Abstract
In Xenopus embryos, the dorso-ventral and antero-posterior axes are established by the Spemann-Mangold organizer. According to the prevalent model of early development, the organizer is induced by the dorsalizing Nieuwkoop signal, which is secreted by the Nieuwkoop center. Formation of the center requires the maternal Wnt pathway, which is active on the dorsal side of embryos. Nevertheless, the molecular nature of the Nieuwkoop signal remains unclear. Since the Nieuwkoop center and the organizer both produce dorsalizing signals in vitro, we asked if they might share molecular components. We find that vegetal explants, the source of Nieuwkoop signal in recombination assays, express a number of organizer genes. The product of one of these genes, chordin, is required for signaling, suggesting that the organizer and the center share at least some molecular components. Furthermore, experiments with whole embryos show that maternal Wnt activity is required in the organizer just as it is needed in the Nieuwkoop center in vivo. We conclude that the maternal Wnt pathway generates the Nieuwkoop center in vitro and the organizer in vivo by activating a common set of genes, without the need of an intermediary signaling step.
Keywords: Nieuwkoop center, Spemann-Mangold organizer, Wnt, dorsalizing, recombinant, chordin, Xenopus
Introduction
The Nieuwkoop recombination experiment was the first to demonstrate the possibility of an inductive mechanism for meso-endoderm formation (reviewed in (Gerhart, 1999). In this experiment, when amphibian animal cells, fated to become neurectoderm, are juxtaposed to endoderm-fated vegetal cells, they switch fate to meso-endoderm. In addition, dorsal vegetal cells induce a “dorsal” type of mesoderm (notochord and muscle), as opposed to the “ventral” type (blood, mesenchyme) induced by ventral vegetal cells. Nieuwkoop’s observations were later incorporated in the three signal model that describes mesoderm formation and patterning in amphibians (reviewed in (De Robertis and Kuroda, 2004; De Robertis et al., 2000; Gerhart, 2001; Harland and Gerhart, 1997; Kimelman, 2006). According to this model, all vegetal cells produce a general mesoderm-inducing signal (first signal), responsible for the mesodermal fate of overlying marginal cells. Dorsal vegetal cells produce in addition a dorsalizing signal (Nieuwkoop signal), required for establishing the Spemann-Mangold organizer in dorsal marginal cells. The third signal emanates at later stages from the organizer and patterns the dorso-ventral axis of the embryo (reviewed in (Niehrs, 2004). The dorsal vegetal cells constitute the Nieuwkoop center (Gerhart et al., 1991), defined as cells producing both mesoderm-inducing and dorsalizing signals. Experimentally, Nieuwkoop center transplants induce an embryonic axis containing dorsal mesoderm, while retaining an endodermal fate (Gimlich, 1985; Gimlich and Gerhart, 1984). Nieuwkoop center transplants appear therefore to induce an organizer in adjacent mesoderm-fated host cells. In contrast, transplanted organizers (dorsal marginal cells at blastula stage or dorsal lip at gastrula stage) become axial dorsal mesoderm (notochord) themselves, and induce host cells to adopt other dorsal fates, such as neural plate and paraxial mesoderm. The spatial localization of the two entities varies somewhat depending on the criteria used to define them: functional (transplantation and recombinants) or morphological and molecular (the dorsal lip of the blastopore in early gastrula and cells expressing a set of genes centered on dorsal marginal cells in blastula and gastrula). Functionally, transplantation experiments with blastomeres from 32 cell stage embryos place the Nieuwkoop center in D1 cells (nomenclature of Nakamura and Kishiyama, Fig. 4, top panel), and the organizer in C1 and B1 cells (Gimlich, 1985; Gimlich, 1986; Gimlich and Gerhart, 1984). Morphologically and molecularly, cell fate experiments localize the organizer to the B1 blastomere (Bauer et al., 1994) or to B1 and part of C1 (Vodicka and Gerhart, 1995). The Nieuwkoop center has become a paradigm of early development after similar signaling centers were also described in zebrafish (reviewed in (Schier and Talbot, 1998; Schier and Talbot, 2005), chick (reviewed in (Boettger et al., 2001), and sea urchin (reviewed in (Davidson et al., 1998).
Figure 4.
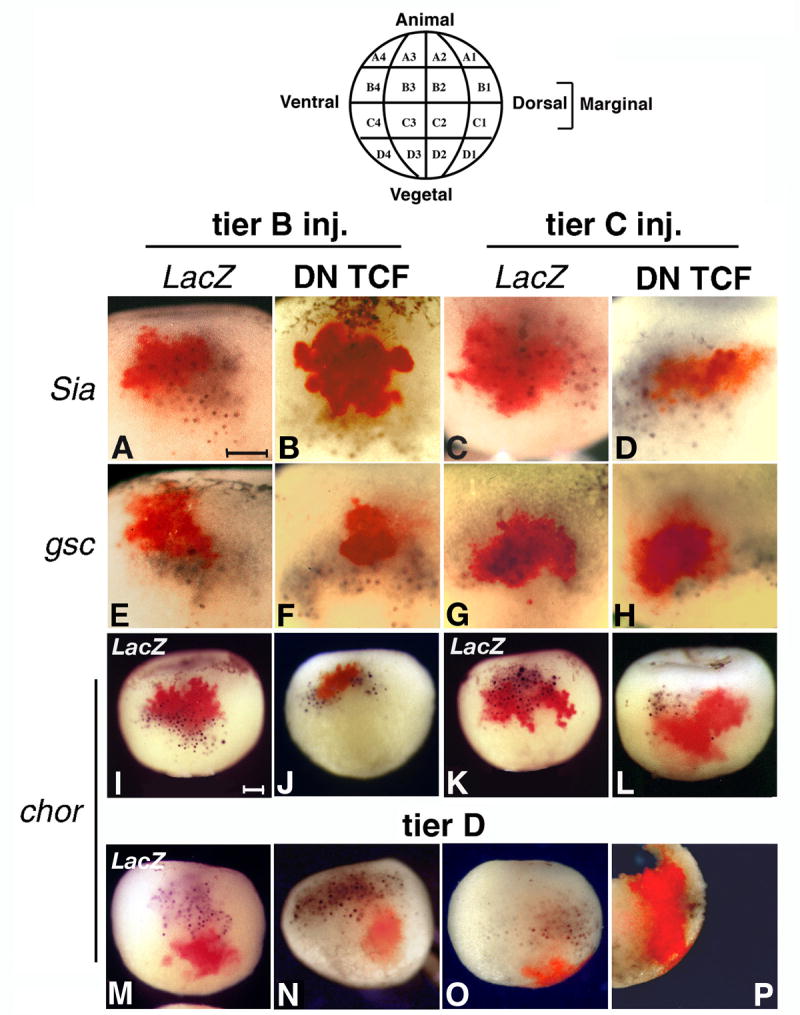
Inhibition of the maternal Wnt pathway by DN XTcf-3 inhibits wild-type expression of organizer genes only in the injected cells. 32 cell embryos were injected in the dorsal cells of the indicated tiers with 1 ng LacZ RNA with or without 100 pg DN XTcf-3 RNA. Double in situ hybridization was performed at stage 9 for LacZ (Fast red) and the indicated genes (BM Purple). The drawing (top) shows the blastomere nomenclature of a 32 cell stage embryo (Nakamura and Kishiyama, 1971). All views are dorsal. A-L. Inhibition of organizer gene expression is restricted to the injected area in the marginal tiers B and C (control and DN Xtcf3 RNA injections, Sia 35 and 22 embryos, gsc 20 and 25 embryos, chordin 22 and 32 embryos, respectively). A, C, E, G, I, K are control injections with LacZ RNA only. M-P. D1 injections of LacZ RNA alone (M) or together with DN Xtcf-3 RNA (20 embryos) do not interfere with the expression pattern of chordin in marginal cellls. P is a sagital section of the embryo in O, showing the internal distribution of injected RNA. Bars in A and I indicate 0.2 mm.
At the molecular level, the Nieuwkoop center was connected to the maternal Wnt pathway, which defines the dorsal pole of the embryo (reviewed in (De Robertis and Kuroda, 2004; De Robertis et al., 2000; Gerhart, 2001; Heasman, 2006; Weaver and Kimelman, 2004). Two types of experiments support the presence of a Wnt activity in the Nieuwkoop center. First, vegetal explants need the maternal Wnt pathway to induce expression of organizer and dorsal mesoderm genes in recombined animal caps or equatorial explants (Agius et al., 2000; Wylie et al., 1996). Second, components of the Wnt pathway induce a secondary axis, or rescue the axis of UV-ventralized embryos, when injected in vegetal cells that do not themselves form dorsal mesoderm, and thus behave like a Nieuwkoop center (Cui et al., 1996; Guger and Gumbiner, 1995; Lemaire et al., 1995; Pierce and Kimelman, 1995; Smith and Harland, 1991; Yamanaka et al., 1998).
An unresolved issue is the molecular nature of the Nieuwkoop signal. Experiments with Nieuwkoop recombinants show that blocking the Wnt pathway in cap cells does not prevent induction of organizer markers (Agius et al., 2000; Xanthos et al., 2002), or of dorsal mesoderm at later stages (Wylie et al., 1996). This implies that the Nieuwkoop signal produced by recombined vegetal cells is not itself a Wnt ligand, and that expression of organizer genes in the induced caps is Wnt-independent. In one study, nodal ligands were identified as Nieuwkoop signal, because their expression is induced by the maternal Wnt pathway in vegetal cells, and they can trigger a dorsalizing signal in Nieuwkoop recombinants and in whole embryos (Agius et al., 2000).
There is, however, an alternative interpretation of the events leading to the formation of the organizer. In the direct induction model, the maternal Wnt pathway is active in organizer cells and directly activates organizer genes (Heasman, 1997; Heasman, 2006; Kodjabachian et al., 1999; Kodjabachian and Lemaire, 1998; Moon and Kimelman, 1998; Weaver and Kimelman, 2004). Evidence for this alternative model comes from both embryonic manipulations and molecular experiments. In the first group, removal of dorso-vegetal cells had no effect on dorso-ventral axis formation (Ding et al., 1998; Kageura, 1995), dorsal-marginal blastomeres generate dorsal axial structures even when isolated at early stages (Gimlich, 1986), and vegetal explants from embryos where cortical rotation was blocked by UV irradiation do not produce a dorsalizing signal, although they have an active maternal Wnt pathway (Darras et al., 1997). An intriguing report on ectopic axis induction by transplanted dorsal vegetal cells (D1) found that cells derived from the animal side of the transplanted D1 blastomeres express the organizer marker chordin and gastrulate themselves, thus raising questions about the type of signaling involved in transplants (Nagano et al., 2000). In molecular experiments, the Wnt pathway can activate intracellularly the promoters of classic organizer genes, such as goosecoid (Laurent et al., 1997) and chordin (Kessler, 1997), through the intermediary of the transcription factors siamois and twin, which are themselves direct Wnt targets (Brannon et al., 1997; Laurent et al., 1997). In whole embryos, expression of organizer genes requires initially only Wnt activity (Delaune et al., 2005; Wessely et al., 2001; Wessely et al., 2004; Zorn et al., 1999), while at later stages nodal signaling is required for expression maintenance (Birsoy et al., 2006; Collart et al., 2005; Wessely et al., 2004).
Nevertheless, experiments contesting the role of the Nieuwkoop center in vivo do not explain the nonautonomous dorsalizing signal in the in vitro setting of Nieuwkoop recombinants. In this study, we first addressed the molecular nature of the Nieuwkoop signal. An implicit assumption of the three signal model is the different identity of the signals produced by the Nieuwkoop center and the organizer. Nevertheless, both signals dorsalize mesoderm in recombination experiments (Carnac et al., 1996). In this, study, we identify the organizer factor Chordin as a component of the Nieuwkoop signal in vitro, and find that organizer formation requires the maternal Wnt pathway exclusively in organizer cells in vivo. Our evidence suggests that, both in vivo and in vitro, no intermediary signaling step is required between the maternal Wnt pathway and the induction of organizer genes.
Materials and Methods
Embryo manipulations
Xenopus laevis embryos obtained by in vitro fertilization were cultured in 0.1x MMR and staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967). RNA injections were done at the 2-4 cell stage in 10 nl volume, and at 32 cell stage, in regularly dividing embryos, in 5 nl volume. For vegetal pole explants, embryos were labeled on the vegetal dorsal side (midline) at the 4 cell stage with Nile Blue crystals, then cut at stage 8.5/9 with a hair knife in 0.5 X MMR as follows: after removal of the vitelline membrane and the animal cap, the marginal zone was sectioned with a hair knife on one side, and removed by cutting along and inside the circumference of the large, white vegetal cells, therefore within vegetal territory to prevent contamination of vegetal explants with marginal cells. For inhibition of the FGF pathway, explants were incubated for two hours in the presence or absence of 20 μM of the MAPK inhibitor U0126 (Promega). For in situ stain of bisected vegetal explants, the left and right halves of explants were separated by sectioning through the Nile Blue dorsal label. Recombination experiments were as in (Agius et al., 2000). Briefly, stage 8.5 animal caps and vegetal explants were recombined in 1:1 CMFM:LCMR. After 2 h, the animal caps were separated and either processed immediately or incubated in 0.5X MMR until stage 20. All experiments were in triplicate, and for each RT reaction we used 7 recombined caps. Morpholino oligonucleotides (MO) for the two chordin alleles (Gene Tools, Philomath, OR) had the published sequence (Oelgeschläger et al., 2003), and were injected in the vegetal pole of each blastomere at the 4 cell stage (15 ng of each MO per blastomere). For β-catenin depletion, MOs were injected at the 4 cell stage on both dorsal blastomeres (20 ng/blastomere), or at the 32 cell stage in B1 and C1 cells (10 ng/blastomere). For fluorescent labeling, C4 or D4 ventral blastomeres were injected with 100 ng Fluoresceine Dextran (Molecular Probes, Eugene, OR), fixed at the indicated time, sectioned with a microsurgery scalpel through the fluorescent spot under UV light, followed by mounting and UV microscopy in the blue (for embryo autofluorescence) and green (for Fluoresceine Dextran) channels.
Plasmids and RNA expression
Expression vectors for β-catenin (Funayama et al., 1995), DN Xtcf-3 and VP16 Xtcf-3 (Vonica et al., 2000) have been described. Other vectors were pSP6nucßgal (from R. M. Harland), pCS2-Xnr-1 (Jones et al., 1995), and pSP35T-chordin (Sasai et al., 1994). Sense RNA for expression was produced with the mMessage mMachine SP6 kit (Ambion, Austin, Tx).
In situ hybridization
Whole mount in situ hybridization followed the protocol of Sive (Sive et al., 1994), with omission of RNAse treatment, and 1.3x SSC concentration in the hybridization buffer (Henrique et al., 1995). For double in situ hybridization we stained with BM Purple (Roche Diagnostic Corp., Indianapolis, IN) for the digoxigenin-labeled marker probes, and with Fast Red or BCIP (Roche Diagnostic Corp., Indianapolis, IN) for the FITC-labeled LacZ and chordin probes. The probes used were: siamois derived from pCS2 siamois (Zeng et al., 1997) by removing the T7 and SP6 promoters, Xbra, Frzb, cerberus, (from A. Salic), noggin (from R. Harland), goosecoid and chordin (from E. M. De Robertis). Antisense RNA was synthesized with T7 or T3 RNA polymerase using digoxigenin or FITC-labeled nucleotide mix (Roche Diagnostic Corp., Indianapolis, IN). Pictures of stained embryos were taken with an AxioCam digital camera (Zeiss) coupled to an AxioVision 4 (Zeiss) image processing software, then transferred in Adobe Photoshop 7.0.
RT-PCR
RT-PCR analysis was as described (Munoz-Sanjuan et al., 2002). For each condition, RNA from 5 recombined animal caps (of a total of 7 recombinants, each experiment repeated three times) was reverse transcribed and 1/10th was used for one PCR reaction. Number of cycles was 21 for ODC, 25 for MyoD, chor, gsc, Xbra and Wnt8, and 30 for cardiac actin and gata1. Primers for chordin were redesigned to differentiate between the injected RNA (open reading frame only) and endogenous RNA: sense 5’ GTAGTCGCTGAGAAGGTGGC 3’ (in 5’ UTR), antisense 5’ CCATCTGGT AAAGTCAGCT 3’. Sequences for the other primers are available at http://xenopus.rockefeller.edu/labprotocols.swf.
Results
Vegetal pole explants express organizer genes
We investigated the possibility that the dorsalizing signals produced by the vegetal Nieuwkoop center and the Spemann organizer have a shared molecular basis. For this purpose, we looked at expression of organizer genes in vegetal explants, similar to those used in Nieuwkoop recombination experiments (Sudarwati and Nieuwkoop, 1971). Previous analyses of gene expression in early gastrula embryos found that a number of organizer genes, including Xnr3, chordin, gsc, and noggin, are expressed in some dorsal vegetal cells, in addition to the dorsal marginal cells that form the organizer (Bouwmeester et al., 1996; Smith et al., 1995; Vodicka and Gerhart, 1995; Wessely et al., 2001; Zorn et al., 1999). We confirmed by double in situ hybridization in stage 10 embryos a substantial overlap between chordin and the exclusively vegetal-endodermal gene Cerberus (Bouwmeester et al., 1996) (Fig. 1 A). We next prepared vegetal explants from stage 8.5 wild-type embryos, taking care to dissect within vegetal territory to prevent contamination of the explant with organizer tissue. After 2 h of incubation, the time required to dorsalize caps in recombination assays (Agius et al., 2000; Wylie et al., 1996), explants were fixed and stained by in situ hybridization for the organizer genes chordin (Sasai et al., 1994), gsc (Cho et al., 1991), noggin (Smith et al., 1991), and Frzb (Leyns et al., 1997; Wang et al., 1997), for the anterior endodermal gene cerberus, and for the mesoderm marker Xbra (Smith et al., 1991) (Fig. 1 B-H). The explants expressed cerberus as expected (Fig. 1 C), but also the organizer genes chordin, gsc, and Frzb (Fig. 1 B, D, E). At least one organizer gene, noggin, and the marginal marker Xbra were absent (Fig. 1 F, H). Together with the presence of the endodermal gene Cerberus, this suggests that the explants contained only vegetal cells. To demonstrate directly that organizer markers were expressed by vegetal cells, we split vegetal explants sagitally and stained each half either for chordin or for the endodermal marker Xsox17β, which is excluded from marginal organizer cells (Zorn et al., 1999) (Fig. 1 G1, G2, G1+2). chordin expression overlapped the area stained with Xsox17β, indicating expression of the organizer marker in vegetal cells.
Figure 1.
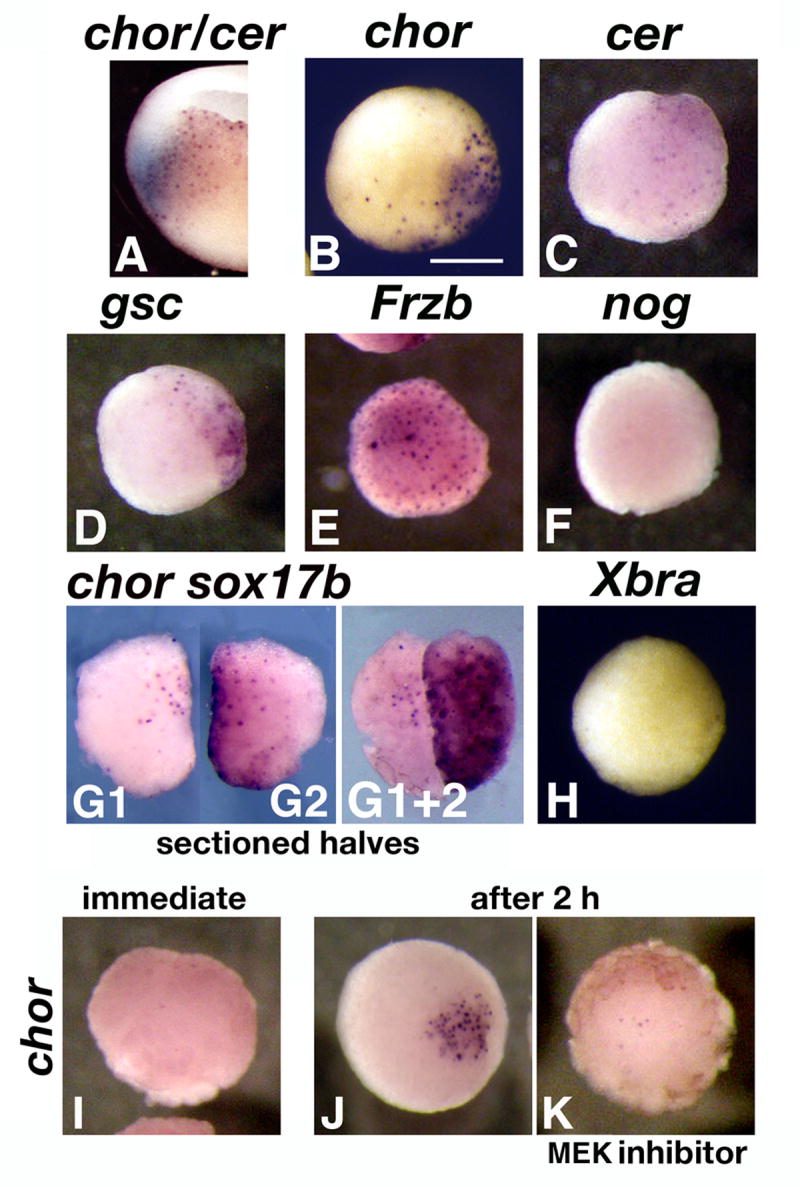
Organizer genes are expressed in vegetal pole explants. A. Double in situ hybridization for chordin (BCIP, blue) and cerberus (BM Purple) in a sectioned stage 10 embryo. The two genes show overlap in endodermal-fated vegetal cells. B-H. Stage 8.5 embryos were dissected and vegetal pole explants were cultured for 2 h before in situ hybridization for chordin (B, n=12), cerberus (C, n=9), gsc (D, n=12), frzb (E, n=15), noggin (F, n=12), and Xbra (H, n=14). All views are animal, dorsal side is to the right. G1, 2. Vegetal explants were sectioned sagitally (n=18), and each half was hybridized in situ for chordin (G1) or Xsox17β (G2). Internal views of the sections, dorsal side up, animal sides facing each other. G1+2. The two halves juxtaposed and viewed from the animal side. I-K. Timing and FGF-dependency of chordin expression in vegetal explants. I. Stage 9 explant, immediately after dissection (n=12). J, K. Explants after 2 hours incubation, in the absence (J, n=15) or presence (K, n=11) of the MEK inhibitor U0126, which blocks the FGF pathway.
Expression of Xbra only partially overlaps the organizer at stage 10, which makes possible the presence of Xbra-negative organizer tissue in stage 10 explants. As an additional control of effective exclusion of marginal tissue from vegetal explants, we stained explants split along the midline with chordin and Xbra at stage 9, when their expression in whole embryos fully overlaps (Figure S1 A 1-3). Only chordin was present in stage 9 explants, similar to what happens at stage 10 (Figure S1 B1-3), when chordin is more vegetally expressed than Xbra in whole embryos.
Organizer markers could be found in vegetal explants because they are normally expressed there, or because their expression is triggered by surgery. We thought the second explanation more likely for two reasons: 1) our explants contained deep vegetal tissues where in situ hybridization of whole embryos does not detect organizer gene expression, and 2) wounding activates the FGF pathway (LaBonne and Whitman, 1997), which is required for chordin expression in whole embryos (Mitchell and Sheets, 2001), and is normally restricted to the marginal zone before stage 10 (Schohl and Fagotto, 2002). To test the activation through wounding hypothesis, we compared stage 9 freshly cut vegetal explants (Fig. 1 I), with explants incubated for 2 h in the absence or presence of the MEK inhibitor U 0126 to block FGF signaling (Fig. 1 J, K). Although chordin is expressed in whole embryos before stage 9 (Wessely et al., 2001), it was absent in freshly cut explants containing only deep vegetal cells, but was expressed after 2 hours, in MEK-dependent manner. These results support a role for surgery in ectopically activating the FGF pathway and organizer gene expression in vegetal explants.
In conclusion, we found organizer genes expressed in vegetal explants, raising the possibility that dorsalizing signals produced by these explants in recombination assays may reflect an organizer-like activity of dorso-vegetal cells.
Chordin is a necessary component of the dorsalizing Nieuwkoop signal in recombinants
Chordin protein is required for ectopic axis induction by implants of organizer tissue in the blastocoel (Oelgeschläger et al., 2003). We therefore investigated a potential role of Chordin in the dorsalizing signal produced by vegetal explants. For this purpose, we blocked translation of endogenous chordin RNA in vegetal cells with specific morpholino oligonucleotides (ChorMO) directed at the two alleles of this gene (Oelgeschläger et al., 2003). When injected dorsally, the MOs produced a ventralized, short axis phenotype as described (Oelgeschläger et al., 2003) that could be rescued by coinjected chordin RNA (Figure S2 B and results not shown). For the Nieuwkoop recombination assay (described in Fig. 2, top panel), we injected 4 cell stage embryos in the vegetal pole of each blastomere with control MOs (ConMO), or specific ChorMOs, with or without chordin RNA (Fig. 2 A-C). Animal caps were recombined at stage 8.5 with the indicated vegetal explants, and separated after two hours of contact. Gene expression was analyzed in caps by RT-PCR immediately (Fig. 2 A) or at stage 20 (Fig. 2 B). In stage 10 caps, organizer markers (chordin, gsc) were induced by wild-type dorso-vegetal poles, but not by ventro-vegetal ones (Fig. 2 A). ChorMOs injected in dorso-vegetal poles decreased the expression of dorsal genes in recombined caps, while coinjection of chordin RNA (1 ng) with Chor MOs restored expression levels. In contrast, the ventral gene Xwnt8 (Christian et al., 1991) was induced by ventro-vegetal explants, and by ChorMO-injected dorso-vegetal explants. The general mesoderm marker Xbra was present in all recombined caps. In stage 20 caps (Fig. 2 B), the dorsal mesodermal markers MyoD and cardiac actin (CA) induced by dorso-vegetal explants were absent when translation of endogenous chordin was blocked by Chor MOs, and restored by coinjected chordin RNA. Expression of the ventral mesodermal gene gata-1 (Zon et al., 1991) changed in a complementary pattern. The molecular profile of transiently recombined caps at stage 20 was reflected in their phenotype (Fig. 2 C). Caps recombined with dorso-vegetal explants were elongated, while caps recombined with MO-injected explants remained round. We conclude that the dorsalizing signal occurring in Nieuwkoop recombinants requires the organizer protein Chordin.
Figure 2.
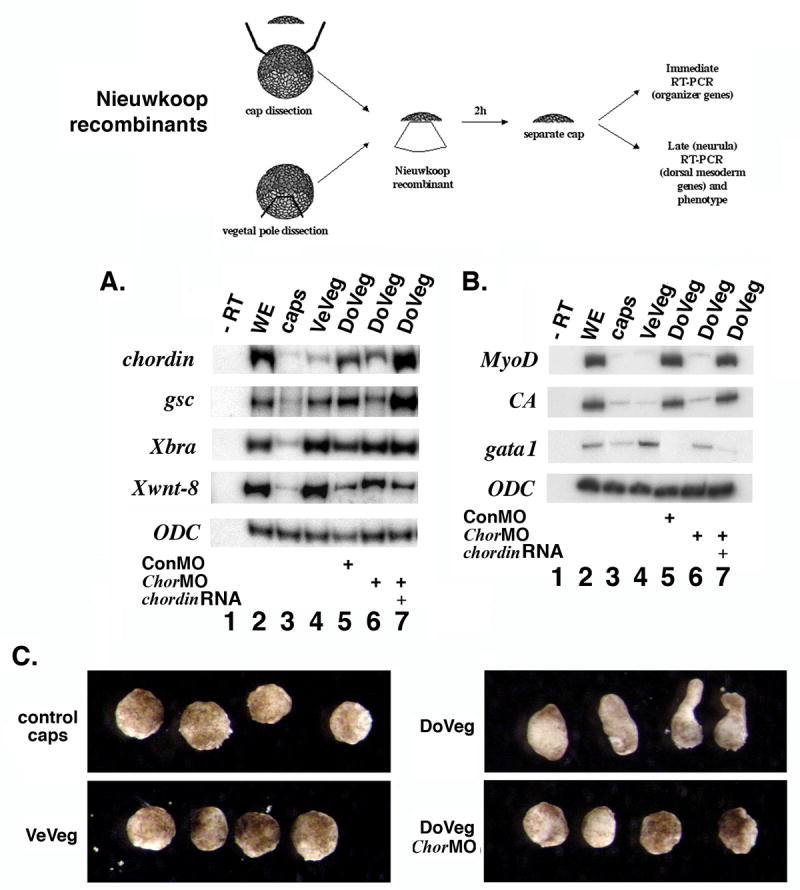
The dorsalizing Nieuwkoop signal requires expression of the organizer protein Chordin in vegetal explants. Nieuwkoop recombinants were made at stage 8.5 from wild-type caps and from ventro-vegetal (VeVeg), or dorso-vegetal (DoVeg) explants injected as indicated with morpholino oligonucleotides for chordin (ChorMO), chordin RNA and MO, and control MO (ConMO). Caps were separated after 2 hours and either prepared immediately for RT-PCR (A) or allowed to develop to stage 20 (B), followed by RT-PCR (n=7 recombined caps for each lane). A. Expression of organizer genes (chordin, gsc) in recombined caps induced by dorsal-vegetal explants is inhibited by chordin MO and restored by chordin RNA. RT-PCR of stage 10 caps. The ventral marker Xwnt8 shows the opposite pattern. Xbra expression indicates general mesoderm induction activity. B. Induction of dorsal mesoderm gene expression in recombined caps requires expression of Chordin protein in vegetal explants. RT-PCR of stage 20 caps. Dorsal mesoderm gene expression (MyoD, Cardiac Actin - CA) is inhibited by ChorMO (lane 6) and rescued by chordin RNA (lane 7). gata1, a ventro-lateral mesodermal gene (blood), has the opposite expression pattern. ODC is used as loading control. C. Phenotype of recombined caps (right panel) at stage 20. Unrecombined caps (control caps), caps recombined with ventral vegetal explants (VeVeg), dorsal vegetal explants (DoVeg), and dorsal vegetal explants injected with ChorMO (DoVeg ChorMO). Elongation implies the presence of dorsal mesoderm (muscle). Depletion of Chordin protein in dorso-vegetal explants prevents the elongation of recombined caps.
In addition, we also tested if Chordin had any role in the vegetal cells of whole embryos (Figure S1, Table S1). Injections of ChorMO in both dorsal vegetal D1 cells of 32 cell stage embryos (Figure 4 top panel) had no effect, while injections in dorsal marginal C1 cells produced a milder version of dorsal 4 cell stage injections (Figure S1 C, D, Table S1). These results indicate that the presence of Chordin in vegetal cells is required for dorsalizing signals only in recombinants.
Chordin is necessary for the nodal-induced dorsalizing signal in Nieuwkoop recombinants
It has been suggested that nodal proteins secreted by vegetal cells in a decreasing dorsal to ventral gradient account for both mesoderm inducing and dorsalizing activities seen in Nieuwkoop recombinants (Agius et al., 2000; De Robertis and Kuroda, 2004). This implies that the first and second signal of the three signal model differ only in intensity, and that nodal is the Nieuwkoop signal. Having demonstrated that the dorsalizing Nieuwkoop signal required Chordin protein, we asked if Chordin is also necessary for nodal-induced dorsalization in Nieuwkoop recombinants.
First, we verified that ectopic expression of Xnr1 in ventral vegetal cells induces ectopic chordin expression in vegetal explants (Fig. 3 A, B). Next, we dissected ventro-vegetal explants from wild-type embryos and embryos injected with Xnr1 RNA in the presence and absence of ChorMO and chordin RNA. Expression of the dorsal mesodermal markers MyoD and CA was analyzed in recombined caps at stage 20 (Fig. 3 C). Explants from embryos injected with Xnr-1 RNA and control MO induce expression of MyoD and CA. Expression is decreased by coinjection with ChorMOs, while addition of chordin RNA restored it. The elongation phenotype of the caps closely matches the molecular profile (Fig. 3 D-I), as the Xnr-1-dependent elongation of recombined caps (Fig. 3 D) is blocked by coinjection of ChorMO (Fig. 3 H). These results demonstrate that the Nieuwkoop signal produced by Xnr1-expressing vegetal cells requires Chordin protein. Therefore, Xnr1, like the maternal Wnt signal, appears to produce a dorsalizing signal of organizer type in vegetal explants.
Figure 3.
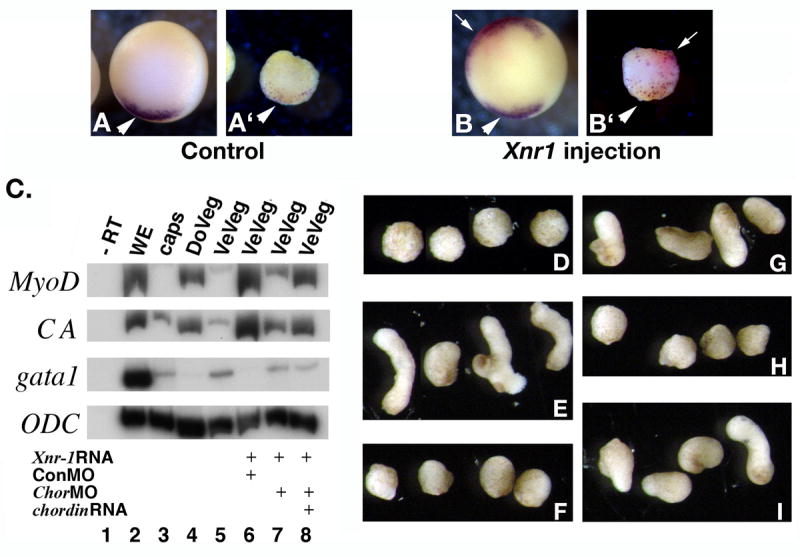
Vegetal explants overexpressing nodal produce a dorsalizing signal that requires expression of Chordin protein. Nieuwkoop recombinants were generated from dorso-vegetal or ventro-vegetal explants, with or without injected RNA and morpholinos. A-B. Vegetal injections of Xnr1 RNA induce ectopic expression of chordin in vegetal explants. 32 cell stage embryos were injected in D4 cells with 500 pg Xnr1 RNA and 1 ng LacZ RNA as tracer. Vegetal explants were dissected at stage 8.5, collected at stage 10 and stained by double in situ hybridization for chordin (purple) and LacZ (red) (A’, B’). Whole embryos are also shown (A, B). Dorsal side is down. Arrow in B’ indicates ectopic chordin, and arrowheads in A, A’, B, B’ indicate wild-type expression. C. Induction of dorsal mesodermal gene expression by Xnr1-expressing vegetal explants requires Chordin. RT-PCR of stage 20 caps (n=7 recombinants for each lane). Expression of dorsal mesodermal genes (MyoD, CA) is present in caps recombined with control dorso-vegetal (DoVeg, lane 4) and ventro-vegetal (VeVeg) explants from embryos injected with Xnr-1 RNA (500 pg) and control morpholinos (ConMO, 60 ng total, lane 6). Coinjection of chordin MOs (60 ng total) with Xnr-1 RNA reduced the dorsalizing signal (lane 7), and addition of chordin RNA (1 ng) restored it (lane 8). The ventro-lateral mesodermal gene gata1 had the opposite expression pattern. B-G. Phenotype of stage 20 caps analyzed in panel A. Cap elongation implies the presence of dorsal mesoderm (muscle). Control caps (B), caps recombined with dorso-vegetal explants (C), ventral-vegetal explants uninjected (D), or injected with Xnr-1 RNA + ConMO (E), Xnr1 RNA + ChorMO (F), Xnr-1 RNA + ChorMO + chordin RNA (G). Caps recombined with Xnr1-injected vegetal explants fail to elongate if Chordin protein has been depleted (F).
The maternal Wnt pathway is required in the Spemann organizer
Our previous experiments suggest that the dorsalizing Nieuwkoop signal is produced by cells expressing organizer genes. Both the organizer and the Nieuwkoop center have been reported to depend on the maternal Wnt pathway (Behrens et al., 1996; Brannon et al., 1997; Kessler, 1997; Molenaar et al., 1996; Wylie et al., 1996). We therefore asked if maternal Wnt/β-catenin pathway activation was required only in cells expressing organizer genes, thus removing the need of an intermediate long-range non-Wnt signal. This was done by both inhibition of the maternal Wnt/β-catenin pathway in the territory of the wild-type organizer, and its ectopic activation.
First, the maternal Wnt pathway was inhibited with a dominant negative (DN) mutant of Xtcf-3 (Molenaar et al., 1996; Vonica et al., 2000). This transcription factor can bind the promoter of Wnt target genes like siamois (Sia) (Brannon et al., 1997; Brannon and Kimelman, 1996; Carnac et al., 1996; Fagotto et al., 1997) and twin (Laurent et al., 1997), and its function in early development is to repress the targets of the maternal Wnt pathway in the absence of signal (Houston et al., 2002; Standley et al., 2006). In addition to Sia, we also monitored the effects of overexpressed DN Xtcf-3 on endogenous expression of the organizer genes gsc and chordin. These genes are direct targets for Sia and twin (Kessler, 1997; Laurent et al., 1997), they precede expression of mesodermal genes like Xbra (Wessely et al., 2001), and they are initially independent of mesoderm-inducing factors (Delaune et al., 2005; Wessely et al., 2001).
Cell fate maps of 32 cell embryos (Vodicka and Gerhart, 1995) show that the organizer is formed mostly from descendants of the dorsal B1 and C1 cells. We injected these blastomeres with DN Xtcf-3 and LacZ RNA, and stained the embryos at stage 9 by double in situ hybridization for Sia, gsc, and chordin (Fig. 4, purple), and for LacZ RNA as tracer (red). LacZ RNA injected alone served as control. Expression of all three markers was absent from cells injected with DN Xtcf-3 RNA, while expression outside this territory was undisturbed (Fig. 4 B, D, F, H, J, L). In control LacZ RNA injections, the tracer overlapped marker expression (Fig. 4 A, C, E, G, I, K). In addition, DN Xtcf-3 RNA injected in vegetal D1 blastomeres had no effect on expression of chordin in adjacent organizer cells (Fig. 4 M-P). Similar results were seen with other Wnt pathway inhibitors, such as overexpressed C-cadherin, which binds the signaling pool of β-catenin (Heasman et al., 1994) (Fig. S3 A, C). Moreover, the same effect was seen when β-catenin was depleted with MOs injected at the 32 cell stage, when expression of both chordin and Xbra was inhibited (Fig. S3 H, J). To show that the effect of DN Xtcf-3 RNA was due to interference with the maternal Wnt pathway, we injected DN Xtcf-3 plasmid DNA (Fig. S3 B, D, F), which expresses DN Xtcf-3 only after midblastula transition (MBT) and therefore does not interfere with the maternal Wnt pathway (Darken and Wilson, 2001; Yang et al., 2002). This construct inhibited expression of Xbra, dependent on zygotic Wnt activity (Vonica and Gumbiner, 2002), but did not inhibit expression of either Sia or chordin, which are dependent on maternal Wnt activity. We also tested if the absence of organizer gene expression had an effect on the fate of the injected B1 and C1 cells. These cells generate notochord and, in the case of B1, neural plate (Bauer et al., 1994; Vodicka and Gerhart, 1995) (Fig. S4, A, C). The injected cells were excluded from the notochord, with B1 descendants being exclusively ectodermal (Fig. S4, B), while C1 descendants are either lateral or anterior to the notochord (Fig. S4 D, E). To demonstrate that DN Xtcf-3 does not repress gene expression nonspecifically, we repeated the experiment of Wylie et al. showing that β-catenin-depleted caps can still respond to signaling in recombinants (Wylie et al., 1996), but used DN Xtcf-3 as Wnt pathway inhibitor (Fig. S5). Such caps show no reduction in chordin and gsc induction at stage 10, supporting the observation that the in vitro Nieuwkoop signal is not a Wnt signal.
Our results indicate that organizer cells with an inhibited Wnt pathway did not express dorsal genes, even though neighboring vegetal cells had an active Wnt pathway. We conclude that cells expressing organizer markers in vivo need an active intracellular maternal Wnt pathway. Reciprocally, blocking the maternal Wnt pathway in C1 or D1 cells, which contain the presumed dorsal vegetal Nieuwkoop center, had no effect on expression of organizer markers by uninjected cells. We cannot, however, rule out the existence of a long range dorsalizing signal from uninjected vegetal cells where the Wnt pathway is still active.
Ectopic activation of the Wnt pathway in ventral blastomeres has an autonomous effect on organizer gene expression
To address the possibility of a long range Nieuwkoop signal, we ectopically activated the Wnt pathway in ventral cells at the 32 cell stage. If intracellular activation of the Wnt pathway can produce an extracellular signal that induces organizer genes in neighboring cells, as suggested by experiments with Nieuwkoop recombinants, the area of expression of the induced genes should be larger than the area covered by injected RNA. We activated the Wnt pathway intracellularly by injecting β-catenin RNA, together with LacZ RNA as tracer, in the ventral blastomeres of tiers B and C (Fig. 5). LacZ RNA injected alone served as control (Fig. 5 B, D, G, I, L, N). Embryos were stained by double in situ hybridization at stage 9 for Sia, gsc, and chordin (purple), and for LacZ, (red). The direct Wnt target gene Sia and the organizer gene chordin were activated exclusively in injected cells in both tiers B and C (Fig. 5 C, E, M, O). gsc was similarly induced in tier C (Fig. 5 J), but not in ventral tier B cells (Fig. 5 H), which lack competence to express this gene (Niehrs, 2004). We conclude that ectopic activation of the Wnt pathway in the ventral cells of tiers B and C leads to expression of organizer genes only in injected cells.
Figure 5.
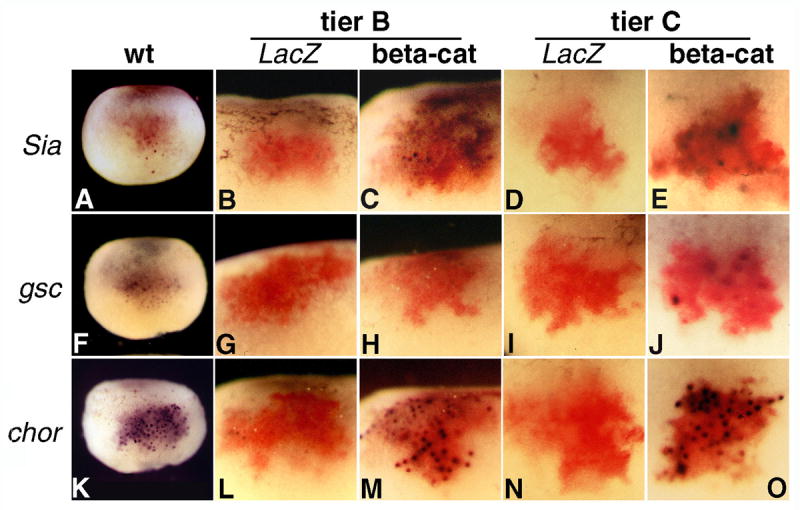
Ectopic activation of the Wnt pathway in tier B and C cells induces expression of organizer genes only in injected cells. Double in situ hybridization of stage 9 embryos for the indicated organizer genes (purple) and coinjected LacZ (red). β-catenin (500 pg RNA) and LacZ (1 ng RNA) were injected at the 32 cell stage in the indicated tiers, on the ventral side of the embryo. A, F, and K are dorsal views showing the wild-type expression pattern for Sia, gsc, and chordin, respectively. Activation of the genes in ventral cells is restricted to the injection site. chordin (21 embryos for tier B, 30 embryos for tier C) and Sia (24 embryos for tier B and 19 embryos for tier C) are activated in both tiers B and C (M, O, and C, E, respectively), but gsc (28 embryos for tier B and 22 for tier C) only in tier C (H, J).
The strongest support for the existence of a Wnt pathway-dependent Nieuwkoop center comes from experiments where intracellular activation of the Wnt pathway in ventro-vegetal cells rescues UV ventralized embryos and induces secondary axes by a nonautonomous mechanism, as shown by the absence of injected cells (β-Gal positive) in organizer-derived axial tissues at tadpole stage (Cui et al., 1996; Guger and Gumbiner, 1995; Lemaire et al., 1995; Pierce and Kimelman, 1995; Wylie et al., 1996). Our overexpression experiments indicate a cell autonomous effect of Wnt activation in tier B and C cells, but it is possible that cells of these tiers lack the competence of vegetal cells to produce a nonautonomous dorsalizing signal. We therefore reexamined the effect of ectopic Wnt activation in ventral D tier blastomeres.
First, we tested whether vegetal injections of Wnt pathway activators in whole embryos induced expression of an organizer gene nonautonomously. While this has never been shown before, it is assumed that a secondary axis produced by such injections would result from the induction of an ectopic organizer. We coinjected β-catenin (500 pg) and LacZ RNA (1 ng) at the 32 cell stage in ventro-vegetal D4 cells (n=120). Half of the embryos were fixed at stage 9 and stained by double in situ hybridization for chordin and LacZ (Fig. 6 A, B), while allowing the other half to develop to tadpole stage. The percentage of embryos positive for ectopic chordin at stage 9 (22%) matched approximately the percentage of axis duplications recorded at tadpole stage (25%, not shown), indicating that secondary axes induced by ventral vegetal injections are likely to be the result of an ectopic organizer. Most of the embryos stained at stage 9 (78%), however, did not have ectopic chordin expression (Fig. 6 A), and the LacZ marker was limited to descendants of D4, as expected from the fate map (Fig. 6 A1, (Bauer et al., 1994). Ectopic chordin expression was seen only in embryos where LacZ RNA was detected outside vegetal territory, in marginal cells (Fig. 6 B, B1). This suggested that β-catenin injections did not induce an organizer at a distance, but instead injected RNA diffused beyond the targeted vegetal D4 blastomere into the neighboring C4, which has a mesendodermal fate (Bauer et al., 1994; Vodicka and Gerhart, 1995).
Figure 6.
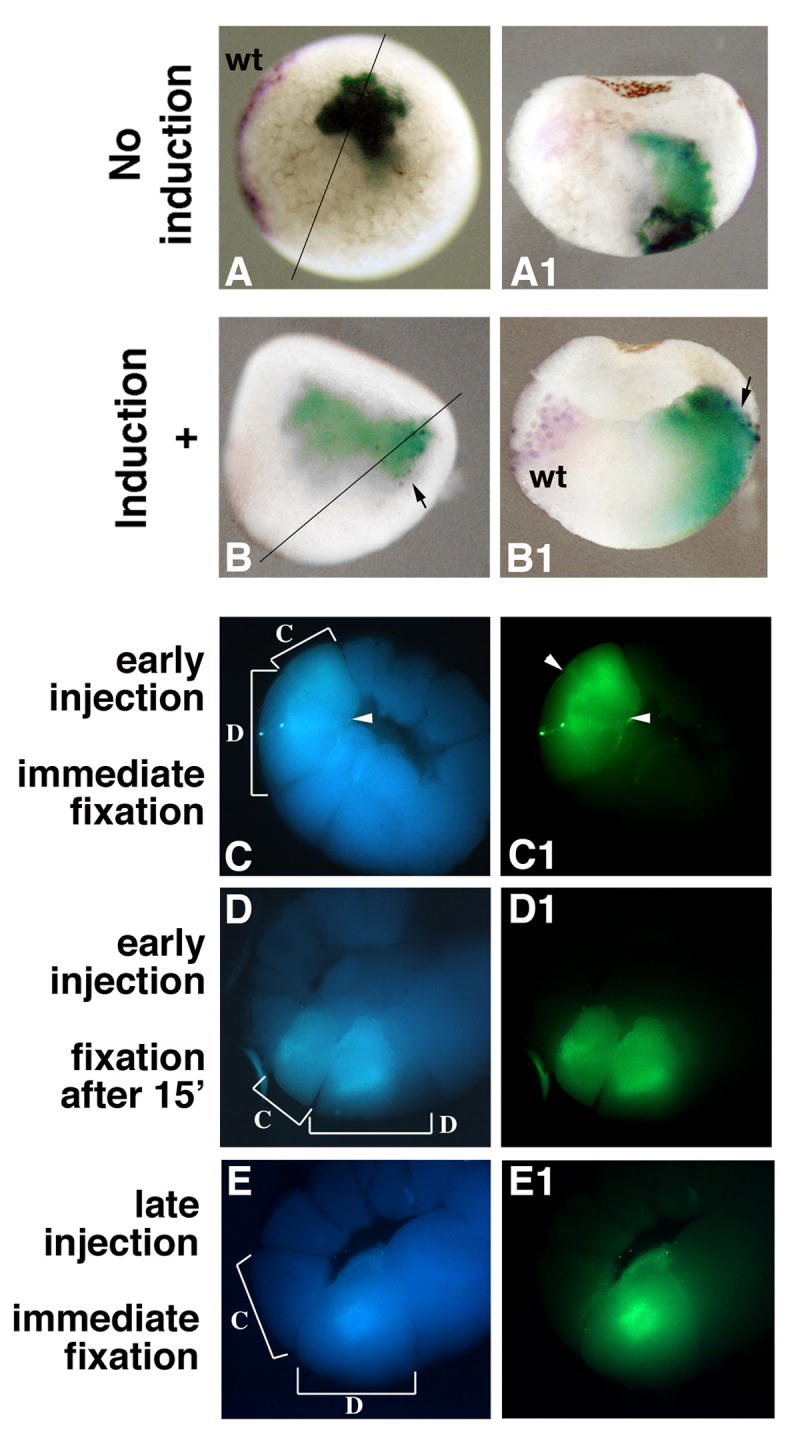
Vegetal injections of an intracellular Wnt activator induce an ectopic organizer by diffusion into marginal cells. A, B Ventro-vegetal injections of β-catenin RNA induce ectopic expression of chordin only when the injected RNA reaches marginal cells. Embryos injected in D4 blastomeres with β-catenin (500 pg) and LacZ (1 ng) RNA were collected at stage 9 and stained by double in situ hybridization (chordin in purple, LacZ in green-blue, n=60). Ectopic chordin expression is absent when the injected RNA is confined to descendants of vegetal tier D cells (A, A1), but present when the injected RNA is found in marginal cells (B, B1, arrow). Embryos are shown whole in vegetal views (A, B, dorsal side to the left) or in sections (A1, B1, section plane indicated in A, B). Wild-type chordin expression is seen on the dorsal side (A, B1). C-E Diffusion of vegetally injected material to the marginal zone is seen in early, but not late, injected D4 cells. Fluoresceine dextran (100 ng) was injected into D4 blastomeres (C, D) immediately after the start of D/C tier separation, followed by fixation either immediately (C, n=10) or after 15 min (D, n=11), or (E) 15 min after the start of the separation (n=15). C, D, E are UV/blue channel pictures, showing the autofluorescence of sectioned embryos. C1, D1, E1 are UV/green channel pictures, showing the position of injected dextran in the same embryos. Brackets in C, D, E, and arrowheads in C and C1 indicate the limits of D4 and C4 blastomeres.
To test whether material injected into the D4 blastomere could diffuse to adjacent C4, we injected fluoresceine dextran in D4 immediately after the separation from C4 became visible (Fig. 6 C, D), or 15 minutes later (Fig. 6 E). In embryos injected early, dextran was present in both the D4 blastomere and C4 blastomeres, regardless of the immediate (Fig. 6 C) or delayed (Fig. 6 D) fixation time, indicating that diffusion into tier C occurred at the moment of injection. At this time, the separation between blastomeres was incomplete (arrowheads in Fig. 6 C, C1). In late injections, when the separation between blastomeres was completed, injected dextran was restricted to the D4 blastomere (Fig. 6 E1). This experiment shows that diffusion of injected material from the injected vegetal to the neighboring marginal blastomere could explain the apparent long-range effect of vegetal mRNA injections.
We next revisited the localization of vegetally injected RNA in the induced ectopic axes (Fig. 7). The previous experiment implies that the coinjected tracer should appear in the axial tissue of ectopic axes induced by vegetal injections. A second implication of the above experiments is that the percentage of ectopic axes induced by vegetal injections should be lower when injections are performed some time after the beginning of the D to C tier separation, as a consequence of limited diffusion from D4 to C4. To address both issues, we coinjected β-catenin RNA with LacZ RNA in D4 cells either immediately after the separation between tiers C and D became apparent, or 15 min later. A high amount of LacZ RNA (5 ng) was used, because it has been suggested that the lower amount commonly used (1 ng) might not be sufficient to fully label the injected cells into tadpole stages (Lane and Sheets, 2002). The amount of β-catenin RNA was the same as that used in a previous study (2 ng, (Guger and Gumbiner, 1995). As positive control, we also injected C4 blastomeres at the same time points. The incidence of secondary axes decreased from 75% in early D4 injections to 35% in late injections, but remained 100% in both early and late C4 injections (Fig. 7 A). In all D4-injected embryos, secondary axes had anterior and axial β-Gal stain (Fig. 7 B), while phenotypically normal embryos had only endodermal stain (Fig. 7 C). Injections in the C4 blastomere always produced secondary axes with β-Gal stain present in dorsal mesoderm (Fig. 7 D, E). These results are in agreement with our previous experiment, supporting the diffusion of injected RNA from the targeted vegetal cells into adjacent marginal cells as a mechanism for the induction of secondary axes by injected intracellular Wnt activators. As the communication between the D and C tiers decreases in time, the opportunity for diffusion, and the induction of ectopic axes, decreases as well. A change in competence to respond to Wnt pathway activation over the 15 min separating the early and late injections appears unlikely, as the percentage of duplicated axes obtained after tier C injections is the same at the early and late time points, and the Nieuwkoop signal is not activated until the stage 8 midblastula transition (Wylie et al., 1996).
Figure 7.
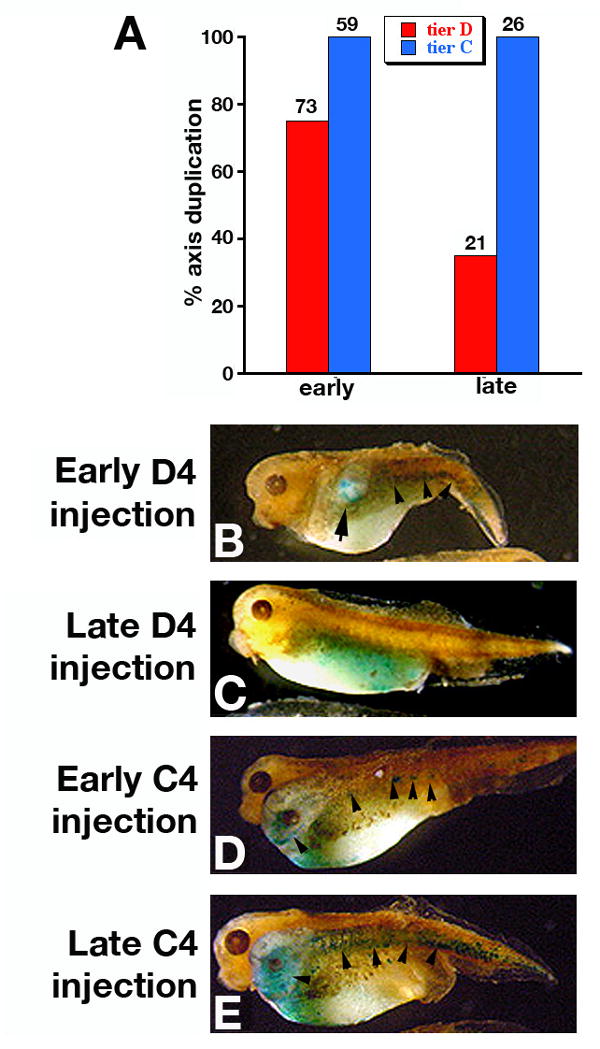
Ectopic axis induction by vegetal overexpression of an intracellular Wnt activator depends on injection timing. Embryos were injected in a ventral tier D blastomere (D4) at the 32 cell stage with LacZ RNA (5 ng) and -catenin RNA (2 ng). A. Induction of secondary axes by ectopic β-catenin injected in vegetal D4 cells depends on the time elapsed from the start of the D/C tier separation. Incidence of secondary axes induced by β-catenin injected in D4 cells decreases from 75% to 35% when the injection is delayed 15 min after the onset of tier D/C separation. The frequency of ectopic axes induced by C4 injections does not change. Numbers above the columns indicate the total number of injected embryos. B-E. β-galactosidase stain of D4 and C4 injected embryos (stage 36). B. Embryo injected in D4 immediately after the start of tier D/C separation. Anterior and axial stain is present in the secondary axis (arrow). C. Late injection in D4 (15 min after start of cell division). Stain is restricted to endoderm and no secondary axis is induced. D, E. Embryos injected in C4 immediately (D) and 15 min (E) after the onset of cells division. Secondary axes are present and stained in axial and anterior tissues. Arrowheads in C, D and E indicate axial β-Gal stain.
Discussion
The Nieuwkoop center is an essential component of the three signal model for early Xenopus development as the source of the dorsalizing second signal. The model implies that the maternal Wnt pathway, active in the vegetal cells of the Nieuwkoop center, induces organizer genes at a distance in the marginal zone through the agency of an intermediate Nieuwkoop signal. We reexamined the molecular nature of the Nieuwkoop dorsalising signal in recombinants, and the spatial relationship between the maternal Wnt pathway and the cells of the organizer.
Molecular nature of the Nieuwkoop signal in vitro
In the first major point of our study we reexamined the nature of the dorsalizing Nieuwkoop signal, and found it shared molecular components with the dorsalising signal produced by the organizer. Vegetal explants used in Nieuwkoop recombinants express a number of dorsal genes, including well-known organizer markers such as chordin, gsc, and Frzb (Fig. 1). Multiple lines of evidence indicate our vegetal explants were not contaminated with organizer cells. The marginal zone and mesoderm marker Xbra was absent even at the stage when it overlaps the expression of organizer genes, while the dorsal endodermal gene cerberus was present. In addition, chordin and the general endodermal marker XSox17β overlapped (Fig. 1). At least one organizer gene, noggin, was not expressed in vegetal explants, suggesting that vegetal cells do not fully reproduce the competence of the organizer. The most likely cause for expression of organizer markers in vegetal explants appears to be the activation by wounding of the FGF pathway, which could synergize with the maternal Wnt pathway in dorsal vegetal cells. At least one of the organizer factors expressed in vegetal explants, Chordin, was required for the Nieuwkoop signal in recombination experiments (Fig. 2). Significantly, a similar requirement for Chordin was reported for secondary axis induction by organizer implants (Oelgeschläger et al., 2003). Furthermore, Nagano and coauthors (Nagano et al., 2000) found that cells descended from the animal side of transplanted D1 blastomeres express chordin and gastrulate, regardless of the orientation of the transplant, and concluded that the animal side of the transplant constitutes a Spemann organizer, and not a Nieuwkoop center. Thus, the experiments of Nagano et al. and our own presented here indicate that the two types of embryonic manipulations that led to the Nieuwkoop signal model can be explained by known organizer signals.
Our findings suggest that, in the experimental conditions of Nieuwkoop recombinants, organizer genes expressed in vegetal explants expand their own expression to the animal caps in an autoinducing loop, as already documented for Chordin in animal cells (Blitz et al., 2000). This is also supported by the ability of the BMP inhibitors chordin and noggin overexpressed in marginal explants to induce dorsal genes in recombination assays (Carnac et al., 1996). Besides chordin, other genes expressed in vegetal explants could also contribute, directly or indirectly, to the dorsalizing signal. For instance, the extracellular Wnt inhibitor frzb was assigned a role in maintaining the effects of BMP inhibition for axis formation (Yasuo and Lemaire, 2001).
The key role of Chordin in the Nieuwkoop signal molecule is also supported by our finding that overexpressed nodal requires Chordin to dorsalize recombined caps. In a previous study (Agius et al., 2000), specific interference with nodal signaling blocked the dorsalizing signal, and soluble nodal protein added to caps produced a concentration-dependent range of mesoderm, with dorsal mesoderm induced at the highest level. Interference with nodal signaling, however, also blocks the mesoderm-inducing signal, which could prevent organizer gene expression or maintenance indirectly. We see that overexpression of Xnr-1 induced ectopic chordin expression in vegetal explants, and that it produced a dorsalizing signal in ventral vegetal cells only in the presence of Chordin protein (Fig. 3). We interpret this effect as an Xnr-1-dependent induction of organizer genes in the vegetal cells, followed by the autoinducing loop described above that would expand the vegetal expression of organizer genes into the recombined caps. This implies that, at least in the case of overexpressed Xnr-1 as nodal ligand, a long distance nodal signal is not sufficient to dorsalize recombined caps. Additional signaling molecules, activated in vegetal cells, must also contribute.
The maternal Wnt pathway is required in the cells of the organizer
As a second major point, we demonstrate with dominant negative inhibitors of the Wnt pathway expressed in whole embryos that Wnt pathway signaling is required autonomously for expression of organizer genes (Fig. 4). In contrast, animal caps depleted of β-catenin still express organizer genes and dorsal mesoderm markers in the conditions of Nieuwkoop recombinants (Wylie et al., 1996; Xanthos et al., 2002), suggesting a different induction mechanism in vitro, such as the organizer autoinduction suggested above.
The autonomous requirement for Wnt pathway activity does not rule out the existence of a parallel, long range non-Wnt dorsalizing signal originating in unaffected, Wnt-activated vegetal cells. We tested this hypothesis in overexpression experiments that ectopically activate the Wnt pathway (Figs. 5-7). We found only local effects on organizer genes expressed in blastomeres of the animal/marginal tiers B and C (Bauer et al., 1994) (Fig. 5), in agreement with previous studies that found autonomous induction of dorsal axial tissues in similar injections (He et al., 1995; Pierce and Kimelman, 1995; Wylie et al., 1996). However, the same autonomous induction was seen for chordin expression (Fig. 6) and axial induction (Fig. 7) in vegetal (tier D) injections, most likely the result of leakage from the injected D tier into the C tier (Fig. 6). This contradicted earlier reports showing nonautonomous rescue of dorsal axis in UV-ventralized embryos, and ectopic axis induction by intracellular Wnt pathway activators, when the β-Gal tracer was absent in dorsal mesoderm (Guger and Gumbiner, 1995; He et al., 1995; Pierce and Kimelman, 1995; Wylie et al., 1996). We note, however, that in some of these and in other studies the efficiency of axis induction was higher in tier C (or equatorial) than in tier D (or vegetal) injections (Brannon et al., 1997; He et al., 1995; Pierce and Kimelman, 1995; Wylie et al., 1996), in contrast to extracellular factors like Wnt 8 and noggin that were more active vegetally (Smith and Harland, 1991; Smith and Harland, 1992).
Earlier experiments used low amounts of tracer LacZ RNA (1 ng vs 5 ng in this study), which may be too low for the detection of LacZ RNA diffused into the marginal zone (Lane and Sheets, 2002). Our analysis of early and late blastulas points to leakage of injected macromolecules as the likely cause for marginal gene activation in vegetal injections, an effect that decreases with the time elapsed after the start of D/C tier separation (Fig. 6). Accordingly, injection timing affected ectopic axis induction in vegetal (tier D) injections, but had no effect on marginal (tier C) injections (Fig. 7). In all cases of secondary axes induced in vegetally injected embryos, the tracer was present in anterior and axial structures. Therefore, our results indicate that ectopic activation of the Wnt pathway in the vegetal cells of whole embryos can induce ectopic organizer genes in blastula, and ectopic axes at tadpole stage, only when injected RNA diffuses into marginal tier C cells.
The recent identification of Xwnt-11 as the maternal Wnt ligand that induces the organizer (Kofron et al., 2007; Tao et al., 2005), offers additional support for a direct role of Wnt signaling in organizer formation. Maternal Xwnt-11, localized to the vegetal pole at cleavage stage (Kofron et al., 2007; Ku and Melton, 1993), is necessary to trigger the maternal Wnt pathway signal and activate organizer genes. This ligand, secreted by vegetal cells, could directly activate the Wnt pathway in dorsal marginal cells.
In conclusion, our study demonstrates that the dorsalizing signals from the Nieuwkoop center in vitro and the Spemann organizer in vivo share a requirement for organizer gene expression. In addition, the organizer in vivo and the Nieuwkoop center in vitro are similarly dependent on Wnt activity.
Supplementary Material
Acknowledgments
We thank Drs. Ali H. Brivanlou, John Gerhart, Chris Wylie, Francesca Spagnoli and Ignacio Munoz-Sanjuan for comments, and Drs. Richard Harland (UC Berkeley), Eddy De Robertis (UCLA), and Adrian Salic (Harvard Med. Sch.) for plasmids. We are also grateful to the members of the Gumbiner and Brivanlou labs for their support. This work was supported by NIH grant (5R01 GM066977) awarded to A. H. B, NIH grant (R37 GM374432) awarded to B. M. G., by NICHD fellowship (PHS HD40724) to A. V., by the Dewitt Wallace Fund for Memorial Sloan-Kettering Cancer Center, and by Cancer Center Support Grant NCI-P30-CA-08784.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–83. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DV, Huang S, Moody SA. The cleavage stage origin of Spemann’s Organizer: analysis of the movements of blastomere clones before and during gastrulation in Xenopus. Development. 1994;120:1179–89. doi: 10.1242/dev.120.5.1179. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–42. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Birsoy B, Kofron M, Schaible K, Wylie C, Heasman J. Vg 1 is an essential signaling molecule in Xenopus development. Development. 2006;133:15–20. doi: 10.1242/dev.02144. [DOI] [PubMed] [Google Scholar]
- Blitz IL, Shimmi O, Wunnenberg-Stapleton K, O’Connor MB, Cho KW. Is chordin a long-range- or short-range-acting factor? Roles for BMP1-related metalloproteases in chordin and BMP4 autofeedback loop regulation. Developmental Biology. 2000;223:120–38. doi: 10.1006/dbio.2000.9740. [DOI] [PubMed] [Google Scholar]
- Boettger T, Knoetgen H, Wittler L, Kessel M. The avian organizer. Int J Dev Biol. 2001;45:281–7. [PubMed] [Google Scholar]
- Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D. A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev. 1997;11:2359–70. doi: 10.1101/gad.11.18.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon M, Kimelman D. Activation of Siamois by the Wnt pathway. Dev Biol. 1996;180:344–7. doi: 10.1006/dbio.1996.0306. [DOI] [PubMed] [Google Scholar]
- Carnac G, Kodjabachian L, Gurdon JB, Lemaire P. The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser activity in the absence of mesoderm. Development. 1996;122:3055–65. doi: 10.1242/dev.122.10.3055. [DOI] [PubMed] [Google Scholar]
- Cho KW, Blumberg B, Steinbeisser H, De Robertis EM. Molecular nature of Spemann’s organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991;67:1111–20. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian JL, McMahon JA, McMahon AP, Moon RT. Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm- inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development. 1991;111:1045–55. doi: 10.1242/dev.111.4.1045. [DOI] [PubMed] [Google Scholar]
- Collart C, Verschueren K, Rana A, Smith JC, Huylebroeck D. The novel Smad-interacting protein Smicl regulates Chordin expression in the Xenopus embryo. Development. 2005;132:4575–86. doi: 10.1242/dev.02043. [DOI] [PubMed] [Google Scholar]
- Cui Y, Tian Q, Christian JL. Synergistic effects of Vg1 and Wnt signals in the specification of dorsal mesoderm and endoderm. Dev Biol. 1996;180:22–34. doi: 10.1006/dbio.1996.0281. [DOI] [PubMed] [Google Scholar]
- Darken RS, Wilson PA. Axis induction by wnt signaling: Target promoter responsiveness regulates competence. Dev Biol. 2001;234:42–54. doi: 10.1006/dbio.2001.0253. [DOI] [PubMed] [Google Scholar]
- Darras S, Marikawa Y, Elinson RP, Lemaire P. Animal and vegetal pole cells of early Xenopus embryos respond differently to maternal dorsal determinants: implications for the patterning of the organiser. Development. 1997;124:4275–86. doi: 10.1242/dev.124.21.4275. [DOI] [PubMed] [Google Scholar]
- Davidson EH, Cameron RA, Ransick A. Specification of cell fate in the sea urchin embryo: summary and some proposed mechanisms. Development. 1998;125:3269–90. doi: 10.1242/dev.125.17.3269. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annual Review of Cell & Developmental Biology. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Larrain J, Oelgeschlager M, Wessely O. The establishment of Spemann’s organizer and patterning of the vertebrate embryo. Nature Reviews Genetics. 2000;1:171–81. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]
- Ding X, Hausen P, Steinbeisser H. Pre-MBT patterning of early gene regulation in Xenopus: the role of the cortical rotation and mesoderm induction. Mechanisms of Development. 1998;70:15–24. doi: 10.1016/s0925-4773(97)00163-9. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Guger K, Gumbiner BM. Induction of the primary dorsalizing center in Xenopus by the Wnt/GSK/beta-catenin signaling pathway, but not by Vg1, Activin or Noggin. Development. 1997;124:453–60. doi: 10.1242/dev.124.2.453. [DOI] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of beta- catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–68. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J. Pieter Nieuwkoop’s contributions to the understanding of meso-endoderm induction and neural induction in chordate development. Int J Dev Biol. 1999;43:605–13. [PubMed] [Google Scholar]
- Gerhart J. Evolution of the organizer and the chordate body plan. Int J Dev Biol. 2001;45:133–53. [PubMed] [Google Scholar]
- Gerhart JC, Doniach T, Stewart R. Organizing the Xenopus organizer. In: Keller R, Clark WHJ, Griffin F, editors. Gastrulation: Movements, Patterns, and Molecules. Plenum Press; New York: 1991. [Google Scholar]
- Gimlich RL. Cytoplasmic localization and chordamesoderm induction in the frog embryo. J Embryol Exp Morphol. 1985;89(Suppl):89–111. [PubMed] [Google Scholar]
- Gimlich RL. Acquisition of developmental autonomy in the equatorial region of the Xenopus embryo. Developmental Biology. 1986;115:340–52. doi: 10.1016/0012-1606(86)90254-x. [DOI] [PubMed] [Google Scholar]
- Gimlich RL, Gerhart JC. Early cellular interactions promote embryonic axis formation in Xenopus laevis. Dev Biol. 1984;104:117–30. doi: 10.1016/0012-1606(84)90042-3. [DOI] [PubMed] [Google Scholar]
- Guger KA, Gumbiner BM. beta-Catenin has Wnt-like activity and mimics the Nieuwkoop signaling center in Xenopus dorsal-ventral patterning. Dev Biol. 1995;172:115–25. doi: 10.1006/dbio.1995.0009. [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann’s organizer. Annu Rev Cell Dev Biol. 1997;13:611–67. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Woodgett JR, Varmus HE, Dawid IB. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature. 1995;374:617–22. doi: 10.1038/374617a0. published erratum appears in Nature 1995 May 18;375(6528):253. [DOI] [PubMed] [Google Scholar]
- Heasman J. Patterning the Xenopus blastula. Development. 1997;124:4179–91. doi: 10.1242/dev.124.21.4179. [DOI] [PubMed] [Google Scholar]
- Heasman J. Patterning the early Xenopus embryo. Development. 2006;133:1205–1217. doi: 10.1242/dev.02304. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–90. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Houston DW, Kofron M, Resnik E, Langland R, Destree O, Wylie C, Heasman J. Repression of organizer genes in dorsal and ventral Xenopus cells mediated by maternal XTcf3. Development. 2002;129:4015–25. doi: 10.1242/dev.129.17.4015. [DOI] [PubMed] [Google Scholar]
- Jones CM, Kuehn MR, Hogan BL, Smith JC, Wright CV. Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation. Development. 1995;121:3651–62. doi: 10.1242/dev.121.11.3651. [DOI] [PubMed] [Google Scholar]
- Kageura H. Three regions of the 32-cell embryo of Xenopus laevis essential for formation of a complete tadpole. Dev Biol. 1995;170:376–86. doi: 10.1006/dbio.1995.1223. [DOI] [PubMed] [Google Scholar]
- Kessler DS. Siamois is required for formation of Spemann’s organizer. Proc Natl Acad Sci U S A. 1997;94:13017–22. doi: 10.1073/pnas.94.24.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D. Mesoderm induction: from caps to chips. Nat Rev Genet. 2006;7:360–72. doi: 10.1038/nrg1837. [DOI] [PubMed] [Google Scholar]
- Kodjabachian L, Dawid IB, Toyama R. Gastrulation in zebrafish: what mutants teach us. Dev Biol. 1999;213:231–45. doi: 10.1006/dbio.1999.9392. [DOI] [PubMed] [Google Scholar]
- Kodjabachian L, Lemaire P. Embryonic induction: is the Nieuwkoop centre a useful concept? Curr Biol. 1998;8:R918–21. doi: 10.1016/s0960-9822(98)00009-8. [DOI] [PubMed] [Google Scholar]
- Kofron M, Birsoy B, Houston D, Tao Q, Wylie C, Heasman J. Wnt11/beta-catenin signaling in both oocytes and early embryos acts through LRP6-mediated regulation of axin. Development. 2007;134:503–13. doi: 10.1242/dev.02739. [DOI] [PubMed] [Google Scholar]
- Ku M, Melton DA. Xwnt-11: a maternally expressed Xenopus wnt gene. Development. 1993;119:1161–73. doi: 10.1242/dev.119.4.1161. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Whitman M. Localization of MAP kinase activity in early Xenopus embryos: implications for endogenous FGF signaling. Dev Biol. 1997;183:9–20. doi: 10.1006/dbio.1996.8497. [DOI] [PubMed] [Google Scholar]
- Lane MC, Sheets MD. Primitive and definitive blood share a common origin in Xenopus: a comparison of lineage techniques used to construct fate maps. Developmental Biology. 2002;248:52–67. doi: 10.1006/dbio.2002.0717. [DOI] [PubMed] [Google Scholar]
- Laurent MN, Blitz IL, Hashimoto C, Rothbacher U, Cho KW. The Xenopus homeobox gene twin mediates Wnt induction of goosecoid in establishment of Spemann’s organizer. Development. 1997;124:4905–16. doi: 10.1242/dev.124.23.4905. [DOI] [PubMed] [Google Scholar]
- Lemaire P, Garrett N, Gurdon JB. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell. 1995;81:85–94. doi: 10.1016/0092-8674(95)90373-9. [DOI] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim S-H, Piccolo S, De Robertis EM. Frzb-1 is a Secreted Antagonist of Wnt Siganling Expressed in the Spemann Organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TS, Sheets MD. The FGFR Pathway is Required for the Trunk-Inducing Functions of Spemann’s Organizer. Dev Biol. 2001;237:295–305. doi: 10.1006/dbio.2001.0385. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–9. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kimelman D. From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus. Bioessays. 1998;20:536–45. doi: 10.1002/(SICI)1521-1878(199807)20:7<536::AID-BIES4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Munoz-Sanjuan I, Bell E, Altmann CR, Vonica A, Brivanlou AH. Gene profiling during neural induction in Xenopus laevis: regulation of BMP signaling by post-transcriptional mechanisms and TAB3, a novel TAK1-binding protein. Development. 2002;129:5529–40. doi: 10.1242/dev.00097. [DOI] [PubMed] [Google Scholar]
- Nagano T, Ito Y, Tashiro K, Kobayakawa Y, Sakai M. Dorsal induction from dorsal vegetal cells in Xenopus occurs after mid-blastula transition. Mech Dev. 2000;93:3–14. doi: 10.1016/s0925-4773(00)00251-3. [DOI] [PubMed] [Google Scholar]
- Nakamura O, Kishiyama K. Prospective fates of blastomeres at the 32-cell stage of Xenopus laevis embryos. Proc Japan Acad. 1971;47:407–412. [Google Scholar]
- Niehrs C. Regionally specific induction by the Spemann-Mangold organizer. Nature Reviews Genetics. 2004;5:425–34. doi: 10.1038/nrg1347. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. ‘Normal table of Xenopus Laevis’. North Holland Publishing Co; Amsterdam, The Netherlands: 1967. [Google Scholar]
- Oelgeschläger M, Kuroda H, Reversade B, Robertis EMD. Chordin Is Required for the Spemann Organizer Transplantation Phenomenon in Xenopus Embryos. Developmental Cell. 2003;4:219–230. doi: 10.1016/s1534-5807(02)00404-5. [DOI] [PubMed] [Google Scholar]
- Pierce SB, Kimelman D. Regulation of Spemann organizer formation by the intracellular kinase Xgsk-3. Development. 1995;121:755–65. doi: 10.1242/dev.121.3.755. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–90. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier AF, Talbot WS. The zebrafish organizer. Curr Opin Genet Dev. 1998;8:464–71. doi: 10.1016/s0959-437x(98)80119-6. [DOI] [PubMed] [Google Scholar]
- Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Annu Rev Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- Schohl A, Fagotto F. Beta-catenin, MAPK and Smad signaling during early Xenopus development. Development. 2002;129:37–52. doi: 10.1242/dev.129.1.37. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. ‘Early Development of Xenopus Laevis’. Cold Spring Harbor; 1994. [Google Scholar]
- Smith JC, Price BM, Green JB, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991;67:753–65. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–40. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith WC, McKendry R, Ribisi S, Jr, Harland RM. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell. 1995;82:37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Standley HJ, Destree O, Kofron M, Wylie C, Heasman J. Maternal XTcf1 and XTcf4 have distinct roles in regulating Wnt target genes. Dev Biol. 2006;289:318–28. doi: 10.1016/j.ydbio.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Sudarwati S, Nieuwkoop PD. Mesoderm induction in the anuran Xenopus laevis. Roux’ Arch Dev Biol. 1971;175:199–204. [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal Wnt11 Activates the Canonical Wnt Signaling Pathway Required for Axis Formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Vodicka MA, Gerhart JC. Blastomere derivation and domains of gene expression in the Spemann Organizer of Xenopus laevis. Development. 1995;121:3505–18. doi: 10.1242/dev.121.11.3505. [DOI] [PubMed] [Google Scholar]
- Vonica A, Gumbiner BM. Zygotic Wnt activity is required for Brachyury expression in the early Xenopus laevis embryo. Developmental Biology. 2002;250:112–27. doi: 10.1006/dbio.2002.0786. [DOI] [PubMed] [Google Scholar]
- Vonica A, Weng W, Gumbiner BM, Venuti JM. TCF is the nuclear effector of the beta-catenin signal that patterns the sea urchin animal-vegetal axis. Dev Biol. 2000;217:230–43. doi: 10.1006/dbio.1999.9551. [DOI] [PubMed] [Google Scholar]
- Wang S, Krinks M, Lin K, Luyten FP, Moos M., Jr Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–66. doi: 10.1016/s0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- Weaver C, Kimelman D. Move it or lose it: axis specification in Xenopus. Development. 2004;131:3491–3499. doi: 10.1242/dev.01284. [DOI] [PubMed] [Google Scholar]
- Wessely O, Agius E, Oelgeschlager M, Pera EM, De Robertis EM. Neural Induction in the Absence of Mesoderm: β-catenin-Dependent Expression of Secreted BMP Antagosnists at the Blastula Stage in Xenopus. Developmental Biology. 2001;234:161–173. doi: 10.1006/dbio.2001.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessely O, Kim JI, Geissert D, Tran U, De Robertis EM. Analysis of Spemann organizer formation in Xenopus embryos by cDNA macroarrays. Dev Biol. 2004;269:552–66. doi: 10.1016/j.ydbio.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Wylie C, Kofron M, Payne C, Anderson R, Hosobuchi M, Joseph E, Heasman J. Maternal beta-catenin establishes a ‘dorsal signal’ in early Xenopus embryos. Development. 1996;122:2987–96. doi: 10.1242/dev.122.10.2987. [DOI] [PubMed] [Google Scholar]
- Xanthos JB, Kofron M, Tao Q, Schaible K, Wylie C, Heasman J. The roles of three signaling pathways in the formation and function of the Spemann Organizer. Development. 2002;129:4027–43. doi: 10.1242/dev.129.17.4027. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Mizuno T, Sasai Y, Kishi M, Takeda H, Kim CH, Hibi M, Hirano T. A novel homeobox gene, dharma, can induce the organizer in a non-cell-autonomous manner. Genes Dev. 1998;12:2345–53. doi: 10.1101/gad.12.15.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Tan C, Darken RS, Wilson PA, Klein PS. Beta-catenin/Tcf-regulated transcription prior to the midblastula transition. Development. 2002;129:5743–5752. doi: 10.1242/dev.00150. [DOI] [PubMed] [Google Scholar]
- Yasuo H, Lemaire P. Role of Goosecoid, Xnot and Wnt antagonists in the maintenance of the notochord genetic programme in Xenopus gastrulae. Development. 2001;128:3783–93. doi: 10.1242/dev.128.19.3783. [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–92. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Zon LI, Mather C, Burgess S, Bolce ME, Harland RM, Orkin SH. Expression of GATA-binding proteins during embryonic development in Xenopus laevis; Proceedings of the National Academy of Sciences of the United States of America; 1991. pp. 10642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn AM, Butler K, Gurdon JB. Anterior endomesoderm specification in Xenopus by Wnt/beta-catenin and TGF-beta signalling pathways. Dev Biol. 1999;209:282–97. doi: 10.1006/dbio.1999.9257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


