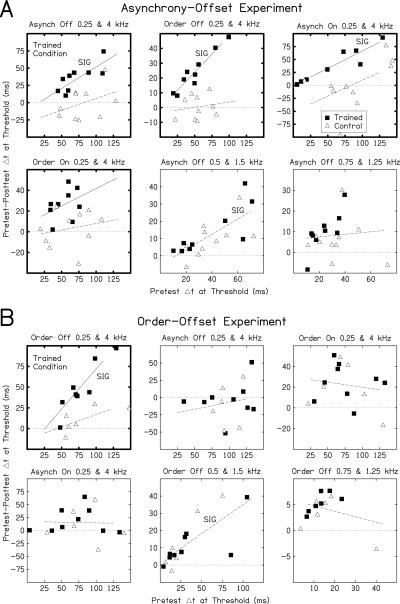
The amount of learning was not correlated with pre-test performance in the majority of cases. To examine the relationship between pre-test threshold and the magnitude of improvement, we determined the linear regression of the amount of learning (pre-test minus post-test threshold, Y-axis) on the pre-test threshold (X-axis) in each condition (Fig. 4). Separate regression lines were fitted for trained listeners and controls only on the conditions for which the two groups were statistically separable (as indicated by darker frames in Fig. 4). For the other conditions, one regression line was fitted to combined data from both groups. Higher pre-test thresholds were associated with greater learning between the pre- and post-tests primarily for trained listeners on the conditions in which they learned more than controls (four out of five regression lines for trained listeners had significantly positive slopes; lines marked “SIG” in Fig. 4). However, in the majority of the remaining cases, there was no consistent relationship between starting value and improvement (only two of the 12 remaining regression lines had significant slopes).
Figure 4.
Pre-test thresholds vs. improvement. The amount of improvement (pre-test threshold subtracted from post-test threshold [Y-axes]) plotted as a function of pre-test threshold (X-axes) for trained listeners (filled squares) and controls (open triangles) in the asynchrony-offset (A) and order-offset (B) experiments. The linear regression of the amount of improvement on the pre-test thresholds was determined for each data set. Separate regression lines were estimated for trained listeners (solid) and controls (dashed) only on the conditions for which analyses of pre- and post-test thresholds indicated a significant difference between the two groups (darker frames). On each remaining condition, the regression line was estimated from the combined data (dashed). The dotted horizontal line at Y = 0 indicates no learning; values above this line indicate that performance improved between the pre- and post-tests. Regression lines with slopes significantly different from zero are marked “SIG.” The regression line for order offset at 0.75 and 1.25 kHz (B, bottom right) was calculated both with (line not shown) and without (line shown) two extreme values (X,Y = 90,30 and X,Y = 41,−34; points not shown). The same conclusions were reached in both cases.
Discussion
Listeners who received multihour training on an asynchrony or order task at sound offset improved on the trained condition. Order-offset training produced learning that was specific to the trained condition, while listeners trained on asynchrony offset generalized their learning to conditions with the untrained task (order offset), the untrained temporal position (asynchrony onset), and the untrained task and temporal position (order onset). These improvements can be attributed to the repeated practice of the trained condition, as there was little or no improvement in listeners who only participated in the pre- and post-testing sessions.
Comparison across four learning experiments
Here we compare the learning patterns obtained at sound offset (present data) with those previously observed on the same tasks at sound onset (Mossbridge et al. 2006). This comparison, which includes several additional analyses not already presented in the results, reveals that some, but not all, of the features of the learning induced by multihour training and pre-test exposure were shared across the four experiments. Based on these results, we draw conclusions about the neural circuitry affected both by extended training on, and single-session exposure to, relative-timing tasks. However, before we present these conclusions, we first consider and reject two alternative explanations for the results.
Possible alternative explanations
Although in all conditions the standard and signal stimuli could be distinguished on the basis of the differing durations of the lower frequency tones (Fig. 1), listeners likely did not use this duration cue. We previously argued that, at sound onset, the duration cue was not used to perform the trained relative-timing conditions for two reasons (Mossbridge et al. 2006). First, listeners trained at sound onset had thresholds on the trained conditions that were well below those reported for duration discrimination with a 500-msec standard in highly trained listeners (Abel 1972). Second, while duration-discrimination learning generalizes to stimuli presented at untrained frequencies (Wright et al. 1997; Karmarkar and Buonomano 2003), learning in the onset experiments did not. Both arguments also hold for the present data obtained at sound offset. Thus, in all four experiments, it appears that listeners made a comparison between the timing of tone onsets or offsets, not a duration judgment.
Further, while confusion could have arisen because the signal and the standard in the order task both served as signals in the asynchrony task, and vice versa, it is unlikely that this potential source of confusion contributed to the observed failures to generalize between the asynchrony and order tasks. Arguing against this “stimulus confusion” explanation, we previously noted that training on order-onset discrimination did not generalize to asynchrony-onset detection, even when the source of potential stimulus confusion was removed by using only one asynchronous signal in the asynchrony-onset condition (E.S. O’Connor and B.A. Wright, unpubl.). Strengthening this argument, the current generalization of learning from asynchrony offset to order-onset and -offset shows that listeners are capable of recategorizing the asynchrony stimuli for use in the order task.
Implications for neural circuitry
Given that the data from all four experiments appear to reflect the ability of listeners to make the tested relative-timing judgments, the learning patterns induced by multihour training provide a window into the circuitry modified as a result of making repeated relative-timing judgments. Trained listeners in all four experiments learned significantly more than controls on the conditions they practiced during training (Fig. 5, top row). Thus, practice can influence at least a portion of the neural circuitry underlying performance on relative-timing conditions at both sound onset and offset.
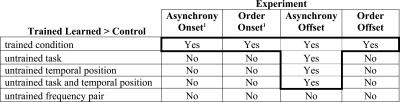
Figure 5.
Training-induced learning in four relative-timing training experiments. Influence of multihour training in each of four relative-timing experiments (columns) on the trained and untrained conditions (rows). In some cases, trained listeners learned more than controls (“Yes”), and in others the performance of trained listeners was statistically indistinguishable from that of controls (“No”).1Data from Mossbridge et al. (2006).
Characteristics of this affected circuitry can be inferred based on the assumption that generalization of learning from a trained condition to an untrained condition occurs if and only if the neural circuitry modified during training also influences performance on the untrained condition (Karni 1996; Karni and Bertini 1997; Wright et al. 1997; Wright and Fitzgerald 2001; Karmarkar and Buonomano 2003; Mossbridge et al. 2006). For the trained asynchrony-onset, order-onset, and order-offset conditions, learning did not generalize to any untrained condition (Fig. 5, columns 1, 2, 4, rows 2–5). Given our assumption, these results suggest that multihour training on each of these three conditions affected three separate neural circuits that specifically process the trained task, temporal position, and frequency pair.
Each of these three conclusions about the specificity of relative-timing processing is supported by other evidence. First, the idea that separate circuitry underlies performance on asynchrony and order tasks has been inferred from visual and audiovisual experiments revealing differences in the influence of parameter manipulations between the two relative-timing tasks (Mitrani et al. 1986; Fujisaki et al. 2004). Further, neurons that respond exclusively either to asynchronous or ordered sounds have been found in at least one species (mustached bat, Portfors and Wenstrup 1999; Leroy and Wenstrup 2000). It is also worth noting that neuronal models that compute asynchrony do not compute order (Jeffress 1948), and vice versa (Lewicki and Konishi 1995; Drew and Abbott 2003). Second, supporting the idea that different circuitry underlies relative-timing judgments made at sound onset versus offset, both performance on relative-timing tasks and physiological responses to auditory stimuli differ between these two temporal positions (for behavior, see Raphael 1972; Pastore et al. 1982; Pastore 1983; Zera and Green 1993; Phillips et al. 2002; for physiology, see Brugge and Merzenich 1973; Pfingst and O’Connor 1981; He et al. 1997; Recanzone 2000; He 2002; Takahashi et al. 2004). Third, in agreement with the idea that relative-timing circuitry processes each frequency combination separately, performance on asynchrony and order (or related sequence-identification) tasks has been shown to differ depending on the frequencies of the tones (for asynchrony, see Portfors and Wenstrup 1999; Leroy and Wenstrup 2000; for order, see Divenyi and Hirsh 1974; Wier and Green 1975; Kelly and Watson 1986; Barsz 1988; Barsz 1996). In parallel, auditory neurons sensitive only to particular frequency combinations have been observed in multiple species (Suga et al. 1979; Margoliash 1983; Margoliash and Fortune 1992; Tian and Rauschecker 1994; Lewicki and Konishi 1995; Ohlemiller et al. 1996; Esser et al. 1997; Brosch et al. 1999; Leroy and Wenstrup 2000; Kilgard and Merzenich 2002).
In contrast to the three other trained conditions, for the asynchrony-offset condition, learning generalized to conditions with the untrained task (order) and temporal position (onset), though this learning was again specific to the trained frequency pair (Fig. 5, column 3, rows 2–5). Thus, an asymmetric generalization pattern emerged: Despite the lack of generalization to asynchrony offset from any of the other three relative-timing conditions considered here, learning generalized from asynchrony offset to these three conditions at the trained frequency pair. Note that the observed generalization from asynchrony offset to order offset is unlikely to simply reflect the apparent need for a determination of asynchrony to precede a judgment of order (Hirsh 1959), because learning on asynchrony at sound onset did not improve performance on order at sound onset (Mossbridge et al. 2006), and learning on order did not improve asynchrony performance at either temporal position. In addition, suggesting that the unique generalization pattern for asynchrony offset did not result from differences in stimulus exposure, the stimuli used at sound onset and offset were mirror images of one another, but the generalization results were not.
There are at least two different arrangements of relative-timing circuitry that could account for the learning patterns observed across the four experiments. One possibility is that four separate frequency-specific circuits underlie performance on the four trained conditions, but there is a unidirectional dependency between the circuitry affected by training on the asynchrony-offset condition and that governing performance on each of the other three relative-timing conditions we trained. Unidirectional dependency was proposed to explain another asymmetric across-task generalization pattern, in which learning on a visual direction-discrimination task generalized to an orientation-discrimination task, but not the reverse (Matthews et al. 1999). Another possibility is that performance on the asynchrony-offset condition relies on a frequency-specific global relative-timing circuit, while performance on the other three conditions can be governed either by this global relative-timing circuit or by one of three separate, specialized, frequency-specific circuits, and that training modifies the global timing circuit only if a specialized circuit is not available. According to this idea, training on asynchrony at sound offset alters the global circuit, resulting in improvements in performance not only on that condition but also on the other relative-timing conditions at the trained frequency pair. However, training on any of the three other conditions affects only the appropriate specialized circuit, and therefore does not influence performance on any of the other conditions.
Note on task difficulty
Interestingly, the only relative-timing condition for which training resulted in broad generalization was also the condition that listeners reported to be the most difficult. Nearly all listeners commented that the condition used for training in the asynchrony-offset experiment was the “hardest” of all the tested conditions. Because all conditions targeted the same level of performance (79.4% correct), we interpret the statements of these listeners to mean that they required more effort to reach the same performance level. In parallel to this anecdotal observation, pre-test thresholds on this condition across the four experiments were significantly higher than those on the other three trained conditions (ANOVA on pre-test thresholds across all four trained conditions: F(3,281) = 18.14, P < 0.001; all post-hoc t-tests between asynchrony-offset thresholds and thresholds on the other three conditions P < 0.0001). Thus, it is possible that the apparent greater effort necessary to perform the trained asynchrony-offset condition contributed to the broader generalization of learning on that condition. If effort can be considered a reflection of task difficulty, the current proposal that greater task difficulty may be associated with broader generalization is the opposite of a previous proposal, based on observations of visual-perceptual learning, that greater task difficulty is associated with less generalization (Ahissar and Hochstein, 1997). Thus, it is possible that either effort is not equivalent to difficulty, or the relationship between difficulty and generalization is not consistent across task types.
Pre-test-induced learning
Regarding the influence of pre-test exposure, there were two differences in the patterns of pre-test-induced learning between the onset and offset experiments, suggesting differences between the neural circuitry affected by single-session exposure to relative-timing tasks at sound onset than at sound offset. First, controls showed more learning from pre- to post-test as a result of exposure to the onset than the offset pre-tests. Indicating that the amount of this pre-test-induced learning differed depending on the pre-test to which controls were exposed, a 2 time (pre-test vs. post-test) by 4 control group (one group for each experiment) × 4 condition ANOVA (four conditions common to all four experiments), with repeated measures on time, revealed a significant interaction between time and control group (F(3,128) = 2.76, P = 0.045). Because the condition order on the pre-tests was randomized, it could not be determined whether the improvement on any single condition was due to exposure to that condition or the transfer of learning from another condition (Wright and Fitzgerald 2001; Mossbridge et al. 2006). Nevertheless, because the pre-tests across the four experiments differed in their task and temporal-position weighting, it was possible to examine whether these factors contributed to the differences in pre-test-induced learning between the onset and offset experiments. We first compared controls who performed pre-tests weighted toward one or the other temporal position in a 2 time (pre-test vs. post-test) × 2 temporal position (two groups of onset controls vs. two groups of offset controls) × 4 shared condition ANOVA. This analysis revealed a significant time × temporal position interaction (F(1,136) = 6.18, P = 0.014), with the onset controls improving more. Thus, pre-test exposure to relative-timing tasks primarily performed at sound onset induced more pre-to-post-test improvement than exposure to tasks primarily performed at sound offset. A similar analysis comparing controls who performed pre-tests weighted toward one or the other task (two groups of asynchrony controls vs. two groups of order controls) yielded a main effect of time (F(1,136) = 17.67, P < 0.001) but no interactions, indicating that the task-weighting of the pre-tests was not a significant factor in determining the amount of pre-to-post-test improvement in controls. These results are consistent with those of a previous investigation in which a single session of training resulted in significant learning on an order-onset, but not an order-offset condition (Bregman et al. 1994).
Second, related to the previous point, there is some evidence that controls improved in all four experiments, but that this learning was retained until the post-test only in the onset experiments. To assess the retention of pre-test-induced learning, for each experiment, we examined performance on the condition used during training. We assumed that, had the thresholds of controls been measured on the day after the pre-test, the improvement of controls would have been equivalent to that of the trained listeners from the pre-test to this day (Mossbridge et al. 2006). Thus, on each condition, if the trained listeners improved from the pre-test to the first training day, but the controls did not improve from the pre- to post-test, it would indicate that controls did not retain the gains induced by pre-test exposure. It appears that such a loss of learning in controls occurred on both offset conditions used during training. In these cases, there were significant improvements in trained listeners between the pre-test and the first day of training (both P-values ≤ 0.006; each measure based on the only or first five threshold estimates) (also see Fig. 2). However, controls did not improve significantly between the pre- and post-tests on these particular conditions (paired t-tests, both P-values ≥ 0.126; each measure based on five threshold estimates). In contrast to this loss of learning in the offset experiments, in the onset experiments, the pre-test-induced learning evident in controls on the post-test was no different from that on the first day of training for trained listeners (Mossbridge et al. 2006). Therefore, there was greater retention of the learning induced by exposure to the onset than the offset pre-tests.
As a final note, it appears that the learning induced by pre-test exposure and that induced by multihour training may have different neural underpinnings. For single-session learning on relative-timing conditions, the temporal position of the disparity (onset vs. offset) appears to have had more influence than the particular task performed (asynchrony vs. order). However, both of these factors influenced the learning patterns induced by multihour training. This difference suggests that there is at least one distinction between the neural processes affected by different amounts of exposure. Evidence that different brain regions are activated as a result of single-session versus multihour training on other tasks (Buckner and Koutstaal 1998; Petersen et al. 1998) is consistent with this conclusion.
Summary
We examined learning patterns on relative-timing conditions at sound offset (present data) and compared them with those previously obtained at sound onset (Mossbridge et al. 2006) to shed light on the characteristics of the underlying neural circuitry. Multihour training on asynchrony and order tasks performed at sound offset resulted in improvements on the condition practiced during training, but the generalization of this learning differed depending on the trained condition. For the order-offset condition, learning did not generalize to the untrained task (asynchrony), temporal position (onset), or any untrained frequency pairs, echoing the specificity of learning previously observed for both the asynchrony and order conditions performed at sound onset (Mossbridge et al. 2006). In contrast, while learning on the asynchrony-offset condition was also specific to the trained frequency pair, learning generalized to the untrained task (order) and temporal position (onset). The results across all four training experiments suggest that, at least in terms of the circuitry affected by the training regimen we used: (1) three separate circuits govern performance on the asynchrony-onset, order-onset, and order-offset conditions; (2) there is an asymmetric relationship between the circuitry that controls performance on the asynchrony-offset condition and that contributing to performance on the other three conditions; and (3) all of the engaged circuits are specialized to process the trained frequency pair. Single-session exposure to relative-timing conditions also yielded learning. The characteristics of this pre-test-induced learning depended on the conditions to which listeners were exposed on the pre-tests. Exposure to pre-tests consisting of primarily onset, as opposed to offset conditions, resulted in greater improvement between the pre-test and a post-test administered 8–13 d later, a difference that may reflect, in part, a longer retention of the learning induced by exposure to the onset pre-tests. This pattern suggests that the circuitry affected by single-session exposure to relative-timing tasks may be specialized to separately process judgments made at sound onset versus offset. Taken together, these data indicate that the circuitry contributing to performance on relative-timing conditions is malleable, and that the nature of the affected circuitry depends on the duration of exposure and the parameters of the condition(s) to which listeners are exposed.
Materials and Methods
The methods were similar to those we used previously in the investigation of learning on auditory relative-timing tasks at sound onset (Mossbridge et al. 2006).
Experimental design
We conducted two experiments referred to as the asynchrony-offset and order-offset experiments, each consisting of a pre-test, a training phase, and a post-test. In the pre- and post-tests, we measured thresholds on six related relative-timing conditions that differed between the experiments, but were the same within each listener. The training phase occurred between the pre- and post-tests. In the training phase, we measured thresholds in a subset of randomly chosen listeners, referred to as trained listeners, for ∼1 h per day for 8–9 d on a single condition, either asynchrony-offset detection (asynchrony-offset experiment) or order-offset discrimination (order-offset experiment), using tones at 0.25 and 4.0 kHz. The remaining listeners, referred to as controls, only participated in the pre- and post-tests. The pre- and post-tests were separated by an average of 12.2 d for the trained listeners in the asynchrony-offset experiment, 13.2 d for the trained listeners in the order-offset experiment, and 13.2 d for the controls in both experiments.
Listeners
Thirty-four paid participants (19 females) between the ages of 18 and 32 yr served as listeners. All reported normal hearing, and none had previously performed psychoacoustic tasks. There were nine trained listeners and 10 controls in the asynchrony-offset experiment, and a separate group of nine trained listeners and six controls in the order-offset experiment. Only individuals who passed an initial tone-detection screening were used as listeners.
Conditions and stimuli
For all conditions, in each of two observation periods, listeners were presented with a standard stimulus and a signal stimulus. The signal was randomly presented in either the first or second observation period. The listener was instructed to press a key on a computer keyboard to indicate which interval contained the signal. Visual feedback as to whether the response was correct or incorrect was given after every trial throughout the entire experiment.
The two trained conditions differed in the task to be performed: asynchrony detection or order discrimination. The signal and standard for both trained conditions were composed of tones at 0.25 and 4 kHz that began simultaneously. In the asynchrony-offset experiment, the trained condition was asynchrony-offset at 0.25 and 4 kHz. In this condition, the listener had to determine which observation period contained two tones that ended nonsimultaneously, rather than simultaneously (Fig. 1C). In the signal, the offset of one tone lagged the other by some temporal disparity (Δt), with the lagging tone selected at random for each signal presentation. In the standard, the two tones ended at the same time. In the order-offset experiment, the trained condition was order-offset at 0.25 and 4 kHz. In this condition, the listener had to determine in which observation period the higher frequency tone ended after the lower frequency tone, rather than the reverse (Fig. 1D). In the signal, the offset of the 4-kHz tone lagged that of the 0.25-kHz tone by a given temporal disparity (Δt), and in the standard, the offset of the 0.25-kHz tone lagged that of the 4-kHz tone by the same absolute temporal disparity (Δt).
There were three categories of untrained conditions on the pre- and post-tests. To examine whether learning generalized between the asynchrony and order tasks, in each experiment we tested performance on the trained condition from the other experiment (described above). To determine whether improvement at sound offset generalized to performance at sound onset, in each experiment we tested the trained and untrained tasks at sound onset with the trained frequency pair. In the asynchrony-onset condition, in the signal, the 0.25- and 4.0-kHz tones began at different times—with the leading tone selected at random for each presentation—and ended simultaneously (Fig. 1A). In the standard, the tones both began and ended simultaneously. In the order-onset condition, the onset of the 4-kHz tone led that of the 0.25-kHz tone in the signal, the onset of the 0.25-kHz tone led that of the 4-kHz tone in the standard, and the tones ended simultaneously (Fig. 1B). Finally, in each experiment, to examine whether learning generalized to untrained frequencies, we tested two conditions that were otherwise identical to the trained conditions, but used untrained frequency pairs (0.75 and 1.25 kHz; 0.5 and 1.5 kHz).
In all conditions, the duration of the higher frequency tone was always 500 msec, including 10-msec raised-cosine rise/fall ramps, and the duration of the lower frequency tone depended on Δt (Hirsh 1959; Pisoni 1980; Pastore et al. 1982; Pastore 1983; Parker 1988). The offsets of the higher frequency tones in the two observation periods were separated by 1000 msec. Each tone was presented at 70 dB SPL. The tones were generated digitally, and delivered through the left earpiece of Sennheiser HD265 headphones. All testing took place in a sound-attenuated chamber.
Procedure
In each block of 60 trials, we adaptively adjusted Δt to estimate threshold. To do so, we used a three-down/one-up algorithm in which we decreased Δt after every three consecutive correct responses and increased Δt after each incorrect response. Values of Δt at which the direction of change reversed from decreasing to increasing or vice versa are referred to as reversals. After discarding the first three reversals, the Δt at the 79.4% correct point on the psychometric function (threshold) was estimated by taking the average value of the greatest even number of remaining reversals as long as four reversals remained (Levitt, 1971). When there were fewer than four reversals, no threshold estimate was obtained on that block. The step size was 10 msec through the third reversal, and was 2 msec thereafter. On the pre-test, we set the initial Δt on the first trial of each condition at 60 msec and, during the training phase, set it at 10 msec above the average threshold obtained in the previous session from that particular listener.
We obtained four or five threshold estimates (240–300 trials) for each condition on the pre- and post-tests from all listeners, and, from the trained listeners, 12 threshold estimates (720 trials) for the trained condition during each training session. For each listener, the same condition order was used on the pre- and post-test, but this order was randomized across listeners. On the pre- and post-tests, all threshold measurements for a given condition were completed before proceeding to the next condition.
Acknowledgments
We thank Karen Banai, Matt Fitzgerald, Julia Huyck, Jeanette Ortiz, Andy Sabin, and Yuxuan Zhang for providing helpful comments on earlier drafts of this paper. We also thank two anonymous reviewers who substantially improved this manuscript. This work was supported by the National Institutes of Health/National Institute for Deafness and Other Communication Disorders.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.573608
References
- Abel S.M. Duration discrimination of noise and tone bursts. J. Acoust. Soc. Am. 1972;51:1219–1223. doi: 10.1121/1.1912963. [DOI] [PubMed] [Google Scholar]
- Ahissar M., Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- Barsz K. Auditory pattern perception: The effect of tonal frequency range on the perception of temporal order. Percept. Psychophys. 1988;48:293–303. doi: 10.3758/bf03207873. [DOI] [PubMed] [Google Scholar]
- Barsz K. Accuracy of same/different judgments of sequences of complex tones differing in tonal order under various levels of fundamental frequency range, listener training, and type of standard sequence. J. Acoust. Soc. Am. 1996;99:1660–1669. [Google Scholar]
- Bregman A.S., Ahad P.A., Kim J. Resetting the pitch-analysis system. 2. Role of sudden onsets and offsets in the perception of individual components in a cluster of overlapping tones. J. Acoust. Soc. Am. 1994;96:2694–2703. doi: 10.1121/1.411277. [DOI] [PubMed] [Google Scholar]
- Brosch M., Schulz A., Scheich H. Processing of sound sequences in macaque auditory cortex: response enhancement. J. Neurophysiol. 1999;82:1542–1559. doi: 10.1152/jn.1999.82.3.1542. [DOI] [PubMed] [Google Scholar]
- Brugge J.F., Merzenich M.M. Responses of neurons in auditory cortex of the macaque monkey to monaural and binaural stimulation. J. Neurophysiol. 1973;36:1138–1158. doi: 10.1152/jn.1973.36.6.1138. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Koutstaal W. Functional neuroimaging studies of encoding, priming, and explicit memory retrieval. Proc. Natl. Acad. Sci. 1998;95:891–898. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divenyi P.L., Hirsh I.J. Identification of temporal order in three-tone sequences. J. Acoust. Soc. Am. 1974;56:144–151. doi: 10.1121/1.1903245. [DOI] [PubMed] [Google Scholar]
- Drew P.J., Abbott L.F. Model of song selectivity and sequence generation in area HVc of the songbird. J. Neurophysiol. 2003;89:2697–2706. doi: 10.1152/jn.00801.2002. [DOI] [PubMed] [Google Scholar]
- Esser K.H., Condon C.J., Suga N., Kanwal J.S. Syntax processing by auditory cortical neurons in the FM-FM area of the mustached bat Pteronotus parnellii. Proc. Natl. Acad. Sci. 1997;94:14019–14024. doi: 10.1073/pnas.94.25.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki W., Shimojo S., Kashino M., Nishida S. Recalibration of audiovisual simultaneity. Nat. Neurosci. 2004;7:773–778. doi: 10.1038/nn1268. [DOI] [PubMed] [Google Scholar]
- Hays W.L. Statistics. Harcourt Brace; Orlando, FL: 1994. [Google Scholar]
- He J. OFF responses in the auditory thalamus of the guinea pig. J. Neurophysiol. 2002;88:2377–2386. doi: 10.1152/jn.00083.2002. [DOI] [PubMed] [Google Scholar]
- He J., Hashikawa T., Ojima H., Kinouchi Y. Temporal integration and duration tuning in the dorsal zone of cat auditory cortex. J. Neurosci. 1997;17:2615–2625. doi: 10.1523/JNEUROSCI.17-07-02615.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh I.J. Auditory perception of temporal order. J. Acoust. Soc. Am. 1959;31:759–767. [Google Scholar]
- Jeffress L.A. A place theory of sound localization. J. Comp. Physiol. Psychol. 1948;41:35–39. doi: 10.1037/h0061495. [DOI] [PubMed] [Google Scholar]
- Karmarkar U.R., Buonomano D.V. Temporal specificity of perceptual learning in an auditory discrimination task. Learn. Mem. 2003;10:141–147. doi: 10.1101/lm.55503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A. The acquisition of perceptual and motor skills: A memory system in the adult human cortex. Brain Res. Cogn. Brain Res. 1996;5:39–48. doi: 10.1016/s0926-6410(96)00039-0. [DOI] [PubMed] [Google Scholar]
- Karni A., Bertini G. Learning perceptual skills: Behavioral probes into adult cortical plasticity. Curr. Opin. Neurobiol. 1997;7:530–535. doi: 10.1016/s0959-4388(97)80033-5. [DOI] [PubMed] [Google Scholar]
- Kelly W.J., Watson C.S. Stimulus-based limitations on the discrimination between different temporal orders of tones. J. Acoust. Soc. Am. 1986;79:1934–1938. doi: 10.1121/1.393200. [DOI] [PubMed] [Google Scholar]
- Kilgard M.P., Merzenich M.M. Order-sensitive plasticity in adult primary auditory cortex. Proc. Natl. Acad. Sci. 2002;99:3205–3209. doi: 10.1073/pnas.261705198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy S.A., Wenstrup J.J. Spectral integration in the inferior colliculus of the mustached bat. J. Neurosci. 2000;20:8533–8541. doi: 10.1523/JNEUROSCI.20-22-08533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J. Acoust. Soc. Am. 1971;49(Suppl 2):467. [PubMed] [Google Scholar]
- Lewicki M.S., Konishi M. Mechanisms underlying the sensitivity of songbird forebrain neurons to temporal order. Proc. Natl. Acad. Sci. 1995;92:5582–5586. doi: 10.1073/pnas.92.12.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D. Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J. Neurosci. 1983;3:1039–1057. doi: 10.1523/JNEUROSCI.03-05-01039.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D., Fortune E.S. Temporal and harmonic combination-sensitive neurons in the zebra finch’s HVc. J. Neurosci. 1992;12:4309–4326. doi: 10.1523/JNEUROSCI.12-11-04309.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N., Liu Z., Geesaman B.J., Qian N. Perceptual learning on orientation and direction discrimination. Vision Res. 1999;39:3692–3701. doi: 10.1016/s0042-6989(99)00069-3. [DOI] [PubMed] [Google Scholar]
- Mitrani L., Shekerdjiiski S., Yakimoff N. Mechanisms and asymmetries in visual perception of simultaneity and temporal order. Biol. Cybern. 1986;54:159–165. doi: 10.1007/BF00356854. [DOI] [PubMed] [Google Scholar]
- Mossbridge J.A., Fitzgerald M.B., O’Connor E.S., Wright B.A. Perceptual-learning evidence for separate processing of asynchrony and order tasks. J. Neurosci. 2006;26:12708–12716. doi: 10.1523/JNEUROSCI.2254-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller K.K., Kanwal J.S., Suga N. Facilitative responses to species-specific calls in cortical FM-FM neurons of the mustached bat. Neuroreport. 1996;7:1749–1755. doi: 10.1097/00001756-199607290-00011. [DOI] [PubMed] [Google Scholar]
- Parker E.M. Auditory constraints on the perception of voice-onset time: the influence of lower tone frequency on judgments of tone-onset simultaneity. J. Acoust. Soc. Am. 1988;83:1597–1607. doi: 10.1121/1.395914. [DOI] [PubMed] [Google Scholar]
- Pastore R.E. Temporal order judgment of auditory stimulus offset. Percept. Psychophys. 1983;33:54–62. doi: 10.3758/bf03205865. [DOI] [PubMed] [Google Scholar]
- Pastore R.E., Harris L.B., Kaplan J.K. Temporal order identification: some parameter dependencies. J. Acoust. Soc. Am. 1982;71:430–436. [Google Scholar]
- Petersen S.E., van Mier H., Fiez J.A., Raichle M.E. The effects of practice on the functional anatomy of task performance. Proc. Natl. Acad. Sci. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst B.E., O’Connor T.A. Characteristics of neurons in auditory cortex of monkeys performing a simple auditory task. J. Neurophysiol. 1981;45:16–34. doi: 10.1152/jn.1981.45.1.16. [DOI] [PubMed] [Google Scholar]
- Phillips D.P., Hall S.E., Boehnke S.E. Central auditory onset responses, and temporal asymmetries in auditory perception. Hear. Res. 2002;167:192–205. doi: 10.1016/s0378-5955(02)00393-3. [DOI] [PubMed] [Google Scholar]
- Pisoni D.B. Adaptation of the relative onset time of two-component tones. Percept. Psychophys. 1980;28:337–346. doi: 10.3758/bf03204393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors C.V., Wenstrup J.J. Delay-tuned neurons in the inferior colliculus of the mustached bat: Implications for analyses of target distance. J. Neurophysiol. 1999;82:1326–1338. doi: 10.1152/jn.1999.82.3.1326. [DOI] [PubMed] [Google Scholar]
- Raphael L.J. Preceding vowel duration as a cue to the perception of the voicing characteristic of word-final consonants in American English. J. Acoust. Soc. Am. 1972;51:1296–1303. doi: 10.1121/1.1912974. [DOI] [PubMed] [Google Scholar]
- Recanzone G.H. Response profiles of auditory cortical neurons to tones and noise in behaving macaque monkeys. Hear. Res. 2000;150:104–118. doi: 10.1016/s0378-5955(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Suga N., O’Neill W.E., Manabe T. Harmonic-sensitive neurons in the auditory cortex of the mustache bat. Science. 1979;203:270–274. doi: 10.1126/science.760193. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Nakao M., Kaga K. Cortical mapping of auditory-evoked offset responses in rats. Neuroreport. 2004;15:1565–1569. doi: 10.1097/01.wnr.0000134848.63755.5c. [DOI] [PubMed] [Google Scholar]
- Tian B., Rauschecker J.P. Processing of frequency-modulated sounds in the cat's anterior auditory field. J. Neurophysiol. 1994;71:1959–1975. doi: 10.1152/jn.1994.71.5.1959. [DOI] [PubMed] [Google Scholar]
- Wier C.C., Green D.M. Temporal acuity as a function of frequency difference. J. Acoust. Soc. Am. 1975;57:1512–1515. doi: 10.1121/1.380592. [DOI] [PubMed] [Google Scholar]
- Wright B.A., Fitzgerald M.B. Different patterns of human discrimination learning for two interaural cues to sound-source location. Proc. Natl. Acad. Sci. 2001;98:12307–12312. doi: 10.1073/pnas.211220498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B.A., Buonomano D.V., Mahncke H.W., Merzenich M.M. Learning and generalization of auditory temporal-interval discrimination in humans. J. Neurosci. 1997;17:3956–3963. doi: 10.1523/JNEUROSCI.17-10-03956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zera J., Green D.M. Detecting temporal onset and offset asynchrony in multicomponent complexes. J. Acoust. Soc. Am. 1993;93:1038–1052. doi: 10.1121/1.405552. [DOI] [PubMed] [Google Scholar]