Abstract
Inositol 1, 4, 5-trisphosphate generated by the action of a phospholipase C (PLC) mediates release of intracellular Ca2+ that is essential for sperm-induced activation of mammalian eggs. Much attention currently focuses on the role of sperm-derived PLCζ in generating changes in egg intracellular Ca2+ despite the fact that PLCζconstitutes a very small fraction of the total amount of PLC in a fertilized egg. Eggs express several isoforms of PLC, but a role for an egg-derived PLC in sperm-induced Ca2+ oscillations has not been examined. Reducing egg PLCβ1 by a transgenic RNAi approach resulted in a significant decrease in Ca2+ transient amplitude, but not duration or frequency, following insemination. Furthermore, over-expressing PLCβ1 by microinjecting a Plcb1 cRNA significantly perturbed the duration and frequency of Ca2+ transients and disrupted the characteristic shape of the first transient. These results provide the first evidence for a role of an egg-derived PLC acting in conjunction with a sperm-derived PLCζin egg activation.
Introduction
A universal feature of fertilization in eggs of all species studied to date is a transient elevation in intracellular Ca2+ concentration. In mammals, the sperm evokes a series of repetitive Ca2+ oscillations that persist for several hours and terminate with pronucleus (PN) formation (Jones et al., 1995; Marangos et al., 2003). This pattern of repetitive Ca2+ oscillations in mice is essential for both early events of egg activation (e.g., resumption of meiosis, CG exocytosis, recruitment of maternal mRNAs) and the developmental program (Ducibella et al., 2002; Ozil and Huneau, 2001; Ozil et al., 2006).
Release of Ca2+ from the inositol 1, 4, 5-trisphosphate (IP3)-sensitive Ca2+ pool is essential for egg activation, because inhibiting its release with a monoclonal antibody that blocks the IP3 receptor (Miyazaki et al., 1992) inhibits egg activation (Xu et al., 1994). Calmodulin-modulated protein kinase II (CaMKII) is likely a major target of the released Ca2+ because expressing a constitutively active form of CaMKII results in egg activation in the absence of any increase in the concentration of intracellular free Ca2+ (Madgwick et al., 2005). CaMKII, whose activity changes in parallel with the changes in intracellular Ca2+ (Markoulaki et al., 2003; Markoulaki et al., 2004), may do this by “summing” the amount of Ca2+ released during the course of egg activation (Ducibella et al., 2002; Ozil et al., 2005).
Fertilization likely triggers the increase in intracellular Ca2+ via PLC-catalyzed production of IP3 in the egg (Runft et al., 2002). The IP3 then acts on type 1 IP3 receptors to initiate Ca2+ release. How the fertilizing sperm activates PLC has been intensively studied for the past 15 years. During this time a paradigm shift occurred from a sperm ligand-egg receptor model using either G protein-coupled or tyrosine kinase receptors to a model proposing that a soluble, sperm-derived protein factor is responsible for activating PLC and thereby evoking Ca2+ oscillations in mammalian eggs (Swann and Lai, 1997). The seminal observations leading to the new model are that mouse sperm and egg fusion precedes the onset of oscillations by ~1-5 min (Lawrence et al., 1997) and that injecting mouse sperm in the egg, which causes fertilization-like Ca2+ oscillations, supports full-term development (Kimura and Yanagimachi, 1995).
A novel, sperm-specific PLC, termed PLCζ, is now thought to be the sperm factor. Expression of PLCζ cRNA in mouse eggs that results in synthesis of PLCζ estimated to be equivalent to the amount in a single sperm triggers Ca2+ oscillations that closely resemble the pattern observed following fertilization (Saunders et al., 2002; Yoda et al., 2004). Furthermore, immunodepleting PLCζ from sperm extracts abolishes the Ca2+ releasing activity, suggesting that PLCζ is the sole Ca2+ releasing component (Saunders et al., 2002). Last, recent biochemical studies using recombinant PLCζ protein demonstrated that its activity is ~70% maximal at 100 nM Ca2+, the resting level of Ca2+ in the egg (Kouchi et al., 2004). This finding supports the model in which the sperm delivers a PLC that is readily activated on exposure to the egg’s cytoplasm. Consistent with this proposal is our observation that reducing sperm PLCζ protein by a transgenic RNAi approach significantly perturbs the number of Ca2+oscillations (but not their amplitude) when eggs are inseminated with these sperm (Knott et al., 2005). Furthermore, no transgenic offspring were derived from founder males mosaic for the transgene suggesting that sperm-derived PLCζ is essential for development to term (Knott et al., 2005).
Several observations suggest a role for an endogenous egg PLC in generating the calcium oscillatory pattern during fertilization. First, it is estimated that only about 50 fg of PLCζ enters the egg, which contains ~25,000,000 fg of protein (Saunders et al., 2002). Second, the egg likely contains significantly greater amounts of numerous PLC isoforms, including β,γ, and δ. Third, these egg PLCs, which are regulated by Ca2+, are likely activated by increases in intracellular Ca2+ that now reach stimulatory concentrations. Taken together, these results suggest a model in which each Ca2+ transient is triggered by a sperm-derived PLCζ resulting in IP3 production and an initial increase in intracellular Ca2+. In turn, this initial increase in Ca2+ activates an egg PLC that results in the further production of IP3 and an increase in intracellular Ca2+ that can last for minutes. Consistent with this proposal is the observation that modulating the amount of PLCζ in eggs affects the frequency but not the amplitude of the Ca2+ oscillations (Saunders et al., 2002).
Except for PLCζ, members of each of the PLC families are expressed in eggs (Table 1) and in theory any of these could be involved in modulating Ca2+ oscillations. Based on the finding that the litter size of null females is normal, we excluded as candidates isoforms β4, δ1, δ4, and ε. We excluded isoforms β2, δ2, and δ3 because their transcripts are not expressed in the oocyte. Microinjecting recombinant PLCγ1 protein into mouse eggs can induce persistent Ca2+ oscillations similar to oscillations induced by sperm (Mehlmann et al., 2001). It is unlikely, however, that PLCγ1 or γ2 collaborate with sperm PLCζ to generate Ca2+ oscillations because a dominant-negative approach employing recombinant SH2 domain to inhibit PLCγ1 and γ2 did not inhibit the Ca2+ oscillatory pattern during fertilization (Mehlmann et al., 1998); these experiments had an appropriate positive control to demonstrate efficacy of PLCγ inhibition.
Table 1.
PLC isoforms expressed in oocytes and knockout phenotype
| PLC isoform | Oocyte Expression | Fertility in knockout (KO) females |
|---|---|---|
| PLCβ1 | Yes (Avazeri et al., 2000; Dupont et al., 1996) | Two KO lines generated: (1) Homozygous females produced smaller litters (L. Upton, personal communication); (2) homozygous females do not reproduce due to behavioral problems (Kim et al., 1997) |
| PLCβ2 | No (Dupont et al., 1996) | |
| PLCβ3 | Yes (Avazeri et al., 2000; Dupont et al., 1996) | Conflicting data; normal litter size (D. Wua, personal communication) but preimplantation lethal for a different knockout line (Wang et al., 1998) |
| PLCβ4 | Yes (our laboratory, unpublished) | Normal litter size (M. Kano, personal communication) |
| PLCγ1 | Yes (Dupont et al., 1996; Mehlmann et al., 1998) | Embryonic lethal E9 (Nakamura et al., 2003) |
| PLCγ2 | Yes (Dupont et al., 1996; Mehlmann et al., 1998) | Conditional KO (Hashimoto et al., 2000) |
| PLCδ1 | Yes (our laboratory, unpublished) | Normal litter size (Nakamura et al., 2003) |
| PLCδ2 | No (our laboratory, unpublished) | |
| PLCδ3 | No (Unigene expression analysis) | |
| PLCδ4 | Yes (our laboratory, unpublished) | Normal litter size (Fukami et al., 2001) |
| PLCε | Yes (our laboratory, unpublished) | Normal litter size (Bai et al., 2004) |
| PLCζ | No (Saunders et al., 2002) |
Because there are conflicting data regarding the fertility of PLCβ3-deficient female mice (Table 1), we tested the ability of egg PLCβ1 to modulate sperm-induced Ca2+ oscillations. We report here that reducing egg PLCβ1 using a transgenic RNAi approach results in a decrease in amplitude of the Ca2+ oscillations but no change in their duration or frequency. Over-expressing PLCβ1 in eggs prior to fertilization does not result in spontaneous Ca2+ oscillations but following fertilization these eggs exhibit changes in the oscillatory pattern including an altered shape of the characteristic first oscillation, shorter duration, and decreased frequency of oscillations.
Materials and Methods
Generation of transgenic mice
A RNAi transgene was designed such that the Zp3 promoter (Millar et al., 1991) directs oocyte-specific expression of Plcb1 double-stranded RNA (dsRNA). To prepare the inverted repeat for generating the dsRNA hairpin, a portion of the Plcb1 coding sequence (nucleotides 301 to 854, Genbank accession no. NM_019677) was amplified by PCR and then ligated 3’ to 3’. The inverted repeat was transferred to the pRNAi-ZP3 cassette (Stein et al., 2003). Transgenic animals were generated by PN injection at the University of Pennsylvania Transgenic and Chimeric Mouse Facility. The zygotes for microinjection were produced by mating of B6SJLF1 mice. Transgenic founders and subsequent generations of transgenic mice were mated to wild-type C57Bl6/J mice. Genotyping was performed by PCR of enhanced green fluorescent protein from tail DNA as previously described (Ma et al., 2006). Experimental oocytes and eggs were obtained from transgenic female offspring, and controls were obtained from their non-transgenic littermates; these will be referred to as “TG” and “NTG” oocytes and eggs, respectively. All animal experiments were approved by the University of Pennsylvania Institutional Animal Use and Care Committee and were consistent with National Institutes of Health guidelines.
Complementary RNA synthesis
The pTRIamp19 vector (Ambion, Austin, TX) was modified for the generation of in vitro transcribed, stable, and polyadenylated mRNAs for microinjection. The 5’ and 3’ Xenopus β-globin untranslated regions were amplified from pXT7 (a generous gift from Dr. Sergei Sokol, Harvard Medical School) and cloned into unique HindIII and EcoRI sites of pTRIamp19, respectively. The 3’ globin untranslated region was engineered to end with a polyA(33) and polyC(12) tail. This expression vector was renamed pIVT (plasmid In Vitro Transcription) and was used to generate the overexpression constructs.
The complete 3651 bp coding region of mouse PLCβ1 (GenBank accession no. NM_019677) was engineered using PCR to contain an N-terminal abbreviated Kozak sequence (Kozak, 1987) and a C-terminal T7 epitope tag (MASMTGGQQMG). A catalytically inactive C-terminal deletion mutant of PLCβ1 (Δ830-1041; GenBank accession no. NP_062651) (Litosch, 2000; Ross et al., 2006) containing a C-terminal T7 epitope tag was generated in similar fashion. The coding region of DsRed-Monomer was amplified by PCR from pDsRed-Monomer-C1 (Clontech, Mountain View, CA). The resulting cDNAs were subcloned into pIVT using the unique SphI and XbaI sites. Complementary RNA (cRNA) was synthesized from linearized plasmid DNA using T3 or T7 RNA polymerase and the mMESSAGE mMACHINE kit (Ambion, Austin, TX) according to the manufacturer’s instructions. The cRNA was cleared and purified by using MEGAclear (Ambion) and MicroPoly(A)Purist (Ambion), respectively. The final cRNA concentration was determined by spectrophotometry, and cRNA integrity was confirmed by analyzing a sample on a formaldehyde gel.
Gamete collection and culture
Female CF-1 mice (6-8 wk old) were obtained from Harlan Sprague-Dawley (Indianopolis, IN). Fully-grown, GV-intact oocytes and metaphase II (MII)-arrested eggs were collected from gonadotropin-treated females as previously described (Manejwala et al., 1986). The collection medium for oocytes was bicarbonate-free minimal essential medium (Earle’s salts) containing 0.01% polyvinyl alcohol (PVA), 25 mM Hepes, pH 7.3, and 2.5 μM milrinone to prevent germinal vesicle (GV) breakdown. After collection, oocytes were cultured in Whitten’s medium (Whitten, 1971) containing 0.01% PVA (Whittens/PVA) and 2.5 μM milrinone. Metaphase II (MII)-eggs were collected 13-14 h post-hCG administration into modified Whitten’s medium containing 15 mM Hepes, pH 7.2, 7 mM Na2HCO3, 10 μg/ml gentamicin, and 0.01% polyvinyl alcohol (modWhittens/PVA). The cumulus cells were removed by brief hyaluronidase treatment. When necessary, zona pellucida (ZP)-free oocytes and eggs were obtained by removing the ZP with acidic Tyrodes solution, pH 1.6 (Bornslaeger and Schultz, 1985). The eggs were cultured until use in Whittens/PVA under light mineral oil. All gametes and embryos were cultured at 37° C in a humidified atmosphere of 5% CO2 in air.
RNA isolation, reverse transcription and real-time PCR
Total RNA was isolated from groups of 20-30 GV-intact oocytes using the Absolutely RNA Microprep kit (Strategene) according to the manufacturer’s protocol. The RNA was reverse transcribed using Superscript II (Invitrogen) and random hexamer primers. Real-time PCR analysis was carried out using an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA). TaqMan probes corresponding to PLCβ1 (ABI assay ID Mm00479987_m1) and histone H2A (ABI assay ID Mm00501974_s1) were used. A single oocyte equivalent of cDNA was used in each reaction and performed in triplicate. The amount of PLCβ1 cDNA was normalized by the comparative Ct method (http://www.ambion.com) using amplification of endogenous histone H2A in the same samples.
Microinjection and in vitro maturation of oocytes
Injections were done in 10 μl drops of modWhittens/PVA containing 2.5 μM milrinone. Approximately 10 pl of cRNA or diethyl pyrocarbonate (DEPC)-treated water was injected into the cytoplasm of GV-intact oocytes using a Harvard Apparatus PLI-100 Pico-Injector on the stage of a Nikon Eclipse TE300 microscope equipped with Hoffman optics and Narishige micromanipulators. After microinjection, oocytes were cultured in the presence of milrinone for 1-6 hr to allow for exogenous protein production. The oocytes were washed free of milrinone and then matured for 16 hr in CZB medium (Chatot et al., 1989).
Immunocytochemical analysis
For PLCβ1 detection, ZP-free MII-arrested eggs were fixed in 3.7% paraformaldehyde in PBS for 1 h at room temperature, permeabilized in PBS containing 0.3% BSA and 0.1% Triton X-100 for 15 min, and then blocked in PBS containing 0.3% BSA and 0.01% Tween-20 (blocking solution). The eggs were incubated in a monocolonal anti- PLCβ1 antibody (50 μg/ml in blocking solution; cat# 05-164, Upstate Biotechnology) for 1 h at room temperature and then washed in blocking solution. The primary antibody was detected using a donkey anti-mouse Cy5-conjugated antibody (final concentration 7 μg/ml; Jackson Immunoresearch) for 1 h at room temperature. For detection of the T7 tag, the same procedure was used except the eggs were fixed in 2.5% paraformaldehyde and the primary antibody was a monoclonal anti-T7 tag antibody (0.2 μg/ml in blocking solution; Novagen). DNA was detected by incubation in 0.02% SITOX® Green (Invitrogen) for 15 min at room temperature. Eggs were mounted in Vectashield (Vector Laboratories) and observed with a laser-scanning confocal microscope (Leica DMRE, Leica Microsystems, Inc.). The images were acquired with Leica confocal software, and processed and quantified using Photoshop (Adobe).
The PLCβ1 immunofluorescence signal intensity was quantified by drawing an elliptical region over the entire egg and determining the mean pixel intensity for that egg. Background pixel intensity was determined by measuring the mean pixel intensity of eggs stained with secondary antibody alone in the same experiment. At least 10 TG and 10 NTG eggs were examined in each of two independent experiments for a total of more than 20 eggs examined in these groups.
In vitro fertilization, embryo culture, and Ca2+ imaging
Sperm were collected from the caudae epididymides of B6SJLF1 male mice (8-12 wks old; Jackson Laboratories, Bar Harbor, ME). The tissues were incised and the sperm allowed to swim out into TYH medium (Toyoda et al., 1971). The sperm were incubated in TYH medium for 90 min to allow capacitation. ZP-intact and ZP-free MII eggs were inseminated with 2 × 105/ml and 2.5 × 104/ml sperm in TYH medium, respectively. Three h after insemination, the eggs were washed free of unbound sperm and transferred into KSOM medium with amino acids (Specialty Media) for culture.
To create an artificial grid for egg placement and to prevent movement artifacts during fertilization and Ca2+ imaging, we used a 71 μm pore nylon mesh (Nippon Rikagaku Kikai Co., Ltd) coated with Cell-Tak (Becton Dickinson Labware) (Takahashi et al., 2003). The coating solution was a fresh mixture of 50 μl Cell-Tak, 1445 μl 0.1 M NaHCO3, pH 8.0, and 5 μl 5N NaOH. Pieces of nylon mesh (6 × 6 mm with 71 μm2 pores) were incubated in the coating solution for 20 min, and then washed with sterile water to remove the bicarbonate. The nylon mesh pieces were air-dried and stored at 4° C until use.
For Ca2+ imaging, ZP-free eggs were incubated for 20 min in Whittens/PVA containing 10 μM fura-2-acetoxymethylester (fura-2; Molecular Probes Inc., Eugene, OR), and 0.025% Pluronic F-127 (Poenie et al., 1986). A piece of Cell-Tak-coated nylon mesh was placed on a cover slip in a Leiden chamber and covered with 500 μl Whittens medium not containing BSA. The fura-2 loaded eggs were transferred into individual spaces on the mesh. The chamber was placed on a temperature-controlled microscope stage at 37° C under laminar flow of 5% CO2 in air. After a 10-min incubation to allow the eggs to stick to the nylon mesh and coverslip, 500 μl of Whittens medium containing 30 mg/ml of BSA was added to achieve a final BSA concentration of 15 mg/ml. For insemination, sperm were added such that the final concentration was 2.5 × 104 sperm/ml. Measurements of intracellular Ca2+ were carried out as described previously (Xu et al., 2003). By using the grid created by the Cell-Tak-coated nylon mesh, different experimental groups of eggs were easily distinguished, and Ca2+ oscillations were measured in eggs simultaneously under exactly the same conditions.
Measurements and statistics
The percentages of eggs to form PNs at various times following in vitro fertilization were compared using the chi-square test. Differences between means were judged by the one-way analysis of variance (ANOVA) followed by Sheffe’s F-test using StatView software (Abacus Concepts). Significant difference was defined as p < 0.05.
Measurements of various parameters of the Ca2+ oscillations (amplitude, duration, frequency, persistence) were expressed as a percentage of the mean for control (NTG or RFP) eggs for the same experimental replicate. Each parameter was compared between the two experimental groups using a T test (Prism 4.0; GraphPad Software, San Diego, CA).
Results
Knockdown of PLCβ1 in eggs from transgenic females
A transgenic RNA interference approach using a long inverted repeat RNA under control of the Zp3 promoter (Fig. 2A) was used to reduce production of PLCβ1 during oocyte growth (Stein et al., 2003). Success of the knockdown approach was determined by performing real time quantitative PCR for Plcb1 mRNA using GV-intact oocytes from each transgenic founder line; oocytes of non-transgenic littermates were used as controls. Oocytes derived from females of one transgenic founder line had ~25% of the amount of Plcb1 mRNA as control oocytes, whereas oocytes from several other founder lines had comparatively more Plcb1 mRNA (data not shown). Targeting of Plcb1 appeared specific because there was no decrease in the amount of Plcb4, a PLC isoform highly related to Plcb1 (data not shown). The percentage PLCβ1 protein knockdown in this founder line was ascertained by obtaining MII-arrested eggs from transgenic females and non-transgenic littermates and immunostaining them for PLCβ1 (Fig. 2, B-D). Quantification of the immunofluorescence signal indicated that after subtracting the background fluorescence present in control eggs, TG eggs from this line contained ~66% of the amount of PLCβ1 when compared to NTG control eggs (Fig. 2E).
Figure 2.
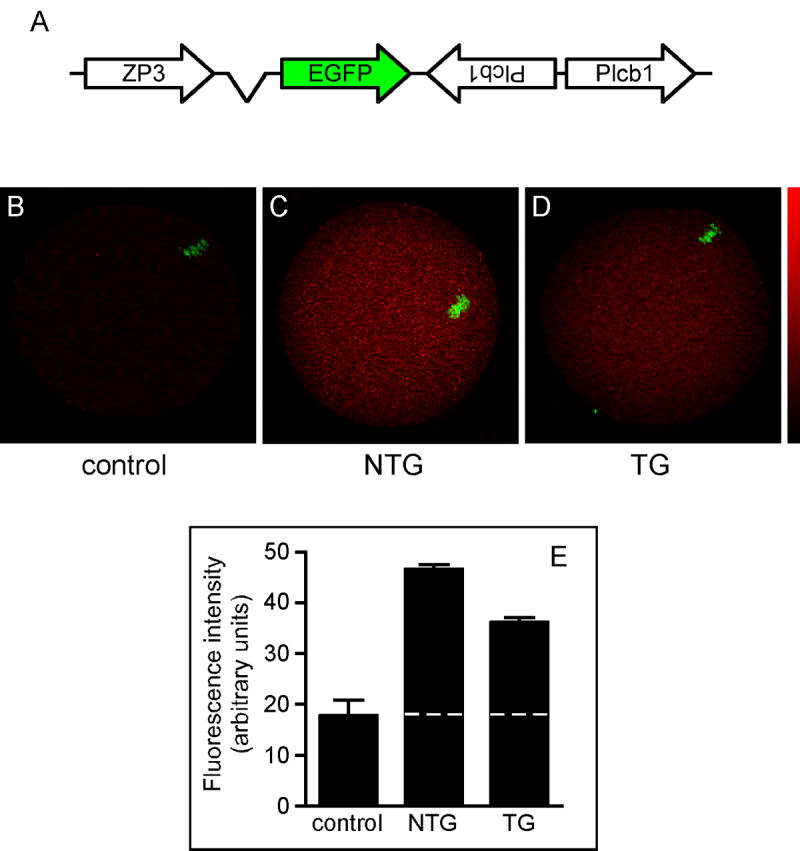
PLCβ1 expression in non-transgenic and transgenic mouse eggs. A. Schematic of construct for generating transgenic mice expressing dsRNA under control of the oocyte-specific Zp3 promoter. B-D. Immunofluorescence analysis of PLCβ1 protein in MII-arrested eggs from non-transgenic (NTG) and transgenic (TG) mice. Controls were MII eggs from NTG mice immunostained with secondary antibody alone. Representative images are shown. Color bar shows pixel intensity range from 0 (black) to 255 (red). B, Control; C, NTG; D, TG. E. Graph representing mean pixel intensity of PLCβ1 immunofluorescence signal ± S.E.M. in 5 control, 21 NTG, and 22 TG eggs in two independent experiments. Dashed line indicates mean background fluorescence level in control eggs.
Sperm-induced calcium oscillations in transgenic PLCβ1 knockdown eggs
To determine if a reduction in egg PLCβ1 protein levels affected sperm-induced Ca2+ oscillations, ZP-free NTG and TG eggs were inseminated simultaneously with capacitated sperm and alterations in intracellular Ca2+ were recorded. Representative examples of the Ca2+ oscillation patterns observed for NTG and TG eggs are shown (Fig. 3, A and B). When the Ca2+ oscillation patterns in each group were analyzed across all eggs, there was no significant difference between TG and NTG eggs in the baseline 340/380 fluorescence ratio, persistence of Ca2+ oscillations, amount of time to the first Ca2+ transient, or length of the first Ca2+ transient. The only parameter that we observed to be modified was peak amplitude, which was decreased ~20% (Fig. 3C). Such changes in peak amplitude can arise artefactually if acquisition settings for fura-2 are changed between experiments (e.g., increasing exposure time at 340 nm while maintaining the 380 nm exposure time). However this could not have been the case here because we kept the exposure times the same across all experiments, and the data were acquired simultaneously from both TG and NTG eggs. The modest, but significant, ~20% reduction in amplitude is consistent with the ~34% reduction in the amount of PLCβ1 protein. These findings support the hypothesis that egg PLCβ1 is involved in modulating the overall Ca2+ oscillatory pattern induced by the sperm at fertilization.
Figure 3.
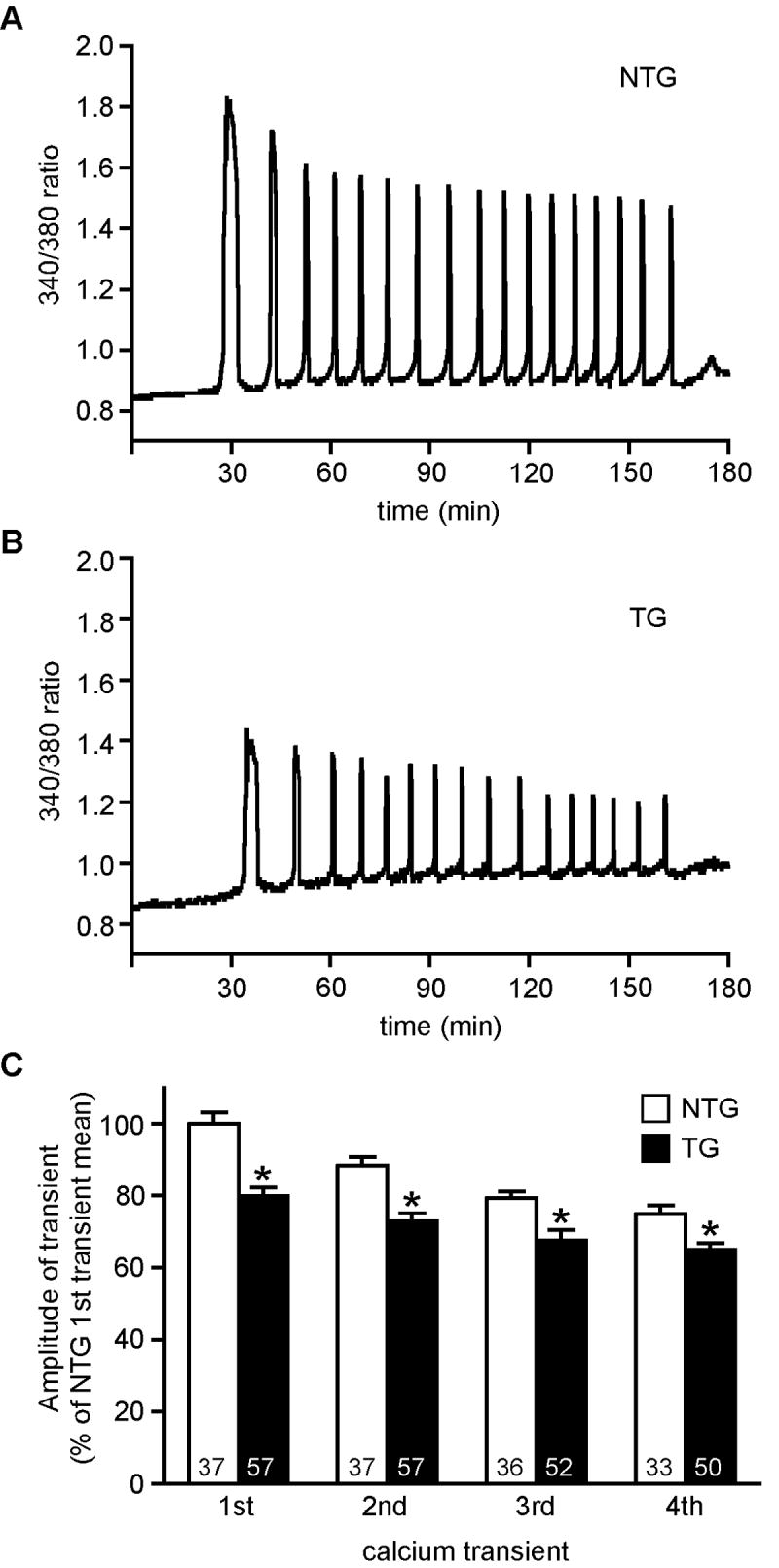
Sperm-induced Ca2+ oscillations in non-transgenic and transgenic mouse eggs. A and B. Representative Ca2+ oscillatory patterns for NTG (A) and TG (B) eggs. C. Graph of amplitude of 1st four Ca2+ oscillations in NTG and TG eggs. Amplitude is expressed as the percentage of the mean amplitude of the 1st Ca2+ transient for NTG eggs for each experimental replicate (mean ± SEM). Asterisks indicate that the mean amplitude of each Ca2+ transient was significantly different in TG as compared to NTG eggs (T test; p < 0.01). Numbers at the base of each column indicate number of transients measured in 3 independent experiments. Time zero is the time of insemination.
Embryo development of fertilized transgenic PLCβ1 knockdown eggs
Because the total Ca2+ signal experienced by the egg regulates completion of fertilization-associated events (Ducibella et al., 2002; Ozil et al., 2005), we hypothesized that the decrease in amplitude of the Ca2+ transients in TG eggs would result in a delay of egg activation as indicated by later PN formation. To test this idea, we performed in vitro fertilization of ZP-free TG and NTG eggs and monitored the timing of PN formation. “Early” and “late” PN formation were determined at 5.5 to 6 h and 7 to 8 h after insemination, respectively. There were no significant differences in the percentage of TG and NTG eggs that had early or late PN formation, or cleavage to the 2-cell stage (Table 2). When these embryos were cultured to the blastocyst stage, no differences in the rate of development or percentage of embryos to develop to fully expanded blastocysts were observed. These results suggested that the observed alterations in peak amplitude in the TG eggs did not affect the total Ca2+ signal enough to alter egg activation events. These findings were confirmed by a six-month mating trial in which no differences in fertility (number of litters and number of pups delivered per litter) were observed when comparing TG females and NTG littermates (data not shown).
Table 2.
Timing of embryo development after fertilization of nontransgenic and transgenic eggs
| Group | # MII eggs | Early PN formation* (%) | Late PN formation** (%) | Cleavage to 2-cell (%) | Fully expanded blastocyst (%) |
|---|---|---|---|---|---|
| NTG | 189 | 35/189 (18.5) | 137/189 (72.5) | 163/189 (86.2) | 116/163 (71.2) |
| TG | 124 | 21/124 (16.9) | 96/124 (77.4) | 102/124 (82.3) | 73/102 (71.6) |
Early PN formation was observed at 5.5-6 hrs after insemination
Late PN formation was observed at 7-8 hrs after insemination
Effect of over-expressing PLCβ1 on sperm-induced calcium oscillations
To explore further our hypothesis that an egg PLC is involved in generating Ca2+ oscillations at fertilization, we examined the effects of over-expressing exogenous PLCβ1 in wild type eggs on the characteristics of the Ca2+ oscillatory pattern induced by sperm. The full-length (~3.6 kb) coding region of Plcb1 with a C-terminal T7 epitope tag (Plcb1-T7) was inserted into the pIVT vector, which allows efficient in vitro transcription of stable polyadenylated cRNA (Fig. 4A). The coding region of a red fluorescent protein (RFP) was inserted into pIVT and cRNA encoding RFP was generated for use as a control. As an additional control, cRNA encoding a C-terminal deletion mutant of PLCβ1 (Δ830-1041) was generated. GV-intact oocytes were microinjected with Plcb1-T7 or RFP cRNA and subsequently matured in vitro. PLCβ1 expression in these MII-arrested eggs was increased over the endogenous protein level as indicated by immunostaining (Fig. 4, B and C). At least some of the exogenous PLCβ1 protein was full length, because the T7 tag was detected in the Plcb1-T7 cRNA-injected eggs (Fig. 4, D and E). RFP was clearly observed by epifluorescence microscopy in the control RFP cRNA injected eggs (data not shown).
Figure 4.
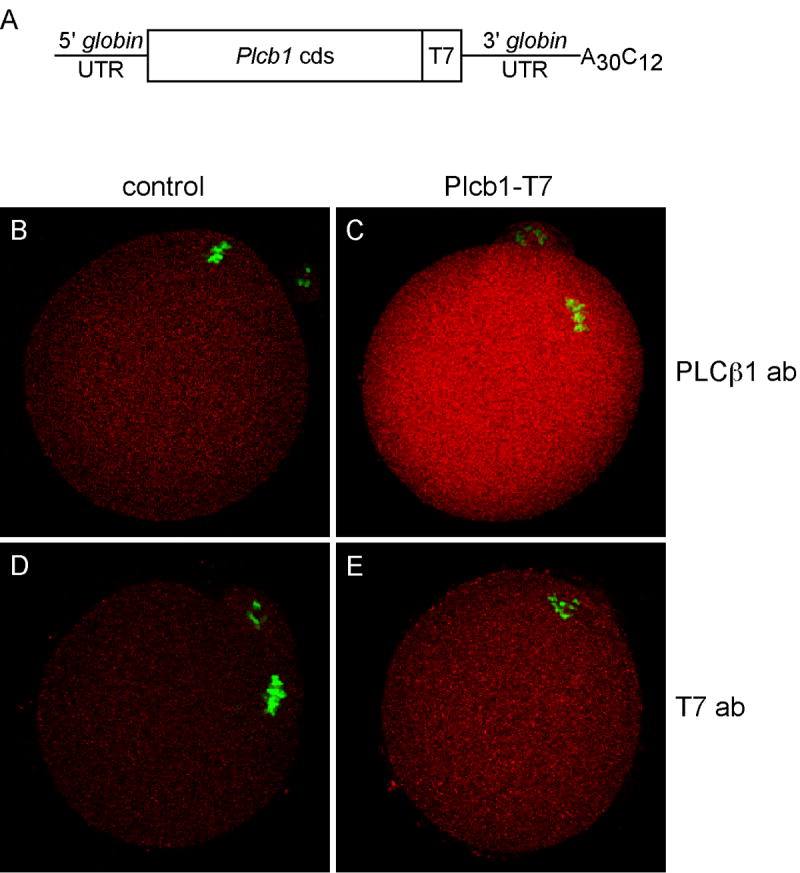
Over-expression of PLCβ1 in wild type MII eggs. A. Schematic of capped cRNA microinjected into GV-intact oocytes. B-E. Immunofluorescent staining of MII eggs for PLCβ1 or T7 epitope tag, as indicated (both shown in red) and DNA (green). B and D, control non-injected eggs; C and E, eggs injected with Plcb1-T7 cRNA. Representative images from two independent experiments are shown.
After maturation in vitro, eggs from each experimental group were simultaneously inseminated and the Ca2+ oscillatory patterns recorded; it should be noted that eggs injected with Plcb1-T7 cRNA but not inseminated displayed no changes in intracellular Ca2+. The majority of eggs over-expressing PLCβ1 (26/28) displayed a distinctly altered Ca2+ oscillatory pattern when compared to RFP-injected eggs (Fig. 5, A and B). We analyzed five parameters of individual Ca2+ oscillations in these eggs: amplitude, duration, frequency, persistence, and shape. There was no difference in either the amplitude or persistence in eggs over-expressing PLCβ1 (data not shown). These eggs, however, had a shorter duration of the first Ca2+ transient and a symmetric shape of each Ca2+ transient (Fig. 5, B and C). In addition, the interval between Ca2+ transients was longer in these eggs (Fig. 5D). We also analyzed the “onset” and “restore” times of the 1st and 2nd Ca2+ transients in these eggs. Onset time refers to the time from an initial increase in Ca2+ from the baseline to when the first sharp change in positive slope occurs. Reciprocally, restore time refers the time at the end of the transient when the sharp reduction in negative slope occurs to the time of return to baseline; these time points were determined by visual examination of each Ca2+ transient (Fig. 6A). Results of these analyses revealed a significant increase in both the onset and restore times in eggs over-expressing PLCβ1 with the restore time exhibiting more than a 10-fold increase (Fig. 6B). These perturbations in Ca2+ oscillatory behavior were not observed when eggs expressing the inactive form of PLCβ1 were inseminated (data not shown). It was also possible that the observed differences were due to reduced calcium stores in eggs over-expressing PLCβ1. This was unlikely because virtually identical amounts of Ca2+ were released in response to the calcium ionophore A23187 when eggs were injected with either Plcb1 or RFP cRNA (data not shown).
Figure 5.
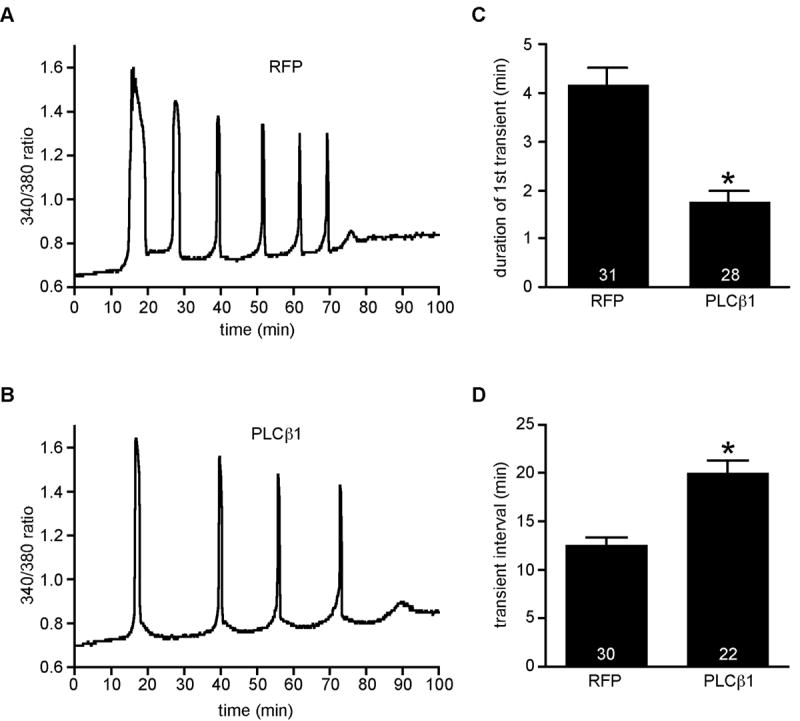
Sperm-induced Ca2+ oscillations in eggs overexpressing red fluorescent protein (RFP) or PLCβ1. A and B. Representative calcium oscillation pattern for RFP (A) and PLCβ1 (B). C and D. Graphs of duration of 1st Ca2+ transient (C) and interval between transients (D), expressed as mean ± SEM. Asterisks indicate significant differences (T test; p < 0.0001). Numbers at the base of each bar indicate total number of eggs analyzed in 3 independent experiments. Time zero is the time of insemination.
Figure 6.
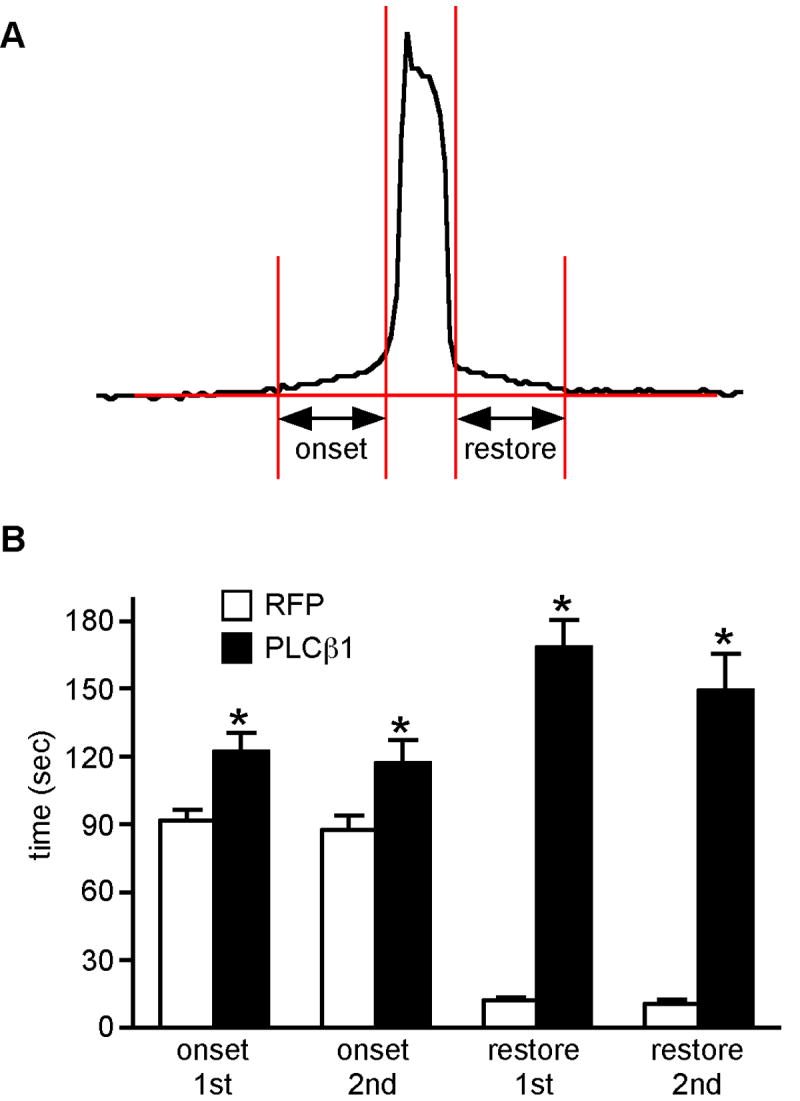
Analysis of onset and restore times of sperm-induced Ca2+ transients in eggs over-expressing RFP or PLCβ1. A. Schematic showing how onset and restore times were measured. B. Graph comparing onset and restore times of 1st and 2nd Ca2+ transients in eggs expressing RFP or PLCβ1, as indicated. Asterisks indicate significant differences (T test; *, p < 0.02; **, p<0.0001). At least 28 1st and 2nd transients were analyzed in 3 independent experiments.
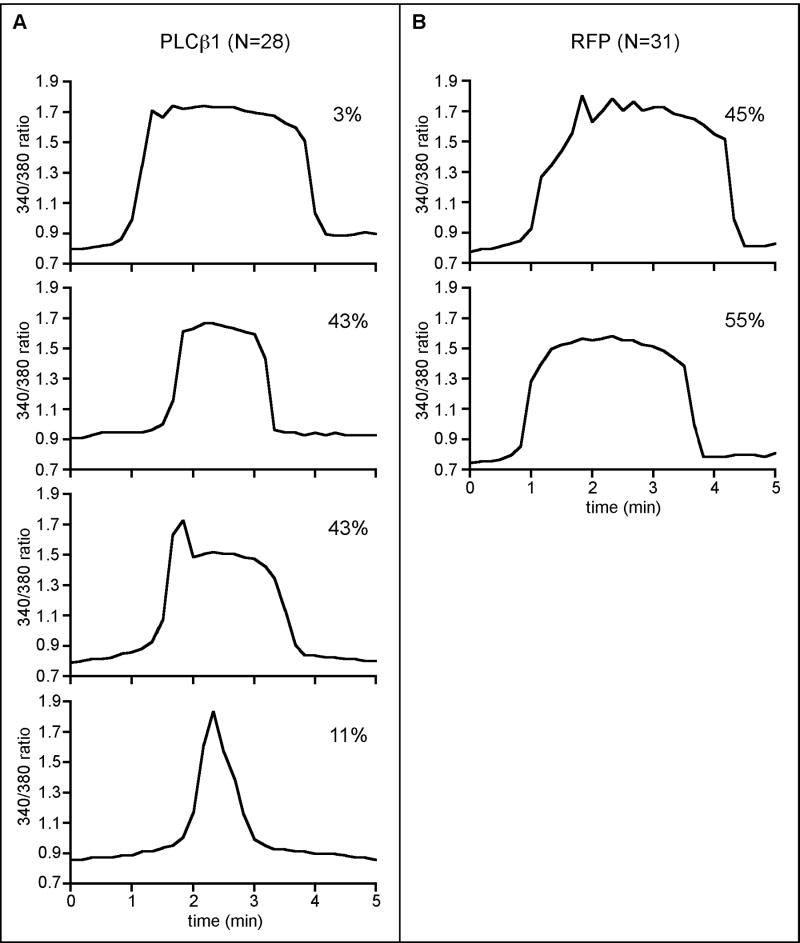
A more detailed analysis of the first Ca2+ transient was undertaken because of the pronounced effects on its shape (Fig. 7). The signature pattern of a normal sperm-induced first transient is characterized by a shoulder that is often followed by a series of initial spikes (Fig. 7, A and B). These features were largely absent in the eggs over-expressing PLCβ1, e.g., only 1 of 28 eggs displayed a transient that had normal spikes but even this one had no shoulder (Fig. 7C). A large fraction (43%) displayed no shoulder or spikes (Fig. 7D), 43% displayed an abnormally large single initial spike followed by a plateau (Fig. 7E), and 11% displayed a single very large spike with no plateau (Fig. 7F).
Figure 7.
Analysis of the first Ca2+ transient in eggs overexpressing RFP or PLCβ1. Graphs representing observed shapes of the first calcium transient for RFP (A, B) and PLCβ1 (C-F) are shown. The percentage of eggs exhibiting each shape and the total number of eggs analyzed are indicated. The arrows point to the shoulder region of the transient.
The results shown above clearly indicated that decreased amounts of total Ca2+ are released in eggs over-expressing PLCβ1. Because events of early egg activation show a graded response to the amount of Ca2+ released (Ducibella et al., 2000; Ozil et al., 2005), we anticipated that the timing of the PN formation would be delayed in these eggs. ZP-free eggs over-expressing either PLCβ1 or RFP were inseminated and the number of eggs that formed PN at “early” or “late” times were counted. As anticipated, the timing of PN formation was slower in eggs over-expressing PLCβ1 than controls as indicated by the smaller number of fertilized eggs that displayed a PN at the early time point (Table 3).
Table 3.
Timing of pronucleus formation after fertilization of eggs overexpressing PLCβ1
| Overexpressed protein | No. MII eggs | Early PN formation* (%) | Late PN formation** (%) |
|---|---|---|---|
| RFP | 78 | 35/94 (37.2) | 65/94 (69.1) |
| PLCb1 | 94 | 14/78 (17.9)† | 32/78 (41.0)† |
Early PN formation was observed at 5.5-6 hrs after insemination
Late PN formation was observed at 7-8 hrs after insemination
Significantly different from RFP group, chi-square, p<0.01
Discussion
The results presented here strongly suggest that the Ca2+ oscillatory pattern observed in a fertilized egg is not solely due to a sperm-derived PLCζ but rather represents a response to both sperm and egg-derived PLCs. Such a model provides a mechanism for amplifying the initial sperm-derived signal, amplification being a common feature of signal transduction pathways. In addition, this mechanism would foster development and implantation of “healthy” eggs, i.e., eggs that have accumulated appropriate amounts of PLC and other signaling components required to generate Ca2+ oscillations sufficient for development to term (Ozil et al., 2006). Although we focused on egg-derived PLCβ1 for the reasons discussed in the Introduction, our results neither address nor exclude a function for other egg PLCs in modulating Ca2+ oscillatory behavior in fertilized eggs.
We observed a modest reduction in the amount of egg PLCβ1 using the transgenic RNAi approach even though PLCβ1 mRNA was substantially reduced. A likely explanation for this finding is that PLCβ1 is accumulated early during oocyte growth and the protein is relatively stable. Nevertheless, even this modest reduction in egg PLCβ1 results in a consistent and statistically significant reduction in amplitude of the Ca2+ oscillations in fertilized eggs. This result suggests that other egg PLCs do not compensate for loss of PLCβ1 function, implying a pivotal role for PLCβ1 in egg activation. In addition, previous work demonstrates that events of early development depend on the total amount of Ca2+ released during the oscillatory period. In our transgenic eggs, there is a modest reduction in amplitude, with no observable change in duration or frequency Ca2+ oscillations. Because neither preimplantation development nor development to term is compromised in embryos derived from transgenic eggs, sufficient amounts of Ca2+ are likely released following fertilization.
An inherent caveat of any over-expression study is that non-physiological amounts of protein are present and this may result in non-physiological responses. Nevertheless, if sperm-derived PLCζ were solely responsible for the signature Ca2+ oscillatory pattern, over-expressing an egg PLC should not significantly change the pattern. Instead, we noted that over-expressing PLCβ1 in mouse eggs has a pronounced effect on sperm-induced Ca2+ oscillations. This result complements the results obtained from the knockdown experiments and provides additional support for involvement of an egg-derived PLC. Particularly noteworthy, is the dramatic effect of over-expressing PLCβ1 on the characteristic shape of the first Ca2+ oscillation (Jones et al., 1998; Miyazaki et al., 1986). Remarkably, little is known regarding the molecular basis why the first Ca2+ transient is of longer duration and exhibits initial spikes, but these properties must reflect, at least in part, Ca2+ release and re-uptake mechanisms active in the fertilized egg. Perhaps the shorter duration and effects on the initial spikes in fertilized eggs over-expressing PLCβ1 reflects faster release of Ca2+ from intracellular stores that in turn stimulates even faster Ca2+ re-uptake than normally occurs. An enhanced re-uptake mechanism may also explain failure to observe an increase in amplitude in these fertilized eggs.
Over-expressing PLCβ1 in eggs results in a substantial reduction in the total amount of Ca2+ released following fertilization. This change is due to the shorter duration, particularly that of the first transient, and decreased frequency of Ca2+ oscillations. Consistent with the finding that experimentally decreasing the total amount of Ca2+ released delays the time course for PN formation (Tóth et al., 2005) is that a similar delay occurs in eggs over-expressing PLCβ1. This finding also minimizes concerns about using electroporation to manipulate intracellular Ca2+ concentrations to study egg activation (Ducibella et al., 2002; Ozil et al., 2005, 2006) because similar results are observed with both methods.
In summary, the results presented here provide the first evidence for a role of an egg-derived PLC in sperm-induced egg activation in mammals. Although these studies focused on PLCβ1, a similar approach could unmask functions for other egg-derived PLCs in egg activation.
Figure 1.
Photograph of nylon mesh creating grid for fura-2-loaded eggs. A. Brightfield image. B. Fluorescence image.
Acknowledgments
The authors thank Dr. Shin Murai for conducting the calcium ionophore experiments reported as data not shown. This research was supported by a grant from the NIH (HD22732 to RMS and CJW). HI was supported in part by a grant from Yamagata University, School of Medicine. JGK was supported by training grant T32 HD007305.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Avazeri N, Courtot AM, Pesty A, Duquenne C, Lefevre B. Cytoplasmic and nuclear phospholipase C-β1 relocation: role in resumption of meiosis in the mouse oocyte. Mol Biol Cell. 2000;11:4369–4380. doi: 10.1091/mbc.11.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornslaeger EA, Schultz RM. Adenylate cyclase activity in zona-free mouse oocytes. Exp Cell Res. 1985;156:277–281. doi: 10.1016/0014-4827(85)90282-4. [DOI] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil J-P. Egg to embryo transition is driven by differential responses to Ca2+ oscillation number. Dev Biol. 2002;250:280–291. [PubMed] [Google Scholar]
- Dupont G, McGuinness OM, Johnson MH, Berridge MJ, Borgese F. Phospholipase C in mouse oocytes: characterization of β and g isoforms and their possible involvement in sperm-induced Ca2+ spiking. Biochem J. 1996;316:583–591. doi: 10.1042/bj3160583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Merriman JA, Whittingham DG, Kono T. Repetitive sperm-induced Ca2+ transients in mouse oocytes are cell cycle dependent. Development. 1995;121:3259–3266. doi: 10.1242/dev.121.10.3259. [DOI] [PubMed] [Google Scholar]
- Jones KT, Soeller C, Cannell MB. The passage of Ca2+ and fluorescent markers between the sperm and egg after fusion in the mouse. Development. 1998;125:4627–4635. doi: 10.1242/dev.125.23.4627. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- Knott JG, Kurokawa M, Fissore RA, Schultz RM, Williams CJ. Transgenic RNA interference reveals role for mouse sperm phospholipase Cζ in triggering Ca2+ oscillations during fertilization. Biol Reprod. 2005;72:992–996. doi: 10.1095/biolreprod.104.036244. [DOI] [PubMed] [Google Scholar]
- Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S. Recombinant Phospholipase Cζ Has High Ca2+ Sensitivity and Induces Ca2+ Oscillations in Mouse Eggs. J Biol Chem. 2004;279:10408–10412. doi: 10.1074/jbc.M313801200. [DOI] [PubMed] [Google Scholar]
- Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987;196:947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- Lawrence Y, Whitaker M, Swann K. Sperm-egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development. 1997;124:233–241. doi: 10.1242/dev.124.1.233. [DOI] [PubMed] [Google Scholar]
- Litosch I. Regulation of phospholipase C-β1 activity by phosphatidic acid. Biochemistry. 2000;39:7736–7743. doi: 10.1021/bi000022y. [DOI] [PubMed] [Google Scholar]
- Ma J, Zeng F, Schultz RM, Tseng H. Basonuclin: a novel mammalian maternal-effect gene. Development. 2006;133:2053–2062. doi: 10.1242/dev.02371. [DOI] [PubMed] [Google Scholar]
- Madgwick S, Levasseur M, Jones KTc. Calmodulin-dependent protein kinase II, and not protein kinase C, is sufficient for triggering cell-cycle resumption in mammalian eggs. J Cell Sci. 2005;118:3849–3859. doi: 10.1242/jcs.02506. [DOI] [PubMed] [Google Scholar]
- Manejwala F, Kaji E, Schultz RM. Development of activatable adenylate cyclase in the preimplantation mouse embryo and a role for cyclic AMP in blastocoel formation. Cell. 1986;46:95–103. doi: 10.1016/0092-8674(86)90863-9. [DOI] [PubMed] [Google Scholar]
- Marangos P, FitzHarris G, Carroll J. Ca2+ oscillations at fertilization in mammals are regulated by the formation of pronuclei. Development. 2003;130:1461–1472. doi: 10.1242/dev.00340. [DOI] [PubMed] [Google Scholar]
- Markoulaki S, Matson S, Abbott AL, Ducibella T. Oscillatory CaMKII activity in mouse egg activation. Dev Biol. 2003;258:464–474. doi: 10.1016/s0012-1606(03)00133-7. [DOI] [PubMed] [Google Scholar]
- Markoulaki S, Matson S, Ducibella T. Fertilization stimulates long-lasting oscillations of CaMKII activity in mouse eggs. Dev Biol. 2004;272:15–25. doi: 10.1016/j.ydbio.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Carpenter G, Rhee SG, Jaffe LA. SH2 domain-mediated activation of phospholipase Cγ is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev Biol. 1998;203:221–232. doi: 10.1006/dbio.1998.9051. [DOI] [PubMed] [Google Scholar]
- Millar SE, Lader E, Liang L-F, Dean J. Oocyte-specific factors bind a conserved upstream sequence required for mouse zona pellucida promoter activity. Mol Cell Biol. 1991;11:6197–6204. doi: 10.1128/mcb.11.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S, Hashimoto N, Yoshimoto Y, Kishimoto T, Igusa Y, Hiramoto Y. Temporal and spatial dynamics of the periodic increase in intracellular free calcium at fertilization of golden hamster eggs. Dev Biol. 1986;118:259–267. doi: 10.1016/0012-1606(86)90093-x. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992;257:251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- Ozil JP, Banrezes B, Toth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol. 2006;300:534–544. doi: 10.1016/j.ydbio.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Ozil JP, Huneau D. Activation of rabbit oocytes: the impact of the Ca2+ signal regime on development. Development. 2001;128:917–928. doi: 10.1242/dev.128.6.917. [DOI] [PubMed] [Google Scholar]
- Ozil JP, Markoulaki S, Toth S, Matson S, Banrezes B, Knott JG, Schultz RM, Huneau D, Ducibella T. Egg activation events are regulated by the duration of a sustained Ca2+cyt signal in the mouse. Dev Biol. 2005;282:39–54. doi: 10.1016/j.ydbio.2005.02.035. [DOI] [PubMed] [Google Scholar]
- Poenie M, Alderton J, Steinhardt R, Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986;233:886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Ross EM, Mateu D, Gomes AV, Arana C, Tran T, Litosch I. Structural determinants for phosphatidic acid regulation of phospholipase C-β1. J Biol Chem. 2006;281:33087–33094. doi: 10.1074/jbc.M606487200. [DOI] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: where it all begins. Dev Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- Stein P, Svoboda P, Schultz RM. Transgenic RNAi in mouse oocytes: A simple and fast approach to study gene function. Dev Biol. 2003;256:187–193. doi: 10.1016/s0012-1606(02)00122-7. [DOI] [PubMed] [Google Scholar]
- Swann K, Lai FA. A novel signalling mechanism for generating Ca2+ oscillations at fertilization in mammals. BioEssays. 1997;19:371–378. doi: 10.1002/bies.950190504. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev. 2003;66:143–152. doi: 10.1002/mrd.10341. [DOI] [PubMed] [Google Scholar]
- Tóth S, Huneau D, Banrezes B, Ozil JP. Egg activation is the result of calcium signal summation in the mouse. Reproduction. 2005;131:27–34. doi: 10.1530/rep.1.00764. [DOI] [PubMed] [Google Scholar]
- Toyoda Y, Yokoyama M, Hosi T. Study on the fertilization of mouse eggs in vitro. I. In vitro fertilization of eggs by fresh epidiymal sperm. Jpn J Anim Reprod. 1971;16:147–151. [Google Scholar]
- Whitten WK. Nutrient requirements for the culture of preimplantation mouse embryo in vitro. Adv Biosci. 1971;6:129–139. [Google Scholar]
- Xu Z, Kopf GS, Schultz RM. Involvement of inositol 1,4,5-trisphosphate-mediated Ca2+ release in early and late events of mouse egg activation. Development. 1994;120:1851–1859. doi: 10.1242/dev.120.7.1851. [DOI] [PubMed] [Google Scholar]
- Xu Z, Williams CJ, Kopf GS, Schultz RM. Maturation-associated increase in IP3 receptor type 1: role in conferring increased IP3 sensitivity and Ca2+ oscillatory behavior in mouse eggs. Dev Biol. 2003;254:163–171. doi: 10.1016/s0012-1606(02)00049-0. [DOI] [PubMed] [Google Scholar]
- Yoda A, Oda S, Shikano T, Kouchi Z, Awaji T, Shirakawa H, Kinoshita K, Miyazaki S. Ca2+ oscillation-inducing phospholipase Cζ expressed in mouse eggs is accumulated to the pronucleus during egg activation. Dev Biol. 2004;268:245–257. doi: 10.1016/j.ydbio.2003.12.028. [DOI] [PubMed] [Google Scholar]