Abstract
An outstanding problem in vertebrate development has been to define the genetic program that specifies the cardiomyocyte lineage. It has been a challenge to define the transcription factors that control specification, since candidate gene knockouts typically cause rather complex morphogenetic defects. In contrast, Drosophila genetics identified single transcription factors that are essential for specification of cardiomyocytes from uncommitted mesoderm. For those vertebrate orthologs, it has been considered that paralogous family members might compensate for the loss-of-function of individual genes. However, this hypothesis had not been formally tested. In zebrafish, defects in gata5 can lead to a loss of myocardial tissue, but most embryos depleted for any single vertebrate Gata4/5/6 transcription factor develop a cardiac morphogenetic defect, and cardiomyocytes are specified and differentiate. Here we show that in zebrafish the gata5 and gata6 genes are redundant for specification of cardiomyocytes. Embryos depleted of these two gene products are heartless. Restoring either gene product is sufficient to rescue cardiomyocyte specification. In contrast, embryos depleted of Gata4 and Gata6, or Gata4 and Gata5, develop defective heart tubes. Our study identifies a specific pair of vertebrate transcription factor paralogs that is essential for cardiomyocyte specification.
Keywords: cardiogenesis, myocardium, GATA factors, mesoderm
Introduction
Defining the transcriptional program that specifies cardiomyocyte fate from uncommitted progenitors is an important goal, since this could impact cellular strategies for treating cardiomyopathies and heart disease. A number of transcription factors, including those from the GATA, NKX2, MEF2, and TBX gene families, are established as key regulators of normal heart development (Brand, 2003; Frasch, 1999). Orthologous genes are essential for cardiomyocyte specification in Drosophila, exemplified by the ‘heartless’ phenotypes of pannier and tinman, mutants for Gata and Nkx2 orthologues, respectively. However, genetic experiments in vertebrate animal models have not revealed essential genes that specify cardiomyocytes. Rather, mouse knockouts and zebrafish mutants for these genes show morphological disruptions and in some cases chamber-specific developmental defects. This is generally interpreted as reflecting genetic compensation by related family members in the context of the more complex vertebrate heart (Xin et al., 2006). Some evidence for this is provided by forced expression of potential dominant-negative isoforms that might block family member function (Fu et al., 1998; Horb and Thomsen, 1999). However, the concept has not yet been demonstrated formally; a challenge is that some of these candidate specification genes have additional early requirements, for example in extra-embryonic tissues.
GATA proteins comprise a small family of zinc finger transcription factors that regulate diverse functions during embryogenesis and in the adult (Patient and McGhee, 2002). The gata4, gata5 and gata6 genes are each implicated in the development of the intestinal and cardiovascular systems (Peterkin et al., 2003). Since they share both spatial and temporal expression patterns throughout embryogenesis, each gene may be required for specification of distinct cell types, or for a different step of cardiac morphogenesis. Alternatively, a total specific amount of GATA factor in particular cells might be needed to direct expression of a specific program. Zebrafish studies provide evidence that each factor fulfills a different stage-specific requirement during cardiogenesis. The gata5 gene appears to have the earliest function for zebrafish cardiogenesis, as revealed by analysis of the faust mutant (Reiter et al., 1999; Reiter et al., 2001; Trinh et al., 2005). The phenotype of faust is variable in expressivity, and while some mutant embryos show a significant loss of myocardium (suggesting a key role in specification or proliferation), in most sibling mutants the cardiac progenitors fail to fuse at the midline of the embryo, leading to a cardia bifid phenotype. Thus, gata5 plays a role in the migration of cardiac progenitors from the anterior lateral plate mesoderm towards the midline of the embryo. Also required for cardiac morphogenesis is gata6, essential for proper cardiac tube formation. Embryos depleted of Gata6 show variable cardiac phenotypes including cardia bifida, partially fused tube, and fused but non-looping tube (Peterkin et al., 2003). There is also an essential role for gata4, required for a relatively late “jogging” and growth step of heart tube morphogenesis. In gata4 morphants, the atrium fails to expand or migrate rostrally, causing defects in heart tube looping (Holtzinger and Evans, 2005).
Gain-of-function experiments suggest that individual vertebrate GATA factors encode activity sufficient to enhance cardiogenesis, for example shown by forced expression of Gata4 in Xenopus embryos (Jiang and Evans, 1996) or P19 embryonal carcinoma cells (Grepin et al., 1997), or Gata5 in zebrafish (Reiter et al., 1999). Gata4 over-expression is sufficient to induce cardiomyocyte differentiation in Xenopus ectodermal explants, suggesting the ability to specify cell fate (Latinkic et al., 2003). However, it is not clear if these effects relate to cell specification, progenitor proliferation, or are non-physiological phenotypes induced by over-expression. If GATA factors are an essential component of the cardiomyocyte specification program, loss-of-function for gata4/5/6 gene activity should result in a heartless phenotype, analogous to pannier (Sorrentino et al., 2005). Here we test this hypothesis using the zebrafish system, which facilitates targeted knockdown of multiple genes. Our results show that gata5 and gata6 function together to specify the cardiomyocyte lineage.
Materials and Methods
Zebrafish Strains and Morpholino Microinjections
Zebrafish embryos were maintained and staged as described (Westerfield, 1993). The morpholino specific to gata4 is a translation blocker (G4 MO) and was described (Holtzinger and Evans, 2005). A morpholino specific to gata5 that blocks a splice site (ssG5 MO) was described (Trinh et al., 2005). To confirm specificity, we also designed a translation blocker (tbG5 MO; 5′-AAGATAAAGCCAGGCTCGAATACAT-3′). The morpholino specific to the “long form” of gata6 (G6 MO) was also described previously (Peterkin et al., 2003). Using individual morpholinos each embryo was injected with: 10ng G4 MO; 20 ng ssG5 MO, 16 ng tbG5 MO, or 2.5 ng G6 MO. Under these conditions the gata5 morpholinos phenocopy the faust mutant, while the gata4 and gata6 morpholinos block completely detectable GFP in gata4:gfp and gata6:gfp transgenic reporter fish, respectively (Holtzinger and Evans, 2005). However, when injected with combinations including 2.5 ng of the gata6 morpholino, embryos did not survive. Therefore, for combinatorial injections, 1.25 ng of the gata6 morpholino was used. This was still sufficient to eliminate GFP in the gata6:gfp reporter fish, and to generate identical gata6 single-morphant phenotypes. The cmlc2:gfp strain was generated originally by H.J. Tsai (Huang et al., 1997), and we generated equivalent lines independently. For rescue experiments, batches of embryos were injected with morpholinos, and injected independently in sibling groups (either before or after) with various amounts of in vitro generated and purified mRNA.
Gene Expression Analysis
Whole-mount in situ hybridization was performed essentially as described (Reiter et al., 1999). Briefly, embryos were treated with 0.003% phenylthiourea (PTU) to prevent pigmentation. After fixation, embryos older than 28 hours were treated with 10 μg/ml proteinaseK. Hybridization was performed at 70C, in 60% formamide buffer with digoxigenin-labeled RNA anti-sense probes. The probes used for in situ hybridization were prepared and used as described, for gata4/5/6 (Heicklen-Klein and Evans, 2004), nkx2.5, cmlc2, vmhc, and amhc (Reiter et al., 1999), pax2.1 (Krauss et al., 1991), lmo2 (Zhu et al., 2005), and no tail (Schulte-Merker et al., 1992). For quantitative RT-PCR, embryos were either uninjected or injected with G4+ssG5 MOs; G4+G6 MOs; ssG5+G6 MOs or tbG5+G6 MO. An equal number of embryos was harvested for each sample and total RNA was isolated (TRI REAGENT, Molecular Research Center, Cincinnati, OH). First-strand cDNA synthesis was performed (Superscript III First-Strand Synthesis System for RT-PCR, Invitrogen) and the cDNA subjected to quantitative RT-PCR (Opticon DNA Engine 2, MJ Research, Watertown, MA) with data analysis as described (Livak and Schmittgen, 2001). Statistical analysis was based on three independent experiments each comprised of triplicate samples. Primers were: beta actin, F: 5′-CAACGGAAACGCTCATTGC, R: 5′-CGCGCAGGAGATGGGAACC; cmlc2, F: 5′-AGACCCAGAGGAAACCATCC, R: 5′-TTGGGTCATTAGCAGCCTCT.
Results and Discussion
The faust (gata5) mutant was described as demonstrating variable expressivity with 0 – 45% of the mutant embryos in a given clutch showing abnormal hearts (Reiter et al., 1999; Reiter et al., 2001). This could be explained by partial to full compensation from other co-expressed GATA factors. Indeed, previous work revealed redundancy between gata4 and gata6 for liver bud formation (Holtzinger and Evans, 2005). Moreover, evidence for cooperation between Gata4 and Gata6 during cardiogenesis was revealed by examining trans-heterozygote mutant mouse embryos (Xin et al., 2006). The faust mutant represents in principle a good starting point for targeting GATA factors, since these embryos express low levels of gata5 mRNA (Reiter et al., 1999). However, analyzing phenotypes in the context of the faust background is complicated for several reasons. First, the mutations are not null, and the remaining levels might contribute to the variable expressivity. Second, the molecular basis for the stronger faus26 allele is not known, which complicates genotyping required to “prove” that any individual embryo is a gata5 mutant. Third, only 25% of the embryos derived from heterozygous faust parents are mutant, limiting our ability to analyze statistically the results of manipulating several different gene combinations in the faust background. For these reasons, we used a morpholino-based approach for depleting combinations of cardiogenic GATA factors. The gata4 and gata6 transcripts were targeted using previously characterized and validated morpholinos (Holtzinger and Evans, 2005; Peterkin et al., 2003).
However, we first sought to confirm that a morpholino approach was valid for use in place of the faust mutant. A gata5-specific morpholino was shown previously to phenocopy the faust mutant (Trinh et al., 2005). Since this morpholino targets a gata5 splice site, its effectiveness can be evaluated by whole-mount in situ hybridization for gata5 transcript levels. Indeed, embryos derived from fertilized eggs injected with this splice site gata5 (ssG5) morpholino show little if any detectable gata5 transcripts, confirming the ability of ssG5 to efficiently inhibit gata5 expression (Fig. 1A,B). We also tested a translation blocker (tbG5 MO) because this morpholino will inhibit also expression from maternal gata5 transcripts. Embryos derived from fertilized eggs injected with either the ssG5 or the tbG5 morpholino were compared to embryos derived from crossing heterozygous faus26 adults, by analyzing transcripts for the cardiac progenitor marker nkx2.5 by whole-mount in situ hybridization. Compared to control (wild-type) embryos (Fig. 1C) all embryos injected with the tbG5 morpholino display a bifid heart (Fig. 1D,E; Table 1). The phenotype of embryos injected with the ssG5 morpholino was overall more variable, consistent with the failure to block translation of maternal-derived gata5 transcripts. While the majority of the embryos still display a cardia bifida (61%), the phenotypes vary primarily between this and a fused or partially fused heart tube (Fig. 1F–H; Table 1). In a low percentage of morphant embryos, nkx2.5 transcripts are largely depleted (Fig. 1I; Table 1). The progeny of a cross between two heterozygous faus26 adults shows the same range of cardiac phenotypes as seen with the ssG5 morphants (Fig. 1, J–M). Considering that only 25% are homozygous faus26, the majority of embryos displaying a relatively normal heart tube are presumed to be wild-type or heterozygous (Table 1). Of the remaining abnormal embryos, the large majority of faus26 embryos display a cardia bifid phenotype (Table 1), although again a very small percentage show a significant loss of nkx2.5 transcripts (Fig. 1M, Table 1), consistent with results reported previously (Reiter et al., 1999). Therefore, while loss of Gata5 can cause depletion of myocardial progenitors, in most embryos this is not the case and myocardial cells are specified and development proceeds, albeit with cardia bifida. Therefore, the specificity and efficiency of two independent gata5 morpholinos was validated and they consistently generate embryos that replicate the phenotype of faust mutants. Therefore, we next sought to test for compensation in the context of the gata5 morphant.
Fig. 1. The gata5 morpholinos can be used to phenotype the faust mutant.
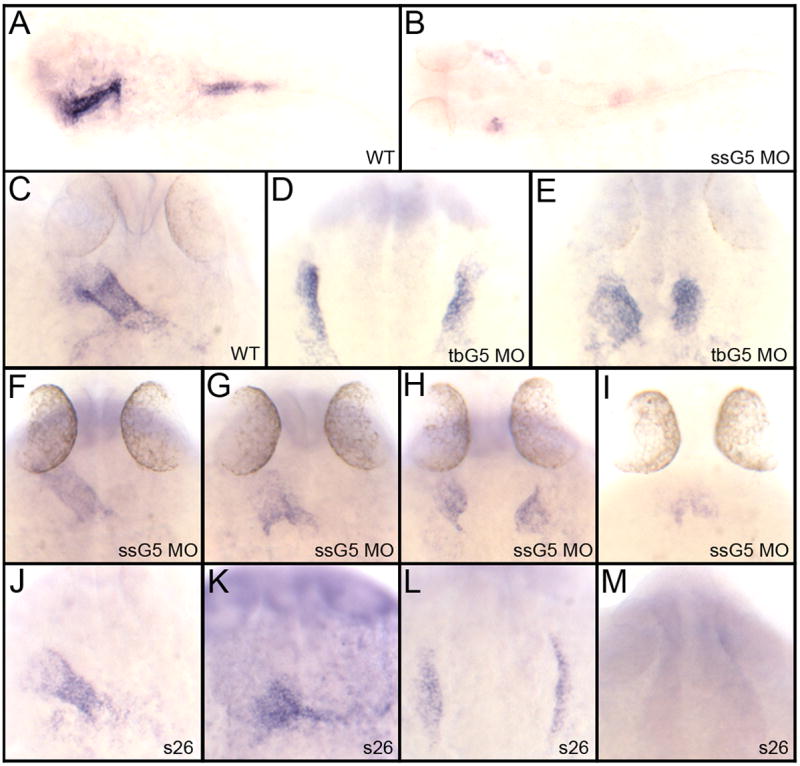
A, B: Shown are representative embryos following in situ hybridization using a gata5-specific probe at 24 hpf analyzing (A) wild type embryos or (B) embryos injected with a gata5 morpholino that targets a splice site (ssG5 MO). Injection of ssG5 MO efficiently blocks accumulation of mature gata5 mRNA. While most embryos lack detectable message, the embryo shown in B represents an example of one that demonstrates a low level of remaining transcripts in a bifid pattern (arrows). C–M: Shown are representative embryos following whole mount in situ hybridization to detect transcripts for the cardiac marker nkx2.5 at 24 hpf. Samples represent results with (C) wild-type embryos (D, E) embryos injected with a translation-blocker morpholino specific to gata5 (tbG5), (F–I) ssG5 morphants, and (J–M) embryos derived from crossing two adult fish heterozygous for the faus26 allele. The ssG5 morphants display the same variable expressivity phenotype as the faus26 mutants, including embryos with a fused heart tube (F,J), a partially fused tube (G,K), cardia bifida (H,L) or a significant reduction of cardiac progenitors (I,M). The tbG5 morpholino more reproducibly generates cardia bifida. See Table 1 for all statistics. In A and B, embryos were flat mounted, views are dorsal, with anterior to the left. All other views are dorsal, with anterior to the top.
Table 1.
Comparison of phenotypes in embryos derived from crossing faus26/+ adults, with those embryos derived from wildtype adults, but injected either with the splice site ssG5 MO or translation-blocker (tbG5 MO) morpholinos targeting gata5. The percentage of embryos is shown for each of 4 characteristic phenotypes (fairly normal heart tube, partially fused heart tube, bifid heart, or significant loss of cardiac tissue.
| Heart tube | Partially fused tube | Cardia bifida | Dramatic decrease or absence of cardiac progenitors | |
|---|---|---|---|---|
| ssG5 MO (51 embryos) | 12% | 24% | 61% | 4% |
| tbG5 MO (31 embryos) | 0% | 0% | 100% | 0% |
| Embryos from s26 hets crossed (68 embryos) | 66% (assumed to be wt and +/s26) | 4% | 27% | 3% |
Gata5 and Gata6 are together essential for cardiomyocyte development
In order to test specific requirements for GATA factors in myocardium, different combinations of morpholinos were injected into fertilized eggs derived from a transgenic reporter strain expressing GFP in cardiomyocytes (cmlc2:gfp). Heart development was analyzed throughout embryogenesis by examining GFP expression in each morphant. The gata4 gene has a relatively late role in heart tube morphogenesis and GFP expression is relatively unaffected in the gata4 morphants (data not shown), while the GFP expression patterns for the gata5 or gata6 morphants reflect heart tube fusion defects (Fig. 2A–G). Embryos injected with morpholinos that target both gata4 and gata5 (gata4+5 morphants) show the same cardiac phenotypes as those injected with the gata5 morpholino alone, primarily cardia bifida (data not shown). Likewise, gata4+6 double morphants display a fused or partially fused heart tube, comparable to the gata6 morphant phenotype (data not shown). In a similar manner, we generated gata5+6 double morphants. In this case we observed a complete lack of GFP expression (Fig. 2H). These observations were reproduced in many experiments analyzing hundreds of embryos for each combination. GFP expression was also absent from gata4+5+6 triple morphants (data not shown).
Fig. 2. Embryos targeted for gata5 and gata6 fail to express GFP in a cmlc2:gfp reporter line.
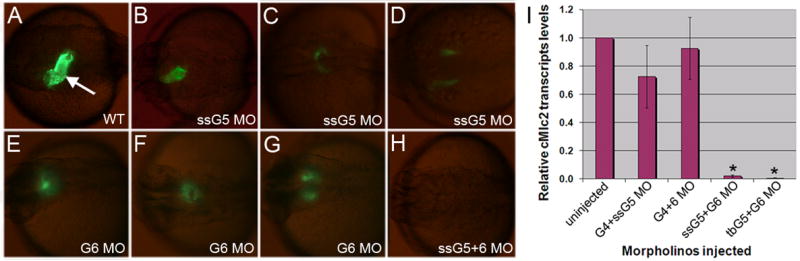
(A) A typical cmlc2:gfp embryo at 30 hpf shows GFP+ cardiomyocytes forming a heart tube (arrow). (B–G) Examples are shown representing cardiac phenotypes generated by injection of the ssG5 MO (B–D) and G6 MO (E–G). Co-injection of ssG5 MO and G6 MO results in an absence of GFP+ cardiomyocytes (H). Brightness of GFP appears lower in the reproduced images of B–G compared to A, because the injected embryos are slightly delayed, but also because the signal is more diffuse in the defective heart tubes. I: Quantitative real time PCR for cmlc2 transcripts. Endogenous cmlc2 transcript levels are reduced to approximately 1% the normal level in ssG5+6 and tbG5+6 double morphants (p<0.01).
The specificity of the cardiomyocyte phenotype was demonstrated in rescue experiments by injecting gata5 and/or gata6 mRNA to restore Gata5 and/or Gata6 proteins to the double morphant embryo. This experiment is complicated because forced expression of Gata5 or (to a lesser extent) Gata6 causes abnormal embryogenesis. This is likely caused by an inappropriate expression pattern since it occurs regardless of whether morpholinos are included, and it is not possible for technical reasons to deliver accurately ectopic RNA back to the endogenous pattern. However, by titrating the amount of exogenous RNA, cardiomyocyte specification is rescued in the double morphant background. For these experiments we used the ssG5 morpholino that does not inhibit expression from the injected in vitro transcribed gata5 mRNA. Morpholinos were injected in embryos derived from cmlc2:gfp reporter fish, and mRNA was injected independently. Over the course of many experiments, control injections lacking RNA yielded embryos that showed no or few GFP+ cardiomyocytes. In a representative set of experiments, 6/81 embryos showed only sparse GFP+ cells (7%). In contrast, GFP+ cardiomyocytes were readily found in 30/47 (64%), 49/79 (62%), 31/75 (41%), or 12/23 (52%) embryos injected with 12.5 pg, 25 pg, 50 pg, or 100 pg of gata5 and gata6 mRNA, respectively. Embryos injected with this RNA were often abnormal, particularly at higher doses, but they did generate cardiomyocytes in the expected embryonic region. In multiple other experiments, similar numbers of embryos (approximately 50%) were rescued using only gata5 or gata6 mRNA, and in some cases cardiac tube formation was restored. Overall, we did not find a statistical difference between rescue using gata5 mRNA, gata6 mRNA, or a combination of both mRNAs.
Since these experiments were done in the context of a transgenic reporter, we also carried out quantitative RT-PCR experiments to analyze at 32 hours post fertilization (hpf) the expression of the endogenous cmlc2 gene (Fig. 2I). The levels of cmlc2 transcripts as measured in different morphants confirmed quantitatively the qualitative observations made in the transgenic reporter fish. There is no significant change in cmlc2 expression in batches of gata4+5 or gata4+6 morphants, although the variability within these two samples likely reflects the phenotypic variations, indicating that some individual morphants have reduced cmlc2 expression. For the analysis of gata5+6 morphants, embryos were injected with the gata6 morpholino in combination with either the ssG5 or tbG5 morpholino. Both combinations showed a striking loss in relative levels of endogenous cmlc2 transcripts (98% and 99% decreased, compared to wild-type, respectively).
Loss of Gata5 and Gata6 causes a heartless phenotype
To more completely evaluate myocardial development we documented at 32 hpf the expression patterns for the atrial differentiation marker Atrial Myosin Heavy Chain (amhc, Fig. 3). The gata4+5 morphants show the same patterns of amhc transcripts as seen in the gata5 morphants, using either the ssG5 or tbG5 morpholino. The phenotypic variability seen in gata6 morphants was similarly observed in the prospective atrium of 32 hpf gata4+6 morphant embryos, and all of the embryos showed amhc transcripts in the abnormal heart tube. In striking contrast, none of the gata5+6 morphants expressed amhc transcripts.
Fig. 3. The gata5 and gata6 genes are functionally redundant for atrial cardiomyocyte differentiation.
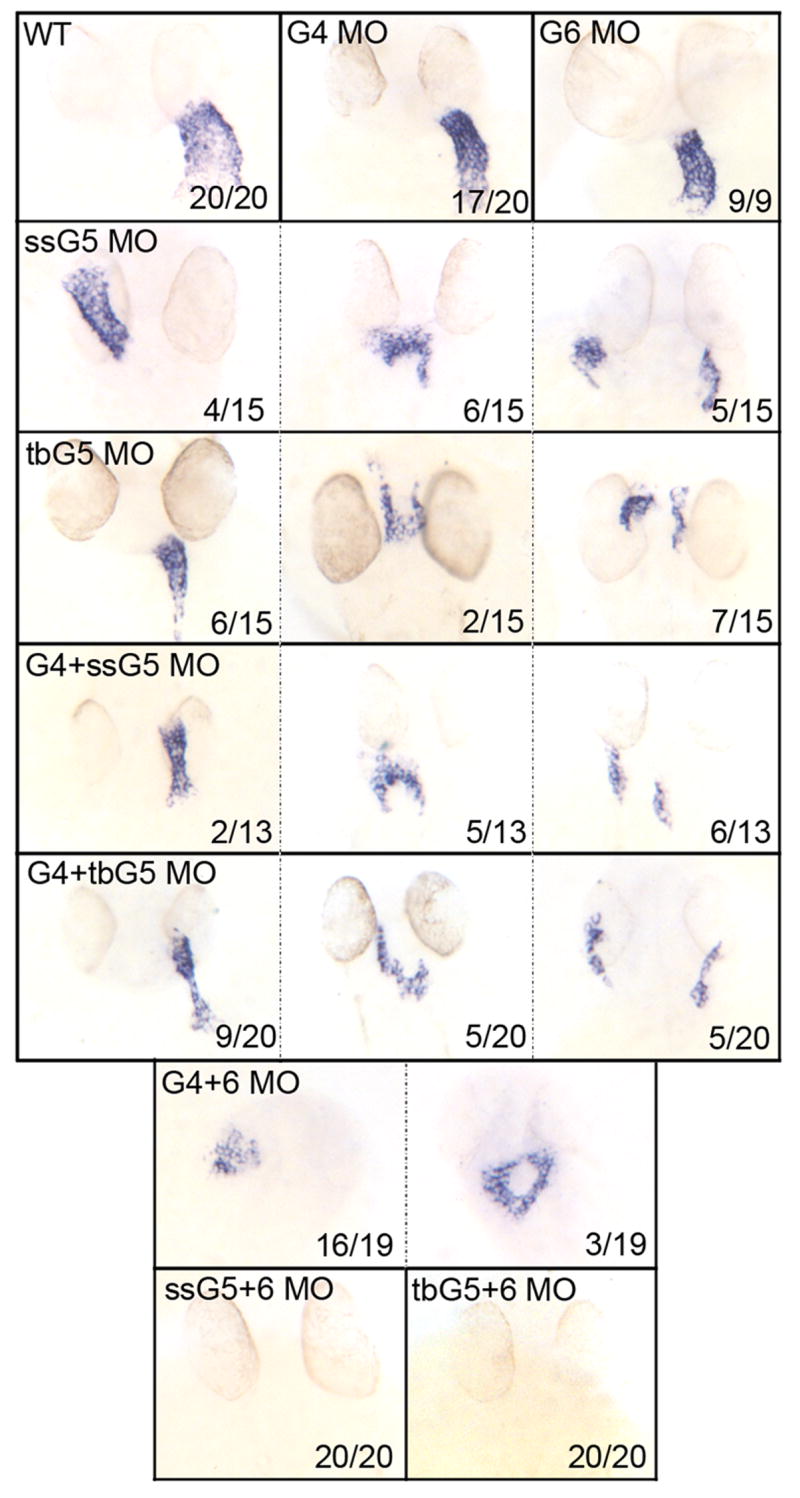
Shown in each panel is a typical representative embryo following processing by whole mount in situ hybridization to detect transcripts for the atrial cardiomyocyte marker amhc at 32 hpf. Samples represent: wild-type embryos (WT), gata4 morphants (G4 MO), gata6 morphants (G6 MO), gata5 morphants (ssG5 MO or tbG5 MO), gata4+5 double morphants (G4+ssG5 MO or G4+tbG5 MO), gata4+6 double morphants (G4+6 MO), and gata5+6 double morphants (ssG5+6 MO and tbG5+6 MO) Despite morphogenetic defects, the atrium is specified in both gata4+6 and gata4+5 double morphants. However, ahmc transcripts are not detectable in gata5+6 double morphants. While these results were reproduced in multiple independent experiments, the number of embryos from the experiment represented in this figure by the phenotype (x) for a given number of embryos (n) is shown in each panel (x/n). Views are either ventral or dorsal, depending on the heart tube position, with anterior to the top.
Similarly, we evaluated in morphants the relative expression pattern of the ventricular differentiation marker Ventricular Myosin Heavy Chain (vmhc, Fig. 4). As was observed for amhc, the gata4+5 morphants at 32 hpf display a range of cardiac phenotypes identical to the single gata5 morphants. In gata4+6 morphants, the presumptive ventricular portion of the heart appears less affected than the atrium, and vmhc transcripts are similar to the single gata6 morphant. Again in contrast, vmhc transcripts are not detected in any of the gata5+6 morphants. Importantly, all the embryos, including the gata5+6 morphants, express apparently normal patterns of vmhc transcripts in the somitic mesoderm, showing that the phenotype is specific to the heart. The gata5+6 double morphants are therefore heartless, lacking cells that express myocardial markers.
Fig. 4. The gata5 and gata6 genes are functionally redundant for ventricular cardiomyocyte differentiation.
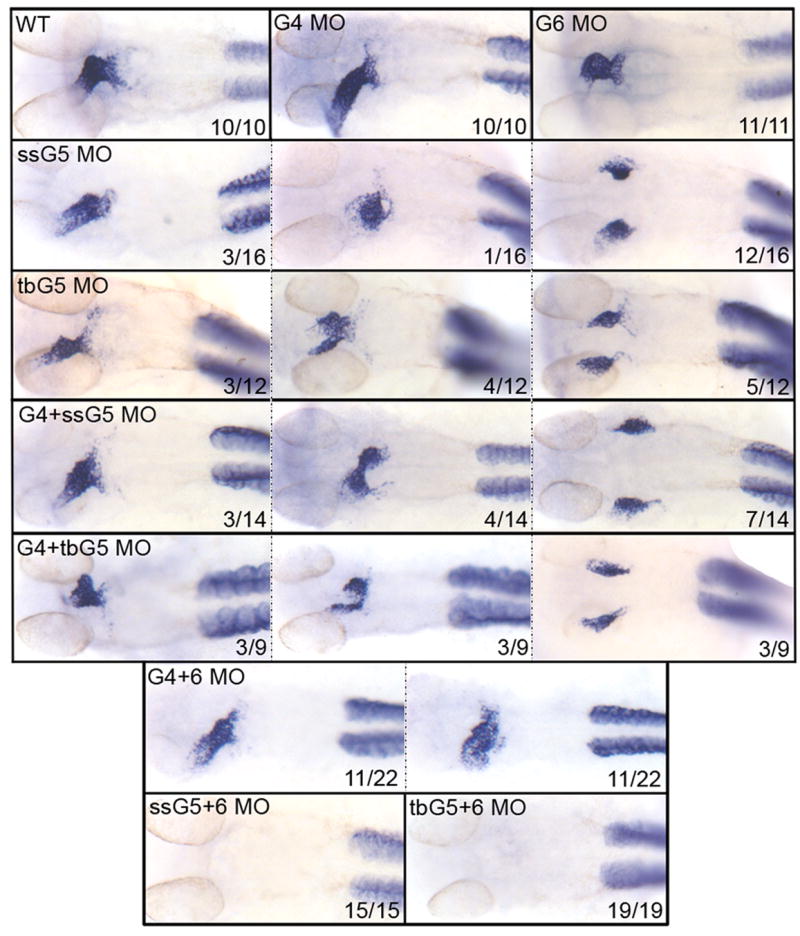
Shown in each panel is a typical representative embryo following processing by whole mount in situ hybridization to detect transcripts for the ventricular cardiomyocyte marker vmhc at 32 hpf. Samples represent: wild-type embryos (WT), gata4 morphants (G4 MO), gata6 morphants (G6 MO), gata5 morphants (ssG5 MO or tbG5 MO), gata4+5 double morphants (G4+ssG5 MO or G4+tbG5 MO), gata4+6 double morphants (G4+6 MO), and gata5+6 double morphants (ssG5+6 MO and tbG5+6 MO) Despite morphogenetic defects, the ventricle is specified in both gata4+6 and gata4+5 double morphants. However, vmhc transcripts are not detectable in gata5+6 double morphants. Note that staining is unperturbed in the somites, showing that the defect is specific to the heart. While these results were reproduced in multiple independent experiments, the number of embryos from the experiment represented in this figure by the phenotype (x) for a given number of embryos (n) is shown in each panel (x/n). Views are ventral, with anterior to the top.
One possibility is that in gata5+6 morphants the cardiogenic program initiates normally, but is not maintained, leading to a failure in differentiation. To evaluate the initiation of the cardiogenic program, injected embryos were analyzed by whole mount in situ hybridization for transcripts encoding the early cardiogenic marker nkx2.5. The gata4+5 morphants show expression of nkx2.5 transcripts at 17 somites in a cardia bifid pattern comparable to gata5 morphants; in the gata4+5 morphants there also appears to be a modest decrease in the levels of nkx2.5 transcripts (Fig. 5A,C,E). The nkx2.5-positive cells are also observed at 12 somites (not shown), indicating that cardiac progenitors are specified. Therefore, gata4 and gata5 are not essential for the initiation of the cardiac program and gata6 alone is sufficient to initiate cardiomyocyte specification. At 17 somites gata4+6 double morphants show normal or slightly reduced levels of nkx2.5 transcripts, similar to gata6 single morphants (Fig. 5A,B,D,F), and also seen in 12-somite embryos (not shown). Therefore, gata5 alone is also sufficient to initiate the cardiogenic program. However, every gata5+6 double morphant fails to express detectable levels of nkx2.5 transcripts at 17 somites (Fig. 5G) or 12 somites (see below). The gata5 and gata6 genes are therefore redundant for cardiomyocyte specification.
Fig. 5. The gata5 and gata6 genes are functionally redundant for cardiomyocyte specification.
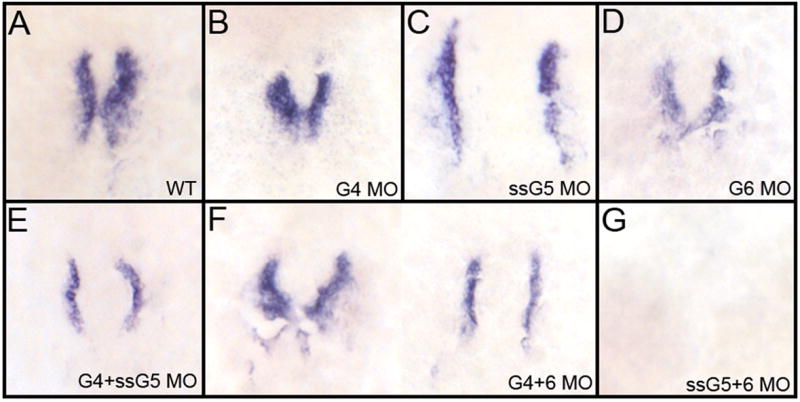
Shown in each panel is a typical representative embryo following processing by whole mount in situ hybridization to detect transcripts for the cardiomyocyte progenitor marker nkx2.5 at the 17 somite stage. (A) wild-type embryos, (B) gata4 morphants, (C) gata5 (ssG5) morphants, (D) gata6 morphants, (E) gata4+5 (ssG5) double morphants, (F) Gata4+6 double morphants and (G) Gata5(ssG5)+6 double morphants. The cardiogenic program is initiated and cardiomyocytes are specified in both gata4+6 and gata4+5 double morphants. However, the early cardiac progenitors are missing in gata5+6 morphants. Embryos were flat mounted; views are dorsal, with anterior to the top. Reproduced in multiple independent experiments, the phenotypes/number of embryos from this experiment represent A:17/17; B: 20/20; C+D combined: 26/26; E: 29/29; F: 25/25; G: 25/25.
The mesodermal defect in gata5+6 morphants is specific to myocardium
The gata5+6 morphants lack mesoderm-derived myocardium, so we tested if this is specific to the heart, or if mesoderm derivatives are more widely deficient in double morphants. We first tested the effect of gata5+6 depletion by analyzing the intermediate mesoderm using in situ hybridization to detect transcripts for the pronephric marker pax2.1. While gata5+6 morphants lack nkx2.5-expressing cardiac progenitors at 12 somites (Fig. 6A,B), the pax2.1 expression pattern is unaffected at the same stage (Fig. 6C,D). A medial mesoderm marker, no tail, was analyzed in 20-somite embryos, and displayed a normal expression pattern in the tail and the notochord in both wild-type and gata5+6 morphants (Fig. 6E,F). Finally, we analyzed the expression of the lateral plate mesoderm marker lmo2, and found that this pattern is also not affected in gata5+6 morphants (Fig. 6G,H). This is consistent with the normal pattern of trunk endothelium seen in gata5+6 morphants using the fli:gfp reporter line that marks vascular endothelium (data not shown). Therefore, the mesoderm defect caused by loss of gata5 and gata6 is specific to the heart. Moreover, there is no obvious expansion of patterns for other mesoderm markers, for example lmo2, in the anterior lateral plate mesoderm. This suggests that prospective myocardium in gata5+6 morphants fails to be specified, rather than being trans-fated into other mesoderm derivatives.
Fig. 6. The defect in gata5+6 morphants is specific to cardiac mesoderm.
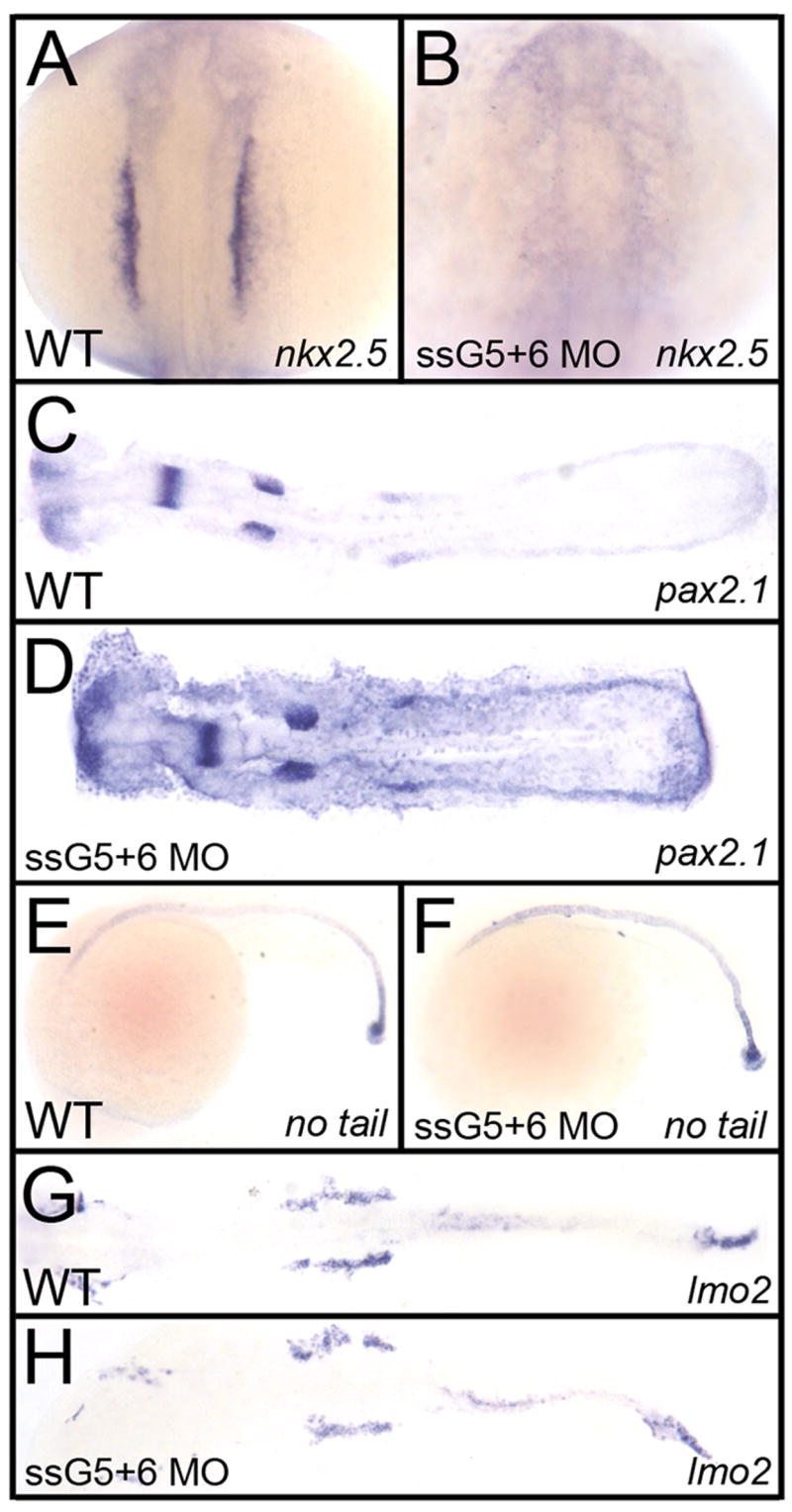
Shown in each panel is a typical representative embryo following processing by whole mount in situ hybridization. Embryos were either wild-type (A, C, E, G) or gata5 (ssG5) +6 double morphants (B, D, F, H). (A, B) The cardiomyocyte progenitor marker nkx2.5 at the 12 somite stage, shows the lack of cardiac progenitors in gata5+6 morphants. (C,D) The pronephric marker pax2.1 shows that the intermediate mesoderm was not affected in morphants. (E,F) The axial mesoderm marker no tail shows that gata5+6 morphants develop a normal notochord. (G, H) The lateral plate mesoderm, marked by the lmo2 probe, is also not altered in gata5+6 morphants. A,B: views are dorsal, with anterior to the top. C,D,G,H: embryos were flat mounted, views are dorsal, with anterior to the left. E,F: Views are from the left side, with anterior to the left. These panels represent patterns seen in A: 39/39; B: 38/38; C: 10/10; D: 23/23; E: 26/26; F: 45/45; G: 28/28; H: 42/42.
Gata4 alone is insufficient to rescue cardiogenesis
Since depletion of gata5 and gata6 together is sufficient to block myocardial development, this implies that the gata4 gene is unable to compensate for their loss. However, as our previous work suggested, it is possible that GATA factors regulate the expression of each other (Holtzinger and Evans, 2005). This could complicate the interpretation, for example if gata4 expression is itself dependent on Gata5 and/or Gata6 protein. Therefore, we examined by in situ hybridization at 12 somites the transcript levels of each factor, in every morphant and double morphant combination and failed to find a significant change in the levels of gata4 transcripts in the gata5+6 morphants (not shown). Thus, the heartless phenotype observed in the gata5+6 morphants is due specifically to the lack of Gata5 and Gata6, and does not require the loss of all three factors. We have thus far not found forced expression of Gata4, by mRNA injection, to rescue the gata5+6 morphant phenotype, with the caveat that this is a negative result.
GATA factors and cardiogenesis
Our study defines a specific pair of transcription factor paralogs that is essential in zebrafish for specification of cardiomyocyte fate from mesoderm progenitors. Similar to Drosophila pannier or tinman embryos that lack expression of a Gata or Nkx2 orthologue, respectively, the gata5+6 morphants are completely heartless. While our study is focused on the cardiomyocyte population, we also found no evidence for endocardium or epicardium in these morphants (our unpublished data). This is not surprising since functions for GATA factors in these tissues are also documented. However, whether the specification of progenitors for endocardium and epicardium are also dependent on one or more specific Gata-4/5/6 genes is not addressed by our experiments. These tissues develop relatively late compared to the myocardial progenitors, and their differentiation and morphogenesis is likely dependent on normal development of the primitive myocardial tube. Further clarification of this issue awaits definition of more definitive specification markers analogous to Nkx2.5 for myocardium. GATA factors also have important functions in endoderm and its derivatives, and so the heartless phenotype could in principle be due to a non-cell-autonomous defect for induction of cardiomyocytes. However, previous studies, for example with the endoderm defective mutant casanova, suggest that endoderm is not essential for cardiomyocyte specification. In addition, the endoderm-derived organ defects seen in gata mutants and morphants are relatively late compared to this step of cardiogenesis.
We note that using a morphant approach the targeted gene levels are reduced but may not be equivalent to a null mutant. Currently there are no null mutants for GATA factors in zebrafish. Therefore, it is possible that complete genetic ablation of either gata5 or gata6 might be sufficient to generate the heartless phenotype, suggested for example by the fact that a small number of faust or gata5 morphant embryos show a significant reduction in myocardium. However, this caveat is less relevant to the issue of defining functional redundancy. We show that the phenotype caused by depletion of Gata5 or Gata6 alone is dramatically distinct from depletion of both genes, showing that they are functionally redundant. We analyzed many hundreds of embryos and essentially 100% of the double morphants lack cardiomyocytes, which is rarely (gata5) or never (gata6) seen with single morphants or mutants. Importantly, this demonstrates formally a concept that has for some time been hypothesized generally for cardiac transcription factor subfamilies in vertebrates.
The shared function for gata5 and gata6 shown here raises the question of whether this functional redundancy is conserved in mammals. We observed that among the key conserved amino acids within the DNA-binding domain that are diagnostic for the three family members from frog, chick, and mammalian species, not all are conserved in fish (Heicklen-Klein et al., 2005). Indeed, the literature has generally considered that the roles of zebrafish and mammalian GATA factors might differ. For example, the mouse Gata4 knockout phenotype shows abnormal cardiac morphogenesis resulting in cardia bifida (Kuo et al., 1997; Molkentin et al., 1997). Since the zebrafish gata5 (faust) mutant also displays a cardia bifida, it was considered that mouse Gata4 might be the ortholog of the zebrafish gata5 gene (Reiter et al., 1999). Consistent with this idea, cardia bifida is not seen in the zebrafish gata4 morphant or the mouse Gata5 knockout. However, the mouse Gata4 knockout phenotype is indirect, due to a primary defect in extra-embryonic endoderm (Morrisey et al., 1998; Narita et al., 1997; Soudais et al., 1995). Rescue of the extra-embryonic defect by tetraploid complementation showed that GATA4 depletion in the mouse embryo proper leads to a cardiac morphogenetic defect (Watt et al., 2004) remarkably similar to that observed in the zebrafish gata4 morphant (Holtzinger and Evans, 2005). The cardia bifida caused in zebrafish by loss of gata5 was not described in the mouse Gata5 knockout (Molkentin et al., 2000). However, the targeted mouse Gata5 mutation deleted only the first exon, leaving an in-frame ATG in exon 2, and so it may not be a null mutant. Unlike in zebrafish (Peterkin et al., 2003), a role for Gata6 in cardiogenesis has not been reported in the mouse knockout (Koutsourakis et al., 1999). However, the redundant function of gata6 with gata4 for liver morphogenesis is conserved between mouse and zebrafish (Holtzinger and Evans, 2005; Zhao et al., 2005). Overall, there is more evidence for conserved functions of GATA factors than for ortholog switching, and the literature thus far does not rule out a redundant function for Gata5 and Gata6 for mammalian cardiogenesis. Our results demonstrate that the function of vertebrate GATA factors for cardiomyocyte specification is conserved with the Drosophila gene pannier, suggesting that similar heartless phenotypes may be revealed by specific combinatorial knockdown of other paralogs, for example from the NKX2, MEF2, and TBX gene families.
Acknowledgments
This work was supported by a grant from the NIH to TE (HL064282). We thank D. Yelon (NYU) and D. Stainier (UCSF) for providing probes, and H.J. Tsai (Taiwan) for the original cmlc2:gfp strain. Spartak Kalinin provided excellent fish husbandry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal p.
References
- Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- Frasch M. Intersecting signalling and transcriptional pathways in Drosophila heart specification. Semin Cell Dev Biol. 1999;10:61–71. doi: 10.1006/scdb.1998.0279. [DOI] [PubMed] [Google Scholar]
- Fu Y, Yan W, Mohun TJ, Evans SM. Vertebrate tinman homologues XNkx2-3 and XNkx2-5 are required for heart formation in a functionally redundant manner. Development. 1998;125:4439–49. doi: 10.1242/dev.125.22.4439. [DOI] [PubMed] [Google Scholar]
- Grepin C, Nemer G, Nemer M. Enhanced cardiogenesis in embryonic stem cells overexpressing the GATA-4 transcription factor. Development. 1997;124:2387–95. doi: 10.1242/dev.124.12.2387. [DOI] [PubMed] [Google Scholar]
- Heicklen-Klein A, Evans T. T-box binding sites are required for activity of a cardiac GATA-4 enhancer. Dev Biol. 2004;267:490–504. doi: 10.1016/j.ydbio.2003.09.042. [DOI] [PubMed] [Google Scholar]
- Heicklen-Klein A, McReynolds LJ, Evans T. Using the zebrafish model to study GATA transcription factors. Semin Cell Dev Biol. 2005;16:95–106. doi: 10.1016/j.semcdb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Holtzinger A, Evans T. Gata4 regulates the formation of multiple organs. Development. 2005;132:4005–14. doi: 10.1242/dev.01978. [DOI] [PubMed] [Google Scholar]
- Horb ME, Thomsen GH. Tbx5 is essential for heart development. Development. 1999;126:1739–51. doi: 10.1242/dev.126.8.1739. [DOI] [PubMed] [Google Scholar]
- Huang WY, Chen JJ, Shih N, Liew CC. Multiple muscle-specific regulatory elements are associated with a DNase I hypersensitive site of the cardiac beta-myosin heavy-chain gene. Biochem J. 1997;327 ( Pt 2):507–12. doi: 10.1042/bj3270507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Evans T. The Xenopus GATA-4/5/6 genes are associated with cardiac specification and can regulate cardiac-specific transcription during embryogenesis. Dev Biol. 1996;174:258–70. doi: 10.1006/dbio.1996.0071. [DOI] [PubMed] [Google Scholar]
- Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. The transcription factor GATA6 is essential for early extraembryonic development. Development. 1999;126:723–32. [PubMed] [Google Scholar]
- Krauss S, Johansen T, Korzh V, Fjose A. Expression pattern of zebrafish pax genes suggests a role in early brain regionalization. Nature. 1991;353:267–70. doi: 10.1038/353267a0. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–60. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- Latinkic BV, Kotecha S, Mohun TJ. Induction of cardiomyocytes by GATA4 in Xenopus ectodermal explants. Development. 2003;130:3865–76. doi: 10.1242/dev.00599. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–72. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Tymitz KM, Richardson JA, Olson EN. Abnormalities of the genitourinary tract in female mice lacking GATA5. Mol Cell Biol. 2000;20:5256–60. doi: 10.1128/mcb.20.14.5256-5260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, Tang Z, Sigrist K, Lu MM, Jiang F, Ip HS, Parmacek MS. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579–90. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita N, Bielinska M, Wilson DB. Wild-type endoderm abrogates the ventral developmental defects associated with GATA-4 deficiency in the mouse. Dev Biol. 1997;189:270–4. doi: 10.1006/dbio.1997.8684. [DOI] [PubMed] [Google Scholar]
- Patient RK, McGhee JD. The GATA family (vertebrates and invertebrates) Curr Opin Genet Dev. 2002;12:416–22. doi: 10.1016/s0959-437x(02)00319-2. [DOI] [PubMed] [Google Scholar]
- Peterkin T, Gibson A, Patient R. GATA-6 maintains BMP-4 and Nkx2 expression during cardiomyocyte precursor maturation. Embo J. 2003;22:4260–73. doi: 10.1093/emboj/cdg400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter JF, Alexander J, Rodaway A, Yelon D, Patient R, Holder N, Stainier DY. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 1999;13:2983–95. doi: 10.1101/gad.13.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter JF, Kikuchi Y, Stainier DY. Multiple roles for Gata5 in zebrafish endoderm formation. Development. 2001;128:125–35. doi: 10.1242/dev.128.1.125. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Ho RK, Herrmann BG, Nusslein-Volhard C. The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development. 1992;116:1021–32. doi: 10.1242/dev.116.4.1021. [DOI] [PubMed] [Google Scholar]
- Sorrentino RP, Gajewski KM, Schulz RA. GATA factors in Drosophila heart and blood cell development. Semin Cell Dev Biol. 2005;16:107–16. doi: 10.1016/j.semcdb.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Soudais C, Bielinska M, Heikinheimo M, MacArthur CA, Narita N, Saffitz JE, Simon MC, Leiden JM, Wilson DB. Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development. 1995;121:3877–88. doi: 10.1242/dev.121.11.3877. [DOI] [PubMed] [Google Scholar]
- Trinh LA, Yelon D, Stainier DY. Hand2 regulates epithelial formation during myocardial diferentiation. Curr Biol. 2005;15:441–6. doi: 10.1016/j.cub.2004.12.083. [DOI] [PubMed] [Google Scholar]
- Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci U S A. 2004;101:12573–8. doi: 10.1073/pnas.0400752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book a guide for the laboratory use of zebrafish Danio (Brachydanio) rerio. Eugene, OR: Institute of Neuroscience University of Oregon; 1993. [Google Scholar]
- Xin M, Davis CA, Molkentin JD, Lien CL, Duncan SA, Richardson JA, Olson EN. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc Natl Acad Sci U S A. 2006;103:11189–94. doi: 10.1073/pnas.0604604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Watt AJ, Li J, Luebke-Wheeler J, Morrisey EE, Duncan SA. GATA6 is essential for embryonic development of the liver but dispensable for early heart formation. Mol Cell Biol. 2005;25:2622–31. doi: 10.1128/MCB.25.7.2622-2631.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Traver D, Davidson AJ, Dibiase A, Thisse C, Thisse B, Nimer S, Zon LI. Regulation of the lmo2 promoter during hematopoietic and vascular development in zebrafish. Dev Biol. 2005;281:256–69. doi: 10.1016/j.ydbio.2005.01.034. [DOI] [PubMed] [Google Scholar]


