Abstract
Carnitine is essential for transport of long-chain fatty acids into mitochondria for their subsequent β-oxidation, but its role in the gastrointestinal tract has not been well described. Recently several genetic epidemiologic studies have shown strong association between mutations in carnitine transporter genes OCTN1 and OCTN2 and a propensity to develop Crohn’s disease. This study aims to investigate role of carnitine and β-oxidation in the GI tract. We have studied the gastrointestinal tract effects of carnitine deficiency in a mouse model with loss-of-function mutation in the OCTN2 carnitine transporter. juvenile visceral steatosis (OCTN2-/-) mouse spontaneously develops intestinal villous atrophy, breakdown and inflammation with intense lymphocytic and macrophage infiltration, leading to ulcer formation and gut perforation. There is increased apoptosis of jvs (OCTN2-/-) gut epithelial cells. We observed an up-regulation of heat shock factor-1 (HSF-1) and several heat shock proteins (HSPs) which are known to regulate OCTN2 gene expression. Intestinal and colonic epithelial cells in wild type mice showed high expression and activity of the enzymes of β-oxidation pathway. These studies provide evidence of an obligatory role for carnitine in the maintenance of normal intestinal and colonic structure and morphology. Fatty acid oxidation, a metabolic pathway regulated by carnitine-dependent entry of long-chain fatty acids into mitochondrial matrix, is likely essential for normal gut function. Our studies suggest that carnitine supplementation, as a means of boosting fatty acid oxidation, may be therapeutically beneficial in patients with inflammation of the intestinal tract.
Keywords: Carnitine, fatty acid β-oxidation, inflammation, Crohn’s disease, NEC
INTRODUCTION
Carnitine is obligatory for transport of long-chain fatty acids into mitochondria for their subsequent β-oxidation. Therefore, carnitine plays a critical role in the energy metabolism of the tissues that derive substantial portion of their metabolic energy from fatty acid oxidation such as heart, skeletal muscle, liver and placenta [1, 2]. Two distinct types of carnitine deficiency states have been identified; primary and secondary. Primary carnitine deficiency arises from defects in the plasma membrane carnitine transporter. Patients with this disorder excrete carnitine in urine due to defective reabsorption, and plasma and tissue levels of carnitine may drop below 10% of normal values [3, 4]. Secondary carnitine deficiency arises from defects in any of the enzymes involved in fatty acid oxidation. Patients with these disorders accumulate organic acids due to defective fatty acid oxidation, and these organic acids enhance urinary excretion of carnitine in the form of acylcarnitine [5].
Role of carnitine in gastrointestinal tract became a topic of discussion recently when several genetic studies linked mutations in genes coding for carnitine transporters OCTN1 (SLC22A4) and OCTN2 (SLC22A5) with Crohn’s disease (CD). Patients with CD have been shown to have a missense substitution 1672C→T in OCTN1 causes amino acid substitution L503F, and a G→C transversion in the promoter region of OCTN2 (-207 G→C), which disrupts a heat shock binding element (HSE). Both these mutations are in strong linkage disequilibrium and create a two-allele risk haplotype. These mutations have been shown to cause a reduced carnitine uptake by OCTN1 and a reduced expression of OCTN2 in in-vitro experiments, thus potentially causing tissue carnitine deficiency [6]. These association studies suggest that carnitine transport deficiency might play a role in the pathogenesis of Crohn’s disease. Though findings of this original report have now been replicated in over 19 studies in ethnically diverse populations, more recent studies have failed to confirm this association [7, 8]. To date, there is no direct evidence linking the reduced function of OCTN1 and OCTN2 to the clinical phenotype observed in Crohn’s disease.
In this report, we present evidence for the occurrence of atrophy, ulceration and onset of inflammation in the small bowel in a mouse model with functional defect in OCTN2 carnitine transporter. We also show that fatty acid β-oxidation enzymes are expressed abundantly in the epithelial cells of intestine and colon, suggesting that fatty acid oxidation facilitated by carnitine may be obligatory for the maintenance of normal gut morphology and function.
METHODS
Animals and preparation of RNA and protein samples
Heterozygous OCTN2+/- mice are viable and fertile. Several pairs of OCTN2+/- heterozygous males and females were mated to obtain homozygous OCTN2-/- mice. Homozygous OCTN2-/- mice in the litter were genotyped using a RFLP based method; [9] they can also be identified by 4-5 weeks of age when small size and weakness become apparent. Wild type (OCTN2+/+) and homozygous (OCTN2-/-) pups were sacrificed at 4 weeks of age to identify any GI tract pathology. Tissues from various parts of GI tract, freed from blood, were put in chilled phosphate-buffered saline (PBS) and snap frozen at − 80° C for further analysis or fixed in 10% neutral buffered formalin solution at 4°C for 24 h prior to paraffin embedding for histological and immunohistochemical studies. Total carnitine in various tissues was measured by tandem mass spectrometry. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Medical College of Georgia, GA, USA.
Immunohistochemistry
Mouse gut samples were fixed in 10% formalin and 2-5 μm thick sections of paraffin embedded tissue were cut, applied to glass slides, deparaffinized in xylene, and rehydrated in an ethanol gradient. Endogenous peroxidase activity was quenched by incubating the specimens in 3% H2O2 in methanol for 30 min. After equilibrating for 5 min in distilled water, the samples were subjected to heat antigen retrieval using citrate buffer (pH 6.0) in a microwave oven for 15 sec.
The slides were then washed and blocked using an Avidin/Biotin blocking kit (Vector Labs, Burlingame, CA) for 30 min followed by a blocking buffer (NEN-Life Sciences, Boston, MA) for 30 min. The blocking buffer was removed, and the sections were exposed to primary rabbit polyclonal antisera against one of the following β-oxidation enzymes; medium-chain acyl CoA dehydrogenase (MCAD), long-chain acyl CoA dehydrogenase (LCAD), very-long-chain acyl CoA dehydrogenase (VLCAD), short-chain L-3-hydroxyacyl CoA dehydrogenase (SCHAD), and long-chain 3-ketoacyl-CoA thiolase (LKAT). The primary antibody was used at following dilutions: MCAD (1:200), LCAD (1:400), VLCAD (1:200), SCHAD (1:200), LKAT (1:400) (Antibodies were kindly supplied by Dr Strauss, Vanderbilt University). The primary antibody was applied with 0.3% Triton X-100 in PBS overnight at 4° C. After two washes with PBS on the following day, secondary goat anti-rabbit biotinylated antibody (NEN Life Sciences Products Inc., Boston, MA) was applied at a dilution of 1:800 for 1 h at room temperature. The tertiary reagent Streptavidin horseradish peroxidase (Dako Corp, Carpinteria, CA) was then applied at a dilution of 1:1000 for 1 h at room temperature followed by application of 3,3-diaminobenzidine substrate for 1 to 5 min. The slides were rinsed, counterstained with Mayer’s hematoxylin, dehydrated in ethanol, cleared with xylene, and mounted with glass coverslips using Histomount (Zymed Laboratories Inc., San Francisco, CA). We stained 3 to 5 sets of tissue for all five FAO enzymes.
Semi-quantitative RT-PCR for HSF-1 and HSPs
Primers were designed using Oligo® primer analysis software 6.0 (National Biosciences Inc. Cascade, CO). The nucleotide sequences of the primers used for RT-PCR are available on request. One microgram each of the total RNA from wild type and homozygous mouse gut scrapings was reverse transcribed with random hexamers and reagents from RNA PCR kit (Perkin Elmer, Norwalk, CT, USA) in a total volume of 20 μl. Consecutive PCR was performed using 1–2 μl of cDNA as template using standard methods. Primer pairs specific for hypoxanthine phosphoribosyl transferase-1 (HPRT) was used as an internal control because its expression in gut is minimally affected [10]. Amplification was carried out for 10 min at 96°C to activate the AmpliTaq Gold polymerase, followed by 30 cycles for 1 min at 95°C, for 1 min at 55°C, and for 1 min at 72°C, and a final extension for 10 min at 72°C. PCR products were visualized on 1.2% agarose gels stained with ethidium bromide, and densitometry was performed using a SpectraImager 5000 Imaging system and AlphaEase 32-bit software (Alpha Innotech, San Leandro, CA, U.S.A.).
Western blot analysis to study apoptosis
Fifty to 100 mg of gut tissue was lysed in RIPA buffer (Sigma, St. Louis, MO) containing 50 mM Tris-HCl, pH 8.0, with 150 mM sodium chloride, 1.0% Igepal CA-630, 0.5% sodium deoxycholate and 0.1% SDS with protease inhibitors using a Polytron homogenizer. The protein concentration of the supernatant was measured by the Lowry method. Twenty five g of protein was analyzed by immunoblotting using 10% SDS-PAGE. The separated proteins were transferred onto nitrocellulose membranes, which were then blocked for 1.5 h at room temperature with TRIS-buffered saline/0.05% Tween–20 containing 5% non-fat milk.
Immunoblots were washed with PBS-T (phosphate-buffered saline with 0.1% tween 20) and subsequently incubated with commercially available antibodies (Santa Cruz Biotechnology, Inc. Santa Cruz, CA) against activated caspase 1, 3, extracellular-signal related kinase 1 (ERK1 and p-ERK1), serine/threonine kinases (Akt1/2 and p-Akt1/2) and β-actin. Primary antibodies were applied at 1:500 dilution at 4° C overnight. Immunoblots were washed with PBS-T and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody as the secondary antibody for 1.5 h and washed, and the proteins visualized by using the ECL Western blot detection system. A total of 4-5 blots were prepared for each enzyme and a representative immunoblot was analyzed with an AlphaImager 3400 (Alpha Innotech Corp. San Leandro CA) using its AlphaEase® image analysis software for densitometry.
Meta-analysis of genetic studies showing association of CD with OCTN transporters
For the meta-analysis, we extracted data from each of the 19 studies as the number of subjects homozygous for TC haplotype (1672C→T in OCTN1 and -207G→C in OCTN2) versus all other genotypes in the cases and controls. We found that only 12 studies provided complete data (incidence of mutations in controls and patients and odds ratio) which could be combined for our analysis. The genetic effect of TC was evaluated using the odds ratio (OR) of the disease for homozygous TC versus other genotypes. The point estimate and the 95% confidence interval (CI) for the odds ratio were estimated for each study from the extracted number of subjects. The pooled OR was estimated by the inverse variance method[11] where the odds ratio estimates from individual studies were weighted proportionate to the sample size for each study. The ORs were assessed for heterogeneity of genetic effect across studies using the Q statistic, which follows a chi-square distribution with k-1 degrees of freedom, where k is the number of studies. The extent of heterogeneity was estimated using the I2 statistic [12], which ranges from 0 to 1 where 0 corresponds to perfect homogeneity among all studies. The standardized log odds ratio from the combined study was used to test for the overall genetic effect of TC haplotype on Crohn’s disease, using the standard normal test [11]. All data are presented as mean ± SEM and comparisons between paired samples were made by student’s ‘t’ test with Bonferroni’s correction where applicable.
RESULTS
IBD5 locus mutations in OCTN1 and OCTN2 carnitine transporters and its association with Crohn’s disease
After the initial association study of IBD5 locus with CD by Rioux et al in 2001 [13], 19 large scale independent studies involving more than 5000 patients with CD and equal numbers of controls have confirmed this association with an odds ratio varying from 1.1 to 20. Our meta-analysis of the combined data from 12 of these studies including 3420 patients and an equal number of matched controls, indicated that the odds of acquiring CD when the risk haplotype was present ranged from 1.5 to 2.0 (Table 1). This analysis formed the basis of our investigation to study the effect of carnitine deficiency in a mouse model where there is a loss-of-function mutation in the carnitine transporter OCTN2.
Table-1.
Homozygosity for mutations in carnitine transporter genes, OCTN1 and OCTN2 and propensity for Crohn’s disease: A meta-analysis of published studies in patients homozygous for TC haplotype (1672C→T in OCTN1 and -207G→C in OCTN2 genes) where data could be combined.
| Reference | Country | No. of Subjects | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|
| Patients | Controls | ||||
| Peltekova et al (discovery) [6] | Canada | 203 | 200 | 3.43 | 1.58 – 7.44 |
| Peltekova et al (replication) [6] | Canada | 300 | 190 | 5.14 | 2.52 – 10.45 |
| Leung et al [20] | New Zealand | 182 | 188 | 1.64 | 0.97 – 2.78 |
| Newman et al (non-jewish) [14] | Canada | 372 | 264 | 1.91 | 1.24 – 2.92 |
| Newman et al (jewish) [14] | Canada | 135 | 88 | 1.48 | 0.74 – 2.97 |
| Noble et al [17] | Scotland, UK | 374 | 294 | 1.83 | 1.20 – 2.80 |
| Torok et al [19] | Germany | 625 | 1012 | 1.65 | 1.28 – 2.13 |
| Babusukumar et al [48] | USA | 264 | 527 | 1.44 | 1.00 – 2.09 |
| Ferraris et al [23] | Italy | 134 | 166 | 1.24 | 0.67 – 2.28 |
| Russell et al [16] | UK | 200 | 256 | 1.48 | 0.91 – 2.40 |
| Vermeire et al [24] | Belgium | 453 | 299 | 1.07 | 0.75 – 1.54 |
| Torkvist et al [26] | Sweden | 178 | 143 | 1.78 | 0.9 – 2.1 |
| Overall total | 3420 | 3627 | 1.76 | 1.5 – 1.99 | |
Test of homogeneity: Chi square = 20.97, df = 11, (P= 0.02), I2 = 52.3%
Test of overall effect: Z = 7.22 (P < 0.00001)
OCTN2 null (OCTN2-/-) mouse is a model of systemic carnitine deficiency
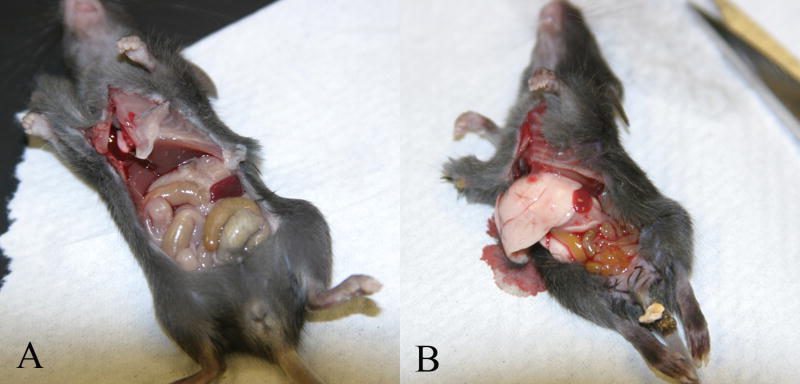
The homozygous OCTN2 null (OCTN2-/-) mice survive for about 4-5 weeks without carnitine supplementation but the heterozygous (OCTN2+/-) mice are viable and fertile. At 3-weeks of age, the body weight of the OCTN2-/- mice is about 50% compared to that of age-matched wild type mice. Furthermore, OCTN2-/- mice develop enlarged fatty liver with steatosis of other organs and hypertrophic cardiomyopathy which led to its original designation as the juvenile visceral steatosis (jvs) mouse [9] (Fig-1).
Figure 1.
Gross pathology in 4-week old jvs (OCTN2-/-) mice. Panel (A) is a wild type (OCTN2+/+) mouse. It weighed 25g with normal looking healthy liver and bowel loops; in contrast the jvs (OCTN2-/-) mouse (panel B) weighed only 12g and had gross fatty infiltration of liver with pale looking atrophied bowel loops and pus in the peritoneal cavity.
Carnitine deficiency leads to gut atrophy, disruption of villous structure and inflammation of GI tract with spontaneous perforations and pus formation
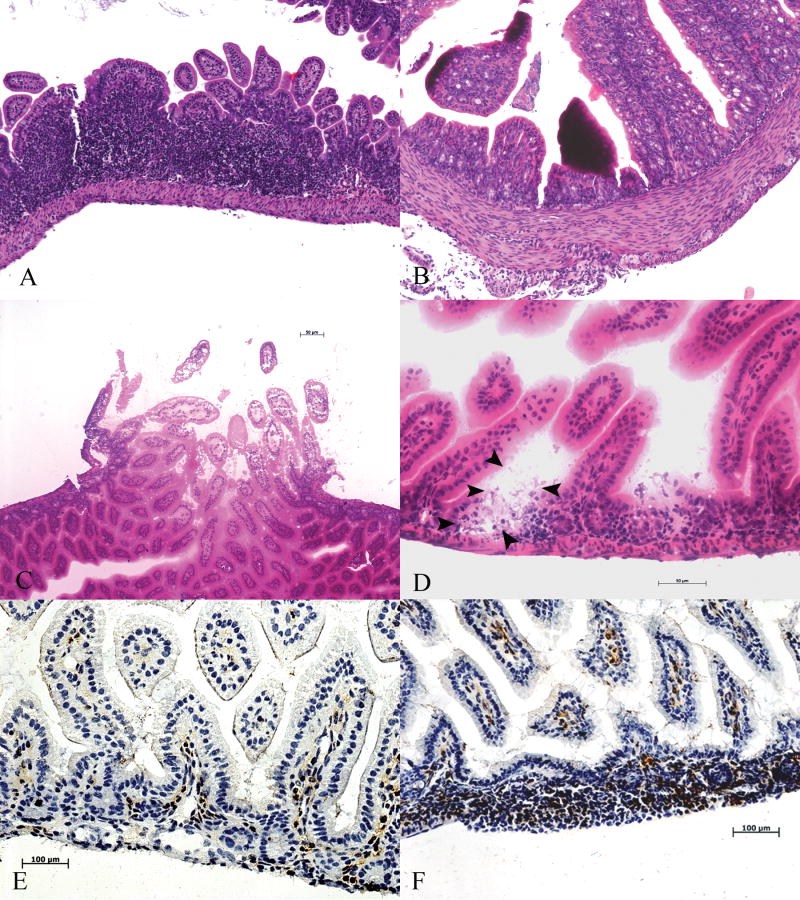
The GI tract pathology of this mouse model was not studied previously, but on close inspection we found that the OCTN2-/- mouse gut is small, thin, and pale with evidence of spontaneous perforations, pus in the peritoneal cavity and abscess formation in some cases (Fig-1). GI tissue carnitine content of the OCTN2-/- null mouse is reduced to less than 5-10% (Table-2) of the wild type mouse. The whole gut epithelium from jejunum to colon is affected in the OCTN2-/- mouse with clearly evident necrotic villi leading to generalized gut atrophy (Fig-2A-F). Several areas characterized by intense lymphocytic infiltration (Fig-3A) with dead or denuded villi in various stages of sloughing (Fig-3B) were observed. In addition, there were spontaneous perforations (Fig-3C) and micro-abscess formation mainly in the jejunum and ileum on frozen section H&E staining (Fig-3D). Immunohistochemical staining for macrophages using antibody against F4/80 antigen showed areas of intense macrophage infiltration in the villous core structure (Fig-3 E&F).
Table-2.
Carnitine content of various tissues from wild type (OCTN2+/+) and homozygous (OCTN2-/-) mice and fatty acid oxidation enzyme activity in the wild-type (OCTN2+/+) mouse gut tissues
| Tissue | Total Carnitine (OCTN2+/+) (nmol/mg) | Total Carnitine (OCTN2-/-) (nmol/mg) | LCHAD activity (OCTN2+/+) (nmol/mg/min) | SCHAD activity (OCTN2+/+) (nmol/mg/min) |
|---|---|---|---|---|
| Jejunum | 6.9 ± 1.3 | 0.9 ± 0.4 | 1328 ± 111 | 1317 ± 122 |
| Ileum | 8.7 ± 3.1 | 0.7 ± 0.3 | 1105 ± 98 | 1012 ± 132 |
| Colon | 7.1 ± 0.2 | 0.1 ± 0.1 | 1196 ± 102 | 1203 ± 116 |
| Liver | 6.8 ± 3.0 | 0.11 ± 0.09 | 302 ± 68 | 161 ± 101 |
| Skeletal Muscle | 5.6 ± 2.1 | 0.05 ± 0.06 | 850 ± 273 | 530 ± 354 |
All data are mean ± SD, (n=36).
Figure 2.
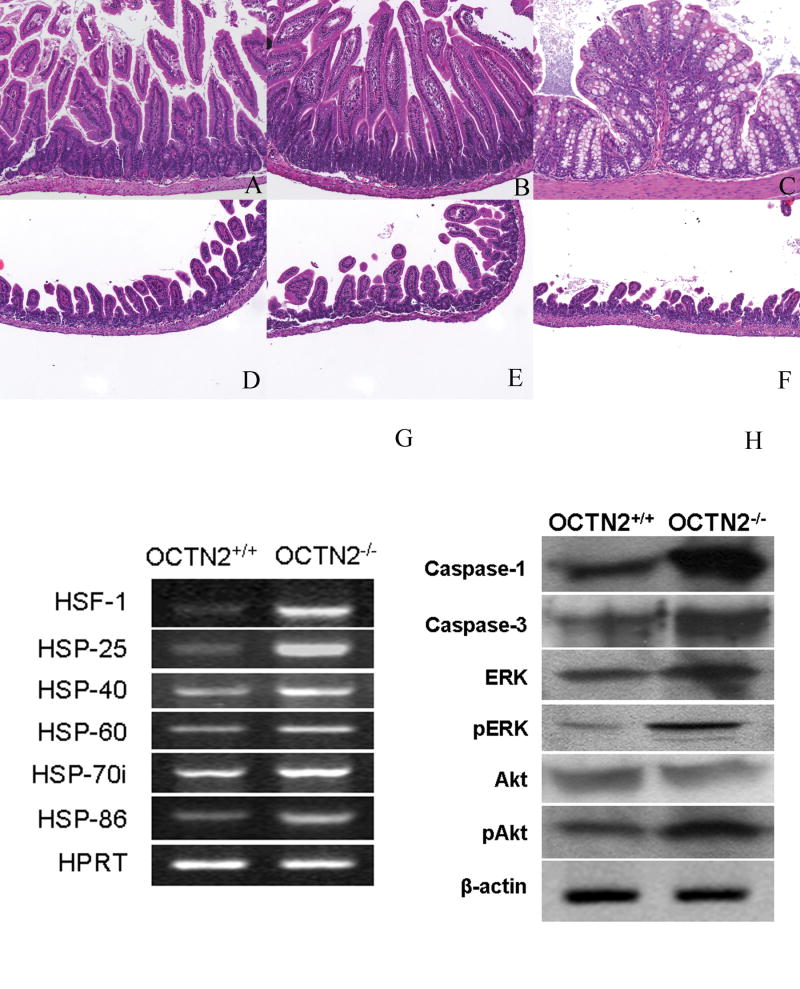
Gut histology in 3-week-old wild type (OCTN2+/+) and jvs (OCTN2-/-) mice (H&E staining x10). Panels (A, B and C) represent photomicrographs of wild type (OCTN2+/+) jejunum, ileum and colon respectively and panels (D, E and F) represent photomicrographs of jvs (OCTN2-/-) jejunum, ileum and colon at the same magnification. Panel (G), a representative RT-PCR gel picture for mRNA levels specific for heat shock proteins in wild type (OCTN2+/+) and jvs (OCTN2-/-) gut. There is significant up-regulation of HSF-1, HSP-25, 40, 60, 70i and 86 in jvs (OCTN2 -/-) mice. Panel (H) is a composite western blot from the wild type (OCTN2+/+) and jvs (OCTN2-/-) gut scrapings from jejunum to ileum. There is an increased expression of caspases 1 and 3 and an increase in phosphorylated ERK and phosphorylated Akt.
Figure 3.
Histopathology of jvs (OCTN2-/-) mouse gut (H&E staining). Panel (A) is H&E staining of formalin-fixed section of OCTN2-/- ileum showing intense lymphocytic infiltration, panel (B) from ileum shows necrotic villi (x20). Panel (C) is a frozen section of jvs (OCTN2-/-) mouse ileum showing an area of spontaneous perforation (bar represents 50 μm) and panel (D) shows areas of abscess formation, outlined by arrow heads (bar represents 50 μm). Lower two panels represent immunohistochemistry for murine macrophages using anti-F4/80 antibody. Panel (E) is wild type (OCTN2+/+) ileum section showing basal presence of macrophages and panel (F) is jvs (OCTN2-/-) ileum section showing intense and widespread macrophage infiltration (Bar represents 100 μm).
Wild type (OCTN2+/+) mouse gut epithelial cells express five key enzymes involved in mitochondrial β-oxidation of fatty acids
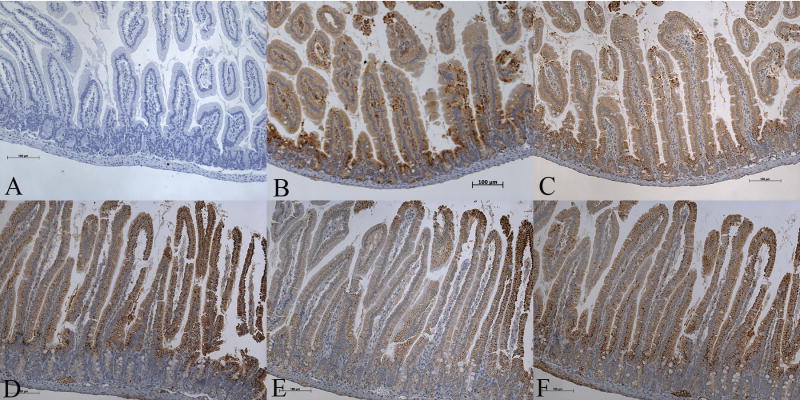
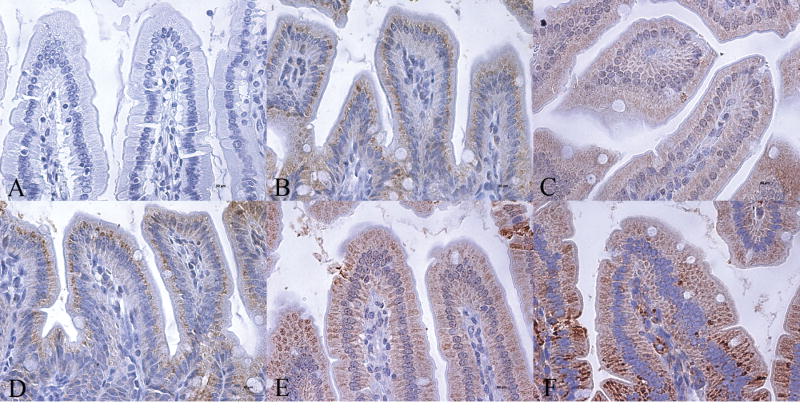
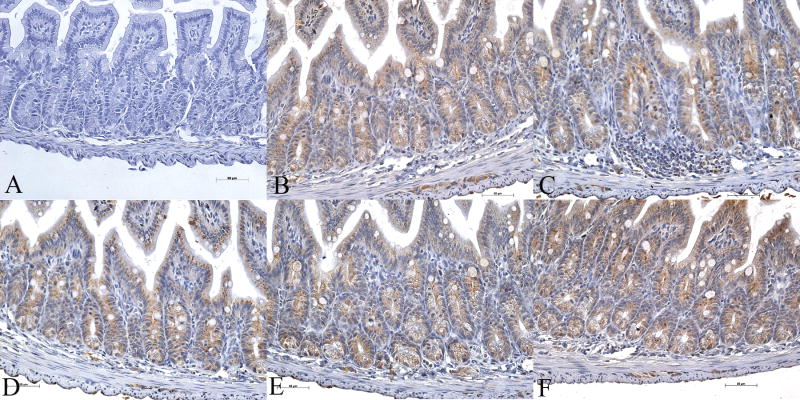
Since carnitine deficiency has such devastating effect on the gut, we examined gut sections of the wild type mice for expression of five key enzymes of the mitochondrial fatty acid β-oxidation pathway by immunohistochemistry: medium-chain acyl CoA dehydrogenase (MCAD), long-chain acyl CoA dehydrogenase (LCAD), very-long-chain acyl CoA dehydrogenase (VLCAD), short-chain L-3-hydroxyacyl CoA dehydrogenase (SCHAD), and long-chain 3-ketoacyl-CoA thiolase (LKAT). We found high expression of all the five enzymes with most intense staining localized to villous epithelial cells of the jejunum (Fig-4). A similar staining pattern was seen in sections of the ileum and colon (Fig-5 & 6). Gut scrapings from jejunum to colon were then used to measure the activities of LCHAD and SCHAD enzymes. The activity of LCHAD in gut scrapings of different regions varied from 1105 ± 98 to 1328 ± 111 nmol/mg/min compared to 302 ± 68 in the liver and 161 ± 101 in the skeletal muscle (Table-2). Likewise SCHAD activity in different gut regions varied between 1012 ± 132 to 1317 ± 122 nmol/mg/min (n=36) compared to 850 ± 273 nmol/mg/min in liver and 530 ± 354 nmol/mg/min in skeletal muscle. Thus activity of both enzymes in the whole gut was at least 3-to 4-fold higher than liver and skeletal muscle (Table-2), two tissues which utilize long-chain fatty acids to generate ATP.
Figure 4.
Immunohistochemical analysis of expression of fatty acid oxidation enzymes in 4-week-old wild type (OCTN2+/+) mouse jejunum showing the complete villous structure (Bar represents 100 μm). Panel A is a control negative section where no primary antibody was used and panels B to F show immunoreactivity for MCAD, LCAD, VLCAD, SCHAD, and LKAT, respectively. All five enzymes are expressed in villous epithelial cells. Expression is less in crypt epithelial cells and negligible in non-epithelial cells of the villous core.
Figure 5.
Immunohistochemical analysis of expression of fatty acid oxidation enzymes in 4-week-old wild type (OCTN2+/+) mouse ileum showing details if villous tip (Bar represents 50 μm). Panel A is a control negative section where no primary antibody was used and panels B to F show immunoreactivity for MCAD, LCAD, VLCAD, SCHAD, and LKAT, respectively. All five enzymes are highly expressed in villous epithelial cells with negligible staining of non-epithelial cells in the villous core.
Figure 6.
Immunohistochemical analysis of expression of fatty acid oxidation enzymes in 4-week-old wild-type (OCTN2+/+) mouse colon (Bar represents 100 μm). Panel A is a control negative section where no primary antibody was used and panels B to F show immunoreactivity for MCAD, LCAD, VLCAD, SCHAD, and LKAT, respectively. All five enzymes are expressed in villous epithelial cells with a higher expression near the tip and lesser staining of crypt epithelial cells.
Carnitine deficiency leads to increased apoptosis of gut epithelial cells and up-regulation of heat shock factor-1 and heat shock proteins
We examined apoptosis in the jvs (OCTN2-/-) mouse gut in comparison to wild type littermates. With β-actin serving as control, we found evidence for activation of caspases 1 and 3 and for an increase in expression of phosphorylated extracellular signal-related kinase (pERK) and serine/threonine (Akt) (Fig-2H). OCTN2 gene expression is regulated by certain heat shock elements in its promoter and we found a significant increase in gene expression of heat shock factor-1 and heat shock proteins 25, 40, 60. 70i (inducible) and 86 in gut scrapings from the jvs (OCTN2-/-) compared to the wild type mice (Fig-2G). Thus, in a state of tissue carnitine deficiency, there may be a compensatory increase in heat shock proteins and heat shock factor-1 in an attempt to up-regulate the expression of OCTN2.
We also monitored the steady-state levels of mRNAs for OCTN1, OCTN3 and ATB0,+ in wild type and jvs (OCTN2-/-) mouse gut. We were able to detect mRNAs for OCTN1 and ATB0,+ in the small intestine and colon, but the levels were not different between wild type mice and jvs (OCTN2-/-) (data not shown). OCTN3 mRNA was not detectable in the small intestine and colon in both groups of mice.
DISCUSSION
Most of the 19 published studies examining mutations in the IBD5 locus containing the OCTN1 and OCTN2 transporter genes showed a higher incidence of Crohn’s disease in the risk haplotype positive group. These studies included diverse populations and originated from Canada [6, 13, 14], the UK [15-18], Germany [19], New Zealand [20], Spain [21], Italy [22, 23], Belgium [24], Greece [25], Sweden [26] and Japan [27, 28]. One Japanese and another Hungarian study with a small number of subjects found no association between OCTN 1&2 transporter polymorphisms and CD [28, 29]; thus there could be ethnic differences in IBD5 locus mutations and propensity for Crohn’s disease [7]. One recent genome-wide association study by the Wellcome Trust Case Control Consortium of UK using GeneChip 500K Mapping Array set did not find any association between mutations of OCTN 1&2 transporters and Crohn’s disease [8]. Our meta-analysis of 12 studies, where complete data was available, revealed a modest association between the IBD5 risk haplotype and CD. In spite of such strong association studies, there was little or no biological basis to suggest that tissue carnitine deficiency could lead to onset or progression of Crohn’s disease [30, 31]. Mutations in the OCTN 1&2 transporters may or may not be associated with Crohn’s disease, but our observations in the jvs mouse represent the first experimental evidence to suggest that a defect in carnitine transporter function in intestinal epithelial cells leads to atrophy of the small intestine and colon, and subsequent onset of inflammation.
The major function of gut epithelium is to digest and absorb nutrients. This is an energy-consuming process which requires constant and reliable availability of ATP. Therefore, it is not surprising that the enzymes of the mitochondrial fatty acid β-oxidation pathway are expressed and highly active in gut epithelium. This ATP-providing metabolic pathway, however, requires availability of carnitine to enable the transport of long-chain fatty acids into the mitochondria for subsequent β-oxidation. Thus, any process which inhibits the ability of enterocytes to utilize fatty acids to generate energy will disturb its internal milieu and have a damaging effect on gut health. The gut epithelial barrier is a dynamic structure and it works to exclude antigens from entering the tissues and stimulate an immune reaction. This barrier is easily breached in the OCTN2-/- mouse with consequent stimulation of an immune response. Inhibition of gut fatty acid β-oxidation by specific inhibitors (i.e. sodium 2-bromo-octanoate) has been shown to produce experimental colitis [32, 33], highlighting the crucial role of fatty acid oxidation in this tissue. Furthermore, several anecdotal reports have shown a beneficial effect of carnitine enemas in some cases of distal inflammatory bowel disease [34-36]. Recently a lot of attention has been focused on short-chain fatty acids (SCFA) in distal gut health [37]. SCFA are substrates for G-protein coupled receptors GPR-41 and GPR-43 and regulate colonic water and bicarbonate secretion and gut motility [38, 39]. Fatty acids not only exert nutritional effect on the gut but also are protective for enterocytes, serve as activators of transcription, and constitute precursors of inflammatory mediators [40, 41]. Fatty acid metabolism related genes are differentially regulated in IBD and are affected by presence of cytokines like TNFα and PPAR-γ ligands in the gut lumen [42].
Additional evidence supporting the role of carnitine in intestinal inflammation include the observation of carnitine deficiency having been implicated in sepsis syndrome due to its regulatory function in immune response and inflammation [43]. Carnitine has also been shown to modulate apoptosis by down-regulating proapoptotic Fas signals and suppressing the generation of ceramide, a key mediator of apoptosis. These factors are likely contributors to the pathology observed in the OCTN2-/- mouse [44]. High turnover of intestinal epithelial cells requires replacement of apoptotic enterocytes to maintain the epithelial barrier function. In the OCTN2-/- mouse the process of apoptosis is accelerated while the inability to replace these dying cells is diminished leading to a breakdown of the epithelial barrier. Intestinal lesions in CD are patchy and the disease runs a relapsing course. In contrast, the extensive pathology observed in the OCTN2-/- mouse represents an extreme inability to oxidize long-chain fatty acids due to severe carnitine deficiency.
Patients with primary carnitine deficiency due to mutations in the OCTN2 gene typically present with progressive cardiomyopathy, episodes of hypoketotic hypoglycemia associated with encephalopathy and hepatomegaly [45]. However, gastrointestinal studies have not been reported in these patients since they are effectively treated with carnitine supplementation. This, along with the observed positive effect of carnitine supplementation on gut health, leads us to postulate that carnitine supplementation may be beneficial to patients with intestinal inflammation. Carnitine supplementation and use of other pharmacologic methods to augment carnitine absorption by alternative transporters such as ATB0+ have been suggested for patients with primary carnitine deficiency [46] and such maneuvers may also be applicable to patients with intestinal inflammation.
It is also of interest to note that absence of a functional OCTN2 in jvs mouse does not lead to any noticeable changes in the expression of OCTN1 and ATB0,+. Some studies have reported that OCTN1 is capable of mediating carnitine uptake [6], but we could not reproduce these results in our laboratory using cloned rat and human OCTN1 (unpublished data). ATB0,+ is a low-affinity transporter for carnitine [47]. The present data showing that neither the expression of OCTN1 nor the expression of ATB0,+ is altered in the jvs (OCTN2-/-) mice show that there is no compensatory up-regulation of other potential carnitine transporters in the intestinal tract when OCTN2 is defective.
Acknowledgments
This work was supported by NIH grant HD048867 to PS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rinaldo P, Matern D, Bennett MJ. Fatty acid oxidation disorders. Annual review of physiology. 2002;64:477–502. doi: 10.1146/annurev.physiol.64.082201.154705. [DOI] [PubMed] [Google Scholar]
- 2.Shekhawat P, Bennett MJ, Sadovsky Y, Nelson DM, Rakheja D, Strauss AW. Human placenta metabolizes fatty acids: implications for fetal fatty acid oxidation disorders and maternal liver diseases. American journal of physiology. 2003;284:E1098–1105. doi: 10.1152/ajpendo.00481.2002. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Ye J, Ganapathy V, Longo N. Mutations in the organic cation/carnitine transporter OCTN2 in primary carnitine deficiency. Proc Natl Acad Sci U S A. 1999;96:2356–2360. doi: 10.1073/pnas.96.5.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiekerkoetter U, Huener G, Baykal T, Demirkol M, Duran M, Wanders R, Nezu J, Mayatepek E. Silent and symptomatic primary carnitine deficiency within the same family due to identical mutations in the organic cation/carnitine transporter OCTN2. Journal of inherited metabolic disease. 2003;26:613–615. doi: 10.1023/a:1025968502527. [DOI] [PubMed] [Google Scholar]
- 5.Longo N, Amat di San Filippo C, Pasquali M. Disorders of carnitine transport and the carnitine cycle. American journal of medical genetics. 2006;142:77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peltekova VD, Wintle RF, Rubin LA, Amos CI, Huang Q, Gu X, Newman B, Van Oene M, Cescon D, Greenberg G, Griffiths AM, St George-Hyslop PH, Siminovitch KA. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat Genet. 2004;36:471–475. doi: 10.1038/ng1339. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg MS, Duerr RH, Brant SR, Bromfield G, Datta LW, Jani N, Kane SV, Rotter JI, Philip Schumm L, Hillary Steinhart A, Taylor KD, Yang H, Cho JH, Rioux JD, Daly MJ. Refined genomic localization and ethnic differences observed for the IBD5 association with Crohn’s disease. Eur J Hum Genet. 2007;15:328–335. doi: 10.1038/sj.ejhg.5201756. [DOI] [PubMed] [Google Scholar]
- 8.The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nezu J, Tamai I, Oku A, Ohashi R, Yabuuchi H, Hashimoto N, Nikaido H, Sai Y, Koizumi A, Shoji Y, Takada G, Matsuishi T, Yoshino M, Kato H, Ohura T, Tsujimoto G, Hayakawa J, Shimane M, Tsuji A. Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter. Nat Genet. 1999;21:91–94. doi: 10.1038/5030. [DOI] [PubMed] [Google Scholar]
- 10.Mamo S, Gal AB, Bodo S, Dinnyes A. Quantitative evaluation and selection of reference genes in mouse oocytes and embryos cultured in vivo and in vitro. BMC developmental biology. 2007;7:14. doi: 10.1186/1471-213X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thakkinstian A, McElduff P, D’Este C, Duffy D, Attia J. A method for meta-analysis of molecular association studies. Stat Med. 2005;24:1291–1306. doi: 10.1002/sim.2010. [DOI] [PubMed] [Google Scholar]
- 12.A D, Deeks JJ, Bradburn MJ. Systematic Reviews in Healthcare: meta-analysis in context. BMJ Books; London: 2001. [Google Scholar]
- 13.Rioux JD, Daly MJ, Silverberg MS, Lindblad K, Steinhart H, Cohen Z, Delmonte T, Kocher K, Miller K, Guschwan S, Kulbokas EJ, O’Leary S, Winchester E, Dewar K, Green T, Stone V, Chow C, Cohen A, Langelier D, Lapointe G, Gaudet D, Faith J, Branco N, Bull SB, McLeod RS, Griffiths AM, Bitton A, Greenberg GR, Lander ES, Siminovitch KA, Hudson TJ. Genetic variation in the 5q31 cytokine gene cluster confers susceptibility to Crohn disease. Nat Genet. 2001;29:223–228. doi: 10.1038/ng1001-223. [DOI] [PubMed] [Google Scholar]
- 14.Newman B, Gu X, Wintle R, Cescon D, Yazdanpanah M, Liu X, Peltekova V, Van Oene M, Amos CI, Siminovitch KA. A risk haplotype in the Solute Carrier Family 22A4/22A5 gene cluster influences phenotypic expression of Crohn’s disease. Gastroenterology. 2005;128:260–269. doi: 10.1053/j.gastro.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 15.Fisher SA, Hampe J, Onnie CM, Daly MJ, Curley C, Purcell S, Sanderson J, Mansfield J, Annese V, Forbes A, Lewis CM, Schreiber S, Rioux JD, Mathew CG. Direct or indirect association in a complex disease: the role of SLC22A4 and SLC22A5 functional variants in Crohn disease. Hum Mutat. 2006;27:778–785. doi: 10.1002/humu.20358. [DOI] [PubMed] [Google Scholar]
- 16.Russell RK, Drummond HE, Nimmo ER, Anderson NH, Noble CL, Wilson DC, Gillett PM, McGrogan P, Hassan K, Weaver LT, Bisset WM, Mahdi G, Satsangi J. Analysis of the influence of OCTN1/2 variants within the IBD5 locus on disease susceptibility and growth indices in early onset inflammatory bowel disease. Gut. 2006;55:1114–1123. doi: 10.1136/gut.2005.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noble CL, Nimmo ER, Drummond H, Ho GT, Tenesa A, Smith L, Anderson N, Arnott ID, Satsangi J. The contribution of OCTN1/2 variants within the IBD5 locus to disease susceptibility and severity in Crohn’s disease. Gastroenterology. 2005;129:1854–1864. doi: 10.1053/j.gastro.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Waller S, Tremelling M, Bredin F, Godfrey L, Howson J, Parkes M. Evidence for association of OCTN genes and IBD5 with ulcerative colitis. Gut. 2006;55:809–814. doi: 10.1136/gut.2005.084574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torok HP, Glas J, Tonenchi L, Lohse P, Muller-Myhsok B, Limbersky O, Neugebauer C, Schnitzler F, Seiderer J, Tillack C, Brand S, Brunnler G, Jagiello P, Epplen JT, Griga T, Klein W, Schiemann U, Folwaczny M, Ochsenkuhn T, Folwaczny C. Polymorphisms in the DLG5 and OCTN cation transporter genes in Crohn’s disease. Gut. 2005;54:1421–1427. doi: 10.1136/gut.2005.066340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung E, Hong J, Fraser AG, Merriman TR, Vishnu P, Krissansen GW. Polymorphisms in the organic cation transporter genes SLC22A4 and SLC22A5 and Crohn’s disease in a New Zealand Caucasian cohort. Immunol Cell Biol. 2006;84:233–236. doi: 10.1111/j.1440-1711.2006.01423.x. [DOI] [PubMed] [Google Scholar]
- 21.Martinez A, Del Carmen Martin M, Mendoza JL, Taxonera C, Diaz-Rubio M, de la Concha EG, Urcelay E. Association of the organic cation transporter OCTN genes with Crohn’s disease in the Spanish population. Eur J Hum Genet. 2006;14:222–226. doi: 10.1038/sj.ejhg.5201529. [DOI] [PubMed] [Google Scholar]
- 22.Palmieri O, Latiano A, Valvano R, D’Inca R, Vecchi M, Sturniolo GC, Saibeni S, Peyvandi F, Bossa F, Zagaria C, Andriulli A, Devoto M, Annese V. Variants of OCTN1-2 cation transporter genes are associated with both Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2006;23:497–506. doi: 10.1111/j.1365-2036.2006.02780.x. [DOI] [PubMed] [Google Scholar]
- 23.Ferraris A, Torres B, Knafelz D, Barabino A, Lionetti P, de Angelis GL, Iacono G, Papadatou B, D’Amato G, Di Ciommo V, Dallapiccola B, Castro M. Relationship between CARD15, SLC22A4/5, and DLG5 polymorphisms and early-onset inflammatory bowel diseases: an Italian multicentric study. Inflamm Bowel Dis. 2006;12:355–361. doi: 10.1097/01.MIB.0000217338.23065.58. [DOI] [PubMed] [Google Scholar]
- 24.Vermeire S, Pierik M, Hlavaty T, Claessens G, van Schuerbeeck N, Joossens S, Ferrante M, Henckaerts L, Bueno de Mesquita M, Vlietinck R, Rutgeerts P. Association of organic cation transporter risk haplotype with perianal penetrating Crohn’s disease but not with susceptibility to IBD. Gastroenterology. 2005;129:1845–1853. doi: 10.1053/j.gastro.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Gazouli M, Mantzaris G, Archimandritis AJ, Nasioulas G, Anagnou NP. Single nucleotide polymorphisms of OCTN1, OCTN2, and DLG5 genes in Greek patients with Crohn’s disease. World J Gastroenterol. 2005;11:7525–7530. doi: 10.3748/wjg.v11.i47.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torkvist L, Noble CL, Lordal M, Sjoqvist U, Lindforss U, Nimmo ER, Lofberg R, Russell RK, Satsangi J. Contribution of the IBD5 locus to Crohn’s disease in the Swedish population. Scand J Gastroenterol. 2007;42:200–206. doi: 10.1080/00365520600842278. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki K, Takazoe M, Tanaka T, Ichimori T, Saito S, Iida A, Onouchi Y, Hata A, Nakamura Y. Association analysis of SLC22A4, SLC22A5 and DLG5 in Japanese patients with Crohn disease. J Hum Genet. 2004;49:664–668. doi: 10.1007/s10038-004-0204-x. [DOI] [PubMed] [Google Scholar]
- 28.Tosa M, Negoro K, Kinouchi Y, Abe H, Nomura E, Takagi S, Aihara H, Oomori S, Sugimura M, Takahashi K, Hiwatashi N, Takahashi S, Shimosegawa T. Lack of association between IBD5 and Crohn’s disease in Japanese patients demonstrates population-specific differences in inflammatory bowel disease. Scand J Gastroenterol. 2006;41:48–53. doi: 10.1080/00365520510023864. [DOI] [PubMed] [Google Scholar]
- 29.Bene J, Magyari L, Talian G, Komlosi K, Gasztonyi B, Tari B, Varkonyi A, Mozsik G, Melegh B. Prevalence of SLC22A4, SLC22A5 and CARD15 gene mutations in Hungarian pediatric patients with Crohn’s disease. World J Gastroenterol. 2006;12:5550–5553. doi: 10.3748/wjg.v12.i34.5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bene J, Komlosi K, Havasi V, Talian G, Gasztonyi B, Horvath K, Mozsik G, Hunyady B, Melegh B, Figler M. Changes of plasma fasting carnitine ester profile in patients with ulcerative colitis. World J Gastroenterol. 2006;12:110–113. doi: 10.3748/wjg.v12.i1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demirkol M, Sewell AC, Bohles H. The variation of carnitine content in human blood cells during disease--a study in bacterial infection and inflammatory bowel disease. Eur J Pediatr. 1994;153:565–568. doi: 10.1007/BF02190659. [DOI] [PubMed] [Google Scholar]
- 32.Roediger WE, Nance S. Selective reduction of fatty acid oxidation in colonocytes: correlation with ulcerative colitis. Lipids. 1990;25:646–652. doi: 10.1007/BF02536016. [DOI] [PubMed] [Google Scholar]
- 33.Roediger WE, Nance S. Metabolic induction of experimental ulcerative colitis by inhibition of fatty acid oxidation. Br J Exp Pathol. 1986;67:773–782. [PMC free article] [PubMed] [Google Scholar]
- 34.Gasbarrini G, Mingrone G, Giancaterini A, De Gaetano A, Scarfone A, Capristo E, Calvani M, Caso V, Greco AV. Effects of propionyl-L-carnitine topical irrigation in distal ulcerative colitis: a preliminary report. Hepatogastroenterology. 2003;50:1385–1389. [PubMed] [Google Scholar]
- 35.Johnson JA. L-carnitine for treatment of distal ulcerative colitis. Gastroenterology. 1992;103:1709–1710. doi: 10.1016/0016-5085(92)91215-p. [DOI] [PubMed] [Google Scholar]
- 36.Johnson JA. L-carnitine for treatment of nonspecific proctosigmoiditis. Dis Colon Rectum. 1993;36:518. doi: 10.1007/BF02050022. [DOI] [PubMed] [Google Scholar]
- 37.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 39.Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–360. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- 40.Canani RB, Terrin G, Cirillo P, Castaldo G, Salvatore F, Cardillo G, Coruzzo A, Troncone R. Butyrate as an effective treatment of congenital chloride diarrhea. Gastroenterology. 2004;127:630–634. doi: 10.1053/j.gastro.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 41.Matthews JB, Hassan I, Meng S, Archer SY, Hrnjez BJ, Hodin RA. Na-K-2Cl cotransporter gene expression and function during enterocyte differentiation. Modulation of Cl-secretory capacity by butyrate. J Clin Invest. 1998;101:2072–2079. doi: 10.1172/JCI1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heimerl S, Moehle C, Zahn A, Boettcher A, Stremmel W, Langmann T, Schmitz G. Alterations in intestinal fatty acid metabolism in inflammatory bowel disease. Biochim Biophys Acta. 2006;1762:341–350. doi: 10.1016/j.bbadis.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Famularo G, De Simone C, Trinchieri V, Mosca L. Carnitines and its congeners: a metabolic pathway to the regulation of immune response and inflammation. Ann N Y Acad Sci. 2004;1033:132–138. doi: 10.1196/annals.1320.012. [DOI] [PubMed] [Google Scholar]
- 44.Andrieu-Abadie N, Jaffrezou JP, Hatem S, Laurent G, Levade T, Mercadier JJ. L-carnitine prevents doxorubicin-induced apoptosis of cardiac myocytes: role of inhibition of ceramide generation. Faseb J. 1999;13:1501–1510. doi: 10.1096/fasebj.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 45.Amat di San Filippo C, Wang Y, Longo N. Functional domains in the carnitine transporter OCTN2, defective in primary carnitine deficiency. J Biol Chem. 2003;278:47776–47784. doi: 10.1074/jbc.M307911200. [DOI] [PubMed] [Google Scholar]
- 46.Amat di San Filippo C, Pasquali M, Longo N. Pharmacological rescue of carnitine transport in primary carnitine deficiency. Hum Mutat. 2006;27:513–523. doi: 10.1002/humu.20314. [DOI] [PubMed] [Google Scholar]
- 47.Nakanishi T, Hatanaka T, Huang W, Prasad PD, Leibach FH, Ganapathy ME, Ganapathy V. Na+- and Cl--coupled active transport of carnitine by the amino acid transporter ATB(0,+) from mouse colon expressed in HRPE cells and Xenopus oocytes. The Journal of physiology. 2001;532:297–304. doi: 10.1111/j.1469-7793.2001.0297f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babusukumar U, Wang T, McGuire E, Broeckel U, Kugathasan S. Contribution of OCTN variants within the IBD5 locus to pediatric onset Crohn’s disease. Am J Gastroenterol. 2006;101:1354–1361. doi: 10.1111/j.1572-0241.2006.00564.x. [DOI] [PubMed] [Google Scholar]