Abstract
Histone modifications contribute to the epigenetic regulation of gene expression, a process now recognized to be important for the consolidation of long-term memory. Valproic acid (VPA), used for many years as an anticonvulsant and a mood stabilizer, has effects on learning and memory and enhances the extinction of conditioned fear through its function as a histone deacetylase inhibitor (HDAC). Here we report that VPA enhances long-term memory for both acquisition and extinction of cued-fear. Interestingly, VPA enhances extinction, but also enhances renewal of the original conditioned fear when tested in a within-subjects design. This effect appears to be related to a reconsolidation-like process since a single CS reminder in the presence of VPA can enhance long-term memory for the original fear in the context in which fear conditioning takes place. We also show that by modifying the intertrial interval during extinction training, VPA can strengthen reconsolidation of the original fear memory or enhance long-term memory for extinction such that it becomes independent of context. These findings have important implications for the use of HDAC inhibitors as adjuncts to behavior therapy in the treatment of phobia and related anxiety disorders.
Histone modification is a fundamental mechanism involved in epigenetic regulation of gene regulation, now recognized to contribute toward the consolidation of long-term memory. Histone modifications are mediated by direct enzymatic interactions with lysine residues on chromatin, and cause dynamic changes in chromatin structure that make an important contribution to the regulation of learning-induced gene expression (Levenson et al. 2004; Bredy et al. 2007). Among the superfamily of mammalian histone-modifying enzymes, prototypical compounds such as trichostatin (TSA), sodium valproate (VPA), and sodium butyrate (NaBt), which inhibit histone deacetylase (HDAC), have been shown to enhance long-term memory (Levenson et al. 2004; Yeh et al. 2004; Wood et al. 2006; Bredy et al. 2007; Lattal et al. 2007; Vecsey et al. 2007). The ability of HDAC inhibitors to modulate behavior makes them potential therapeutic targets for treating cognitive disorders, particularly disorders of fear-related learning including phobias and post-traumatic stress disorder (PTSD).
The extinction of conditioned fear, the reduction in responding to a feared cue when the cue is repeatedly presented without any adverse consequence, is new learning that inhibits the expression of a conditioned association rather than erasing it. For example, conditioned fear shows “spontaneous recovery” after the passage of time (Baum 1988), “reinstatement” after presentations of the unconditioned stimulus (US) alone (Rescorla and Heth 1975), and “renewal” when the feared cue is presented in a context different from that of extinction training (Bouton and King 1983). Another important learning-related phenomenon is reconsolidation, in which memories become labile and sensitive to modification upon retrieval until restabilized over time (Przybyslawski and Sara 1997; Nader et al. 2000; Eisenberg et al. 2003; Suzuki et al. 2004; Lee et al. 2006). Efforts toward understanding the mechanisms of these forms of learning and memory consolidation have increased recently, particularly since they may play an important role in anxiety disorders and their treatment.
Recently we demonstrated that VPA and NaBt, when administered systemically, enhance long-term memory for the extinction of cued-fear when tested 7 d later (Bredy et al. 2007). Using a contextual fear-conditioning paradigm, Lattal et al. (2007) went on to show that systemic administration or direct infusion of an HDAC inhibitor into the dorsal hippocampus also strengthens long-term memory for extinction. In that report, the issue of whether HDAC inhibitor-induced enhancement of extinction would persist at longer post-training time intervals was raised. Given the observation of different return of fear phenomena, particularly spontaneous recovery and renewal and their implication for the treatment of disorders of fear-related learning and memory, perhaps a more relevant question would be whether strengthened extinction memory inhibits fear after not only long training-test intervals (spontaneous recovery) but also after a return to the original training context (renewal).
Here we report that VPA enhances both acquisition and extinction of cued-fear, effects that persist at least 7 d after training, and are strongest in the context in which training occurs. Paradoxically, VPA enhances extinction, but also enhances memory for the original conditioned fear when tested within the same animal; an effect that is also dependent on context. This effect appears to be related to a reconsolidation-like process since even a single CS presentation in the presence of VPA, in a neutral context, can enhance long-term memory for the original fear in the context in which fear conditioning takes place. Finally, we show that by modifying the intertrial interval during training in a protocol that normally induces no extinction, VPA strengthens reconsolidation of the original fear memory (with a massed CS exposure protocol) and enhances long-term memory for extinction (with a spaced CS exposure protocol) such that it becomes independent of context. These findings have implications for the use of cognitive enhancers as adjuncts in the treatment of phobia or PTSD, disorders recognized to be prone to relapse.
Results
VPA, given prior to training, enhances long-term memory for conditioned fear
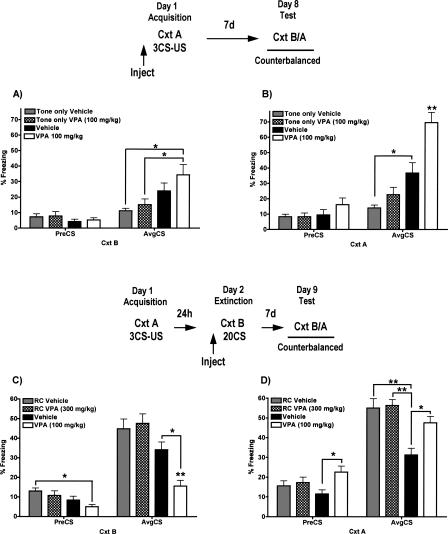
Valproic acid (VPA) is used as an anticonvulsant and a mood stabilizer (Winterer and Hermann 2000); however, it is also a potent HDAC inhibitor (Phiel et al. 2001). In addition, pentyl-4n-valproic acid, a derivative of VPA, enhances spatial learning and long-term memory for avoidance conditioning (Murphy et al. 2001; Foley et al. 2004). We therefore injected VPA or vehicle prior to a partial fear acquisition protocol using a weak (0.4 mA) footshock. VPA had no significant effect on long-term fear memory (LTM) for the CS (measured by behavioral freezing) when injected on day 1 in the absence of footshock (Fig. 1A, cf. tone-only groups), nor on freezing to context B or A prior to tone presentation (Pre-CS). When tested in context B, VPA treatment yielded significant LTM relative to tone-only treated mice, while vehicle treatment did not (Fig. 1A; n = 15–16/group, F(3,60) = 4.67, P < 0.01; Bonferroni post hoc analysis, VPA [100 mg/kg] vs. tone-only vehicle and tone-only VPA [100 mg/kg], Ps < 0.05; all other comparisons nonsignificant). When tested in context A, the VPA effect was stronger. While the vehicle-injected group showed significant fear conditioning relative to its tone-only control, VPA-treated mice showed significantly greater LTM than those conditioned with vehicle (Fig. 1B; n = 15–16/group, F(3,60) = 21.10, P < 0.0001; Bonferroni post hoc analysis, VPA [100 mg/kg] vs. all other groups, Ps < 0.001; vehicle vs. tone-only vehicle, P < 0.001; all other comparisons nonsignificant). These results suggest that VPA has an effect on both CS and context conditioning in the context in which VPA is given, a notion strongly supported and extended by the next experiments.
Figure 1.
Effect of VPA on long-term memory for conditioned fear after partial acquisition when tested 7 d after training (A) in context B and (B) in context A, and after partial extinction when tested 7 d after training (C) in context B and (D) in context A. VPA (100 mg/kg, i.p.), given prior to fear conditioning, enhances long-term memory for fear in both contexts. VPA (100 mg/kg, i.p.), given prior to extinction training, enhances long-term memory for extinction in context B but enhances memory for fear in the original training context A (n = 15–16/group; [RC] retention control; *P < 0.05; **P < 0.001). The context B data presented in panel C are from Bredy et al. (2007).
VPA, given prior to partial extinction training, enhances long-term memory for both the extinction of conditioned fear and the original fear in the respective training context
Chronic administration of VPA reduces fear responding after nonreinforced exposures to an aversive context (Li et al. 2006). This reduction in contextual freezing with VPA treatment persists up to 14 d post-training and suggests a long-lasting effect of VPA on the extinction of contextual fear. Given our finding that acute administration of VPA during cued-fear conditioning can enhance long-term memory for fear (Fig. 1A,B), we wondered if the memory-enhancing effect of VPA might also extend to the extinction of conditioned fear in a discrete cued-fear paradigm. We therefore injected VPA before a partial extinction protocol as previously described (Cain et al. 2003; Ponnusamy et al. 2005). By itself, this partial extinction protocol normally yields no significant long-term memory for extinction. An analysis of Pre-CS freezing revealed a significant main effect of treatment on LTM for context extinction (n = 15–16/group, Pre-CS, F(3,60) = 3.48, P < 0.05; Fig. 1C, left) as VPA (100 mg/kg) treated mice showed reduced pre-CS freezing relative to vehicle-treated retention control (RC) mice (Bonferroni post hoc analysis, P < 0.05). When tested for cued-fear in context B, VPA enhanced long-term memory for the extinction of conditioned fear relative to all other mice, including vehicle-treated mice that underwent extinction training (Fig. 1C, right; F(3,60) = 4.67, P < 0.01; Bonferroni post hoc analysis, extinction VPA [100 mg/kg] vs. extinction vehicle, P < 0.05; VPA [100 mg/kg] vs. RC vehicle and RC VPA (300 mg/kg), Ps < 0.001; all other comparisons nonsignificant). These data and the corresponding figure (Fig. 1C) regarding the effect of VPA on LTM for extinction in context B have previously been published (Bredy et al. 2007). The context A data were collected in the first series of experiments by Bredy et al. (2007), but the emphasis in that manuscript was on the effect of HDAC inhibitors on extinction in relation to correlated effects on BDNF gene expression, and the context A data were not presented at that time. We here examine the effect of VPA given during either fear acquisition or extinction on freezing to the CS in both acquisition and extinction contexts. In order to provide all the data necessary for readers to make this two-way comparison, we have included the previously published panel in Figure 1C. Unexpectedly, when the same mice were tested in context A, an analysis of Pre-CS freezing also revealed a significant main effect of treatment on LTM for fear (F(3,60) = 3.12, P < 0.05) as VPA-treated mice showed increased context freezing relative to vehicle-treated mice (Bonferroni post hoc analysis, vehicle vs. VPA [100 mg/kg], P < 0.05). When tested for cued-fear in context A, vehicle-treated mice showed strong residual extinction relative to retention control mice, while VPA-treated mice (during extinction training in context B) showed a significant return of conditioned fear (Fig. 1D, right; F(3,60) = 21.10, P < 0.0001; Bonferroni post hoc analysis, vehicle vs. RC groups, P < 0.001; vehicle vs. VPA [100 mg/kg], P < 0.05; all other comparisons nonsignificant). The effect in VPA-treated mice resembles renewal, a phenomenon believed to represent the context dependence of extinction learning (Bouton and King 1983).
VPA, given prior to a single CS reactivation, transiently enhances reconsolidation of conditioned fear
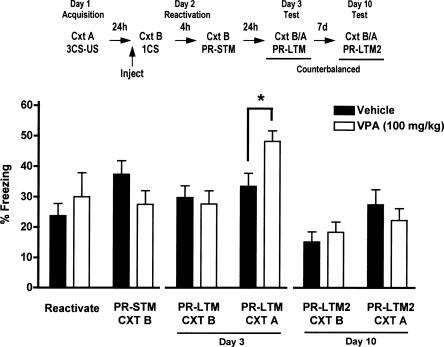
A two-way repeated measures ANOVA revealed a significant interaction between time and treatment on LTM for fear (F(5,145) = 5.15, P < 0.05). VPA, given prior to a single CS reactivation, had no effect on freezing, nor on freezing when tested 4 h after reactivation (PR-STM). However, 24 h after reactivation (PR-LTM), there was a significant interaction between treatment and context (n = 8/group, F(1,58) = 4.52, P < 0.05), such that VPA-treated mice showed increased freezing in context A relative to vehicle-treated mice, an effect not present when tested in context B (Fig. 2, PR-LTM Cxt A) (Bonferroni post hoc analysis, vehicle vs. VPA [100 mg/kg], P < 0.05; all other comparisons nonsignificant). This increase in fear memory was not present when mice were tested 7 d after reactivation (PR-LTM2).
Figure 2.
Effect of VPA on reconsolidation of cued-fear after a single CS reactivation trial (LTM) when tested 24 h (PR-LTM) and 7 d (PR-LTM2) later in context B and context A. VPA (100 mg/kg, i.p.), given 90 min prior to a single CS reactivation, enhances long-term memory for fear in the original acquisition context A (n = 8/group; *P < 0.05).
VPA, given prior to massed or spaced CS protocols, enhances reconsolidation and extinction of conditioned fear, respectively
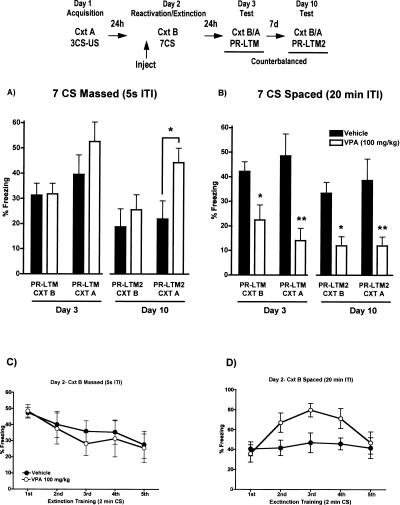
Using a massed (5-sec ITI) CS partial extinction protocol that yields no LTM for extinction by itself, a two-way repeated measures ANOVA revealed a significant main effect of time on LTM for extinction (F(3,42) = 6.42, P < 0.01) as fear decreased from day 3 to day 10 (Fig. 3A). VPA enhanced reconsolidation of conditioned fear when tested in context A, 7 d after training (PR-LTM2, F(1,14) = 4.54, P < 0.05, Bonferroni post hoc analysis, vehicle vs. VPA [100 mg/kg], P < 0.05). There was no effect of treatment of within-session extinction on day 2.
Figure 3.
Effect of VPA on reconsolidation and extinction of conditioned fear using (A) a massed CS exposure protocol or (B) a spaced CS exposure protocol, when tested 24 h (PR-LTM) and 7 d (PR-LTM2) after training in context B and context A. VPA (100 mg/kg, i.p.), administered prior to training, enhances fear reconsolidation in a massed CS presentation protocol but enhances fear extinction in a spaced CS presentation protocol. On day 2, VPA had (C) no effect on within-session extinction with massed CS presentations, but (D) significantly increased freezing during spaced CS presentations (n = 8/group; *P < 0.05; **P < 0.001).
With spaced (20-min ITI) CS presentation, there was a significant main effect of treatment on LTM for extinction (F(3,42) = 24.11, P < 0.0001) as VPA enhanced extinction of conditioned fear when tested both 24 h (n = 8/group, F(1,14) = 16.0, P < 0.0001; Bonferroni post hoc analysis, Cxt B vehicle vs. VPA [100 mg/kg], P < 0.05; Cxt A vehicle vs. VPA [100 mg/kg], P < 0.01) and 7 d after training (n = 8/group, F(1,14) = 19.96, P < 0.0001, Bonferroni post hoc analysis, Cxt B vehicle vs. VPA [100 mg/kg], P < 0.05; Cxt A vehicle vs. VPA [100 mg/kg], P < 0.01; Fig. 3B). During training on day 2, VPA-treated mice showed a significant increase in freezing (F(1,56) = 4.31, P < 0.05; Fig. 3D). These data suggest that VPA, given prior to spaced CS presentations, enhances LTM for extinction and renders this memory independent of context—effects due, in part, to increased freezing in response to the CS during spaced extinction training.
Discussion
This study generated four main findings: (1) VPA enhances long-term memory for the acquisition and extinction of cued-fear; (2) when given before partial extinction training, VPA strengthens both extinction memory in the extinction context and memory for the original conditioned fear in the training context, when tested within the same animal; (3) VPA enhances reconsolidation of conditioned fear; and (4) depending on the intertrial interval, VPA potentiates long-term memory (LTM) for extinction and renders it independent of context. We believe that these results are primarily due to VPA’s function as an HDAC inhibitor since VPA and the prototypical HDAC inhibitor NaBt, but not the non-HDAC inhibitor VPA derivative Valpromide, enhance LTM for the extinction of conditioned fear (Bredy et al. 2007).
VPA enhances long-term memory for conditioned fear and its extinction
When administered prior to training, VPA enhances long-term memory for both the acquisition and extinction of conditioned fear (Fig. 1). These findings are consistent with several studies demonstrating that HDAC inhibitors facilitate memory (Levenson et al. 2004; Yeh et al. 2004; Wood et al. 2006; Bredy et al. 2007; Lattal et al. 2007; Vecsey et al. 2007). Interestingly, we observe that VPA acts, in part, to increase contextual discrimination associated with different learning protocols. For example, the effect of VPA on cued-fear is most pronounced in the context in which fear conditioning took place (Fig. 1B). Conversely, VPA-treated mice also show reduced freezing to CS after extinction, but again, only in the context in which extinction training took place (Fig. 1C). An analysis of pre-CS freezing behavior further suggests an important role for context as VPA-treated mice also show decreased pre-CS freezing in the extinction training context, and increased pre-CS freezing in the original training context, relative to vehicle-treated mice. In addition, after extinction training in context B while under the influence of VPA, mice show increased long-term memory for conditioned fear when tested in context A (Fig. 1D). These data demonstrate the presence of two distinct memory traces after extinction learning (one for cued-fear and one for fear extinction), each sensitive to the effects of VPA and both modulated by context. Together, the findings support the long-standing view that extinction is new learning and not simply erasure of the original fear association (for review, see Bouton et al. 2006). Furthermore, by using an extinction paradigm that assesses long-term memory for a discrete cue after a contextual shift, this A-B-(A ↔ B) protocol offers a way to simultaneously investigate two opposing memory processes and their biochemical signature through concurrent behavioral and transgenic approaches. For example, this protocol could be combined with the use of Arc labeling or transgenic (TetTag) mice to assess distributed neuronal circuitry associated with retrieval of two separate memory traces (Guzowski et al. 2006; Reijmers et al. 2007).
VPA enhances reconsolidation of conditioned fear
At first glance, the finding that long-term memory for fear is paradoxically enhanced in context A after extinction training in context B, suggests an effect of VPA on contextual renewal of cued-fear (Fig. 1D). Given the design of the partial extinction protocol and our observation of no significant extinction and no renewal in vehicle-treated mice, we hypothesized that the effects were not due to renewal, but due, in part, to an enhanced reconsolidation-like process. Numerous studies have shown that a brief reminder such as a tone CS after fear conditioning renders the memory for that fear temporarily labile and sensitive to modification, requiring re-stabilization of the memory trace through reconsolidation (Przybyslawski and Sara 1997; Przybyslawski et al. 1999; Nader et al. 2000). Additionally, there is evidence that a reactivated fear memory (such as that which occurs during partial extinction), can be biased toward extinction (new learning) or reconsolidation, by factors such as stimulus intensity, training to test interval, or duration of reminder (Cain et al. 2003; Eisenberg et al. 2003; Pedreira et al. 2004; Suzuki et al. 2004; Lee et al. 2006; Tronson et al. 2006).
To explore the hypothesis that VPA modulates reconsolidation of a cued-fear memory, we performed a standard reconsolidation experiment using a single CS reactivation under the influence of VPA, a protocol that induces no extinction. It has been suggested that if concurrent stimuli are not strong (or aversive) enough upon reactivation of the original memory trace, then their influence is minimal, new information is not encoded, and the original fear memory is unaffected (Dudai and Eisenberg 2004; Tronel et al. 2005). HDAC inhibition at the time of memory reactivation enhanced long-term memory for the CS such that VPA-treated mice showed increased fear in context A, when tested 24 h after reactivation (PR-LTM, Fig. 2). Once again, HDAC inhibition at the time of learning (in this case, reactivation) served to enhance the CS–context association for the original fear even though the memory was reactivated in a context different from that during initial training when no extinction learning occurred. However, the VPA-induced enhancement of fear memory was not present when tested 7 d after reactivation. These data suggest that after a single CS reactivation, subsequent reconsolidation of cued-fear is a transient process allowing for temporary destabilization of the original association in order to incorporate new information into the existing memory trace (for review, see Alberini et al. 2006). Using a spatial memory task, Morris et al. (2006) found reactivated memories to be labile only under conditions in which there was an opportunity to encode new information. Our finding that reconsolidation of cued-fear can be positively modulated (also see Lee et al. 2006; Tronson et al. 2006) and that the effect is both transient and dependent on context may assist in the interpretation of studies that have found reversal of reconsolidation-blockade-induced contextual memory impairment (Lattal and Abel 2004; Biedenkapp and Rudy 2004; Power et al. 2006). It is possible that in those studies examining contextual learning and memory, there wasn’t sufficiently new or salient information available at the time of reactivation to be encoded, and therefore less opportunity to permanently disrupt the reconsolidation of the original contextual memory trace. Furthermore, our findings offer a cautionary note for targeting the reconsolidation process as a method of memory modification in the treatment of fear-related learning disorders. Reconsolidation, like extinction, may be less robust than the original memory trace and have a stronger relationship with context than currently appreciated.
Depending on the intertrial interval, VPA can enhance long-term memory (LTM) for extinction and render it independent of context
To further explore factors that contribute to the effect of VPA on reconsolidation or extinction of conditioned fear, we performed two experiments using either seven massed or seven spaced non-reinforced CS presentations, protocols that again induce no significant extinction in vehicle-treated mice (Cain et al. 2003). The rationale for these studies was based on finding that the duration of CS presentation biases reactivated fear memory toward reconsolidation (short duration) or extinction (long duration) (Pedreira et al. 2004; Lee et al. 2006). In our experiments, rather than manipulate the duration of the CS, we modified the intertrial interval using either a massed (5-sec ITI) or spaced (20-min ITI) CS training protocol, and examined the effect of VPA when administered prior to training. When tested 7 d after massed CS presentations in the presence of VPA, long-term memory for conditioned fear was enhanced in the fear conditioning context, again suggesting that HDAC inhibition at the time of retrieval enhanced reconsolidation of the original fear memory trace. These effects appeared to be contingent on training context since fear was not enhanced in context B, where reactivation occurred (Fig. 3A). The findings again agree with previous studies showing that reconsolidation of conditioned fear can be positively modulated (Lee et al. 2006; Tronson et al. 2006).
When the same experiment was performed using a spaced CS presentation protocol, the outcome was the complete opposite. Long-term memory for conditioned fear was virtually eliminated after spaced CS presentations (with a 20-min ITI) in the presence of VPA (Fig. 3B). With a long ITI, the reactivated memory trace and the discrimination associated with the tone-CS, in particular, were biased toward new extinction learning. Furthermore, long-term memory for extinction was enhanced such that it inhibited the expression of the original fear memory in both the extinction-training context (B), and in the original fear acquisition context (A). Spaced extinction training in the presence of VPA may have enhanced memory of the CS–no US relationship by incorporating some other factor, perhaps time, into the associative memory trace. ITI can influence learning as one element of the extinction memory trace, and time itself can be used as a discriminative contextual stimulus (Bouton and García-Gutiérrez 2006; for review, see Bouton et al. 2006). Thus, allowing sufficient time for a new CS–no US relationship to be formed may have facilitated a re-evaluation of the original CS-US association; an idea supported by the observation that VPA-treated mice showed increased freezing during spaced CS presentations on day 2 (Fig. 3D). However, in these experiments, we did not control for total time spent in context B in massed versus spaced extinction training. Therefore, it remains possible that VPA rendered memory for extinction independent of context, not because of CS presentations but simply because of total time spent in the extinction context. However, since exposure to context without CS had no effect on long-term fear memory in either condition, it is unlikely that context presentation alone was the source of the VPA effect on memory.
Our data support and extend the idea that there are competing memory traces whose expression, according to the “trace dominance hypothesis,” is dictated by the strength and duration of the stimulus at the time of reactivation (Eisenberg et al. 2003; Dudai and Eisenberg 2004; Eisenberg and Dudai 2004; Pedreira et al. 2004; Suzuki et al. 2004; Lee et al. 2006). In combination with our previous work (Cain et al. 2003), we would add ITI during reactivation as a factor that influences which memory will be expressed at the time of test. Like yohimbine and sulpiride (which is also an HDAC inhibitor) (Cain et al. 2004; Ponnusamy et al. 2005), VPA overcomes the reconsolidation-like incubation of fear by spaced CS presentations, to allow effective extinction to take place. We hypothesize that VPA may amplify or minimize contextual influences contingent on the learning system engaged at the time of training in the presence of VPA. Thus, in the case of contextual shift after massed training, processes associated with reconsolidation of the original fear memory are engaged, and the original memory trace is enhanced in compound with the training context A. Conversely, under spaced training conditions, the processes governing extinction are preferentially activated, and the contribution of context (including context A) is minimized. In this regard, renewal (the context dependence of extinction memory) may not be necessarily due to the context dependence of extinction learning, but the very strong influence of context in reviving the original trace. Potentially, this may also explain why A-B-C renewal has been so much more difficult to observe than A-B-A renewal, and future studies will directly examine the role of HDAC inhibitors in different renewal protocols.
The observation that VPA, an HDAC inhibitor, renders long-term extinction memory independent of context has implications for the use of VPA in conjunction with behavior therapy for the treatment of human anxiety disorders. Perhaps administering VPA in combination with brief, spaced cue exposures may yield a more effective and more generalized therapeutic result.
Materials and Methods
Subjects
Naive 10–12-wk-old C57BL/6 male mice (Taconic Farms, Oxnard, CA) were housed four per cage, maintained on a 12-h light/dark schedule, and allowed free access to food and water. All testing was conducted during the light phase in illuminated testing rooms following protocols approved by the Institutional Animal Care and Use Committee of the University of California, Los Angeles.
HDAC inhibitor
Sodium valproate (100 mg/kg, i.p.; Sigma) was dissolved in 0.1 M PBS. Mice were injected with vehicle or drug 2 h before behavioral testing. Drug pre-treatment time was chosen based on previous reports that indicate peak histone acetylation time-course post-injection (Tremolizzo et al. 2002).
Conditioning apparatus
Two contexts (A and B), in separate rooms, were used for all behavioral fear testing. Shuttle box compartments (Med Associates) measuring 20.3 × 15.9 × 21.3 cm served as context A, and conditioning boxes (Med Associates) measuring 30.5 × 24.1 × 21 cm served as context B. Both contexts had two transparent walls and stainless steel grid floors (3.2 mm in diameter, 8-mm centers); however, the grid floors in context B were covered with flat white acrylic inserts to minimize context generalization. Context A was wiped down before testing with 10% ethanol, and context B was wiped down with 10% methanol. Individual videocameras were mounted in the ceiling of each chamber and connected via a quad processor to a standard videocassette recorder and monitor for videotaping and scoring of freezing. Grid floors were connected to a scrambled shock source (Med Associates). Auditory stimuli (Med Associates) were delivered via a speaker in the chamber wall. Delivery of stimuli was controlled with a personal computer and Med-PC software through a SmartCTL Interface System (DIG-716; Med Associates). Background white noise was maintained at 62 dB throughout behavioral testing.
Histone deacetylase inhibitor interaction with partial acquisition or partial extinction of conditioned fear
Experiments investigating the effects of VPA on the acquisition and extinction of cued-fear consisted of three phases: fear acquisition (context A), fear extinction (context B), and testing (contexts A and B). Testing occurred 7 d after extinction training to allow for complete memory consolidation. In all experiments, cued-fear was induced in untreated, naive mice with three pairings of a 2-min, 80-dB, white noise CS coterminating with a 2-sec, 0.4-mA footshock for acquisition and 0.7 mA for extinction experiments (2-min ITI). For extinction, mice were matched into equivalent treatment groups based on freezing during the third training CS. One day later, after injections, mice were placed in context B and allowed to acclimate for 2 min. Partial extinction training comprised 20 non-reinforced 2-min CS presentations (5-sec ITI) and was chosen because it yields, by itself, no significant long-term memory for extinction, based on our previous work using partial extinction to test the ability of different compounds to facilitate extinction (Cain et al. 2003; Ponnusamy et al. 2005). Because of the limited range of freezing and the inherent variability in behavioral experiments, it is impossible to measure facilitation of fear acquisition and facilitation of fear extinction using the same fear acquisition protocol. To avoid ceiling effects, we used a weak-intensity footshock (0.4 mA) to generate submaximal fear acquisition in order to assess potentiation of conditioned fear. Conversely, a stronger-intensity footshock (0.7 mA) in combination with a partial extinction protocol allows for manipulation of the extinction process by providing a higher level of baseline freezing and to avoid a floor effect that might obscure potentiated extinction. As controls, fear-conditioned mice without extinction were injected with vehicle or drug and placed in context B for a time equal to that spent there by extinguished mice but were not exposed to any CS presentations. For the behavioral tests, 7 d after fear acquisition or extinction, all mice were returned and tested in contexts A and B, in the drug-free state, and in a counterbalanced design. After a 2-min acclimation, freezing was assessed during two 2-min CS presentations (2-min ITI). As described in the Results, the extinction data were collected in the first series of experiments by Bredy et al. (2007), where we examined the effect of HDAC inhibitors on extinction in relation to correlated effects on BDNF gene expression. For this reason, we did not include the context A test in the methods of that manuscript. We here examine the effect of VPA given during either fear acquisition or extinction on freezing to the CS in both acquisition and extinction contexts, and the methods directly reflect this behavioral analysis.
Histone deacetylase inhibitor interaction with reconsolidation of conditioned fear
Experiments investigating the effect of VPA on the reconsolidation of cued-fear consisted of three phases: fear acquisition (context A), reactivation (context B), and testing (contexts A and B). Testing occurred 7 d after reactivation to allow for complete memory consolidation. In all experiments, cued-fear was induced in untreated, naive mice with three pairings of a 2-min, 80-dB, white noise CS coterminating with a 2-sec, 0.7-mA footshock (2-min ITI). For reconsolidation, mice were matched into equivalent treatment groups based on freezing during the third training CS. One day later, after injections, mice were placed in context B and allowed to acclimate for 2 min. Reactivation was induced with exposure to a single CS (2 min) followed by a test for short-term memory 4 h later. For the behavioral tests, 24 h and 7 d after fear reactivation, all mice were returned and tested in contexts A and B, in the drug-free state, and in a counterbalanced design. After a 2-min acclimation, freezing was assessed during two 2-min CS presentations (2-min ITI).
Histone deacetylase inhibitor interaction with massed versus spaced extinction of conditioned fear
Experiments investigating the effect of VPA extinction of cued-fear consisted of three phases: fear acquisition (context A), reactivation (context B), and testing (contexts A and B). Testing occurred 7 d after reactivation to allow for complete memory consolidation. In all experiments, cued-fear was induced in untreated, naive mice with three pairings of a 2-min, 80-dB, white noise CS coterminating with a 2-sec, 0.7-mA footshock (2-min ITI). For extinction training, mice were matched into equivalent treatment groups based on freezing during the third training CS. One day later, after injections, mice were placed in context B and allowed to acclimate for 2 min. Extinction training proceeded with exposure to either seven massed non-reinforced CSs (2-min duration, 5-sec ITI), or seven spaced non-reinforced CSs (2-min duration, 20-min ITI). For behavioral tests, 24 h and 7 d after massed extinction training, all mice were returned and tested in contexts A and B, in the drug-free state, and in a counterbalanced design. After a 2-min acclimation, freezing was assessed during two 2-min CS presentations (2-min ITI).
Data analyses
Behavioral freezing, the absence of all nonrespiratory movements, was rated during all phases by an experienced investigator blind to subject treatment, using a 5-sec instantaneous time-sampling technique. The percentage of observations with freezing was calculated for each mouse, and data represent mean ± SEM freezing percentages for groups of mice during specified time bins. Total session means were analyzed with one-way ANOVA for data in Figure 1, and by two-way ANOVA for data in Figures 2 and 3. Bonferroni post hoc analyses for all comparisons were used for behavioral data.
Acknowledgments
This work was supported in part by grants from the NIMH and the Tennenbaum Family Foundation (M.B.) and by postdoctoral fellowships from NSERC and CIHR (T.W.B.).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.801108
References
- Alberini C.M., Milekic M.H., Tronel S. Mechanisms of memory stabilization and de-stabilization. Cell. Mol. Life Sci. 2006;63:999–1008. doi: 10.1007/s00018-006-6025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum M. Spontaneous recovery from the effects of flooding (exposure) in animals. Behav. Res. Ther. 1988;26:185–186. doi: 10.1016/0005-7967(88)90118-0. [DOI] [PubMed] [Google Scholar]
- Biedenkapp J.C., Rudy J.W. Context memories and reactivation: Constraints on the reconsolidation hypothesis. Behav. Neurosci. 2004;118:956–964. doi: 10.1037/0735-7044.118.5.956. [DOI] [PubMed] [Google Scholar]
- Bouton M.E., García-Gutiérrez A. Intertrial interval as a contextual stimulus. Behav. Processes. 2006;71:307–317. doi: 10.1016/j.beproc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Bouton M.E., King D.A. Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. J. Exp. Psychol. Anim. Behav. Process. 1983;9:248–265. [PubMed] [Google Scholar]
- Bouton M.E., Westbrook R.F., Corcoran K.A., Maren S. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biol. Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bredy T.W., Wu H., Crego C., Zellhoefer J., Sun Y.E., Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn. Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C.K., Blouin A.M., Barad M. Temporally massed CS presentations generate more fear extinction than spaced presentations. J. Exp. Psychol. Anim. Behav. Process. 2003;29:323–333. doi: 10.1037/0097-7403.29.4.323. [DOI] [PubMed] [Google Scholar]
- Cain C.K., Blouin A.M., Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn. Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y., Eisenberg M. Rites of passage of the engram: Reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44:93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Dudai Y. Reconsolidation of fresh, remote, and extinguished fear memory in Medaka: Old fears don’t die. Eur. J. Neurosci. 2004;20:3397–3403. doi: 10.1111/j.1460-9568.2004.03818.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Kobilo T., Berman D.E., Dudai Y. Stability of retrieved memory: Inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Foley A.G., Gallagher H.C., Murphy K.J., Regan C.M. Pentyl-4-yn-valproic acid reverses age-associated memory impairment in the Wistar rat. Neurobiol. Aging. 2004;25:539–546. doi: 10.1016/j.neurobiolaging.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Guzowski J.F., Miyashita T., Chawla M.K., Sanderson J., Maes L.I., Houston F.P., Lipa P., McNaughton B.L., Worley P.F., Barnes C.A. Recent behavioral history modifies coupling between cell activity and Arc gene transcription in hippocampal CA1 neurons. Proc. Natl. Acad. Sci. 2006;103:1077–1082. doi: 10.1073/pnas.0505519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal K.M., Abel T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc. Natl. Acad. Sci. 2004;101:4667–4672. doi: 10.1073/pnas.0306546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal K.M., Barrett R.M., Wood M.A. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav. Neurosci. 2007;121:1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.L., Milton A.L., Everitt B.J. Reconsolidation and extinction of conditioned fear: Inhibition and potentiation. J. Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J.M., O’Riordan K.J., Brown K.D., Trinh M.A., Molfese D.L., Sweatt J.D. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Li S., Murakami Y., Wang M., Maeda K., Matsumoto K. The effects of chronic valproate and diazepam in a mouse model of posttraumatic stress disorder. Pharmacol. Biochem. Behav. 2006;85:324–331. doi: 10.1016/j.pbb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Morris R.G., Inglis J., Ainge J.A., Olverman H.J., Tulloch J., Dudai Y., Kelly P.A. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–489. doi: 10.1016/j.neuron.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Murphy K.J., Fox G.B., Foley A.G., Gallagher H.C., O’Connell A., Griffin A.M., Nau H., Regan C.M. Pentyl-4-yn-valproic acid enhances both spatial and avoidance learning, and attenuates age-related NCAM-mediated neuroplastic decline within the rat medial temporal lobe. J. Neurochem. 2001;78:704–714. doi: 10.1046/j.1471-4159.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- Nader K., Schafe G.E., Le Doux J.E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Pedreira M.E., Perez-Cuesta L.M., Maldonado H. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn. Mem. 2004;11:579–585. doi: 10.1101/lm.76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiel C.J., Zhang F., Huang E.Y., Guenther M.G., Lazar M.A., Klein P.S. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Ponnusamy R., Nissim H.A., Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn. Mem. 2005;12:399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power A.E., Berlau D.J., McGaugh J.L., Steward O. Anisomycin infused into the hippocampus fails to block “reconsolidation” but impairs extinction: The role of re-exposure duration. Learn. Mem. 2006;13:27–34. doi: 10.1101/lm.91206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyslawski J., Sara S.J. Reconsolidation of memory after its reactivation. Behav. Brain Res. 1997;84:241–246. doi: 10.1016/s0166-4328(96)00153-2. [DOI] [PubMed] [Google Scholar]
- Przybyslawski J., Roullet P., Sara S.J. Attenuation of emotional and nonemotional memories after their reactivation: role of β adrenergic receptors. J. Neurosci. 1999;19:6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers L.G., Perkins B.L., Matsuo N., Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- Rescorla R.A., Heth C.D. Reinstatement of fear to an extinguished conditioned stimulus. J. Exp. Psychol. Anim. Behav. Process. 1975;1:88–96. [PubMed] [Google Scholar]
- Suzuki A., Josselyn S.A., Frankland P.W., Masushige S., Silva A.J., Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremolizzo L., Carboni G., Ruzicka W.B., Mitchell C.P., Sugaya I., Tueting P., Sharma R., Grayson D.R., Costa E., Guidotti A. An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc. Natl. Acad. Sci. 2002;99:17095–17100. doi: 10.1073/pnas.262658999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S., Milekic M.H., Alberini C.M. Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLoS Biol. 2005;3:e293. doi: 10.1371/journal.pbio.0030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson N.C., Wiseman S.L., Olausson P., Taylor J.R. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat. Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Vecsey C.G., Hawk J.D., Lattal K.M., Stein J.M., Fabian S.A., Attner M.A., Cabrera S.M., McDonough C.B., Brindle P.K., Abel T., et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J. Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G., Hermann W.M. Valproate and the symptomatic treatment of schizophrenia spectrum patients. Pharmacopsychiatry. 2000;33:182–188. doi: 10.1055/s-2000-12981. [DOI] [PubMed] [Google Scholar]
- Wood M.A., Attner M.A., Oliveira A.M., Brindle P.K., Abel T. A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn. Mem. 2006;13:609–617. doi: 10.1101/lm.213906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh S.H., Lin C.H., Gean P.W. Acetylation of nuclear factor-κB in rat amygdala improves long-term but not short-term retention of fear memory. Mol. Pharmacol. 2004;65:1286–1292. doi: 10.1124/mol.65.5.1286. [DOI] [PubMed] [Google Scholar]