Abstract
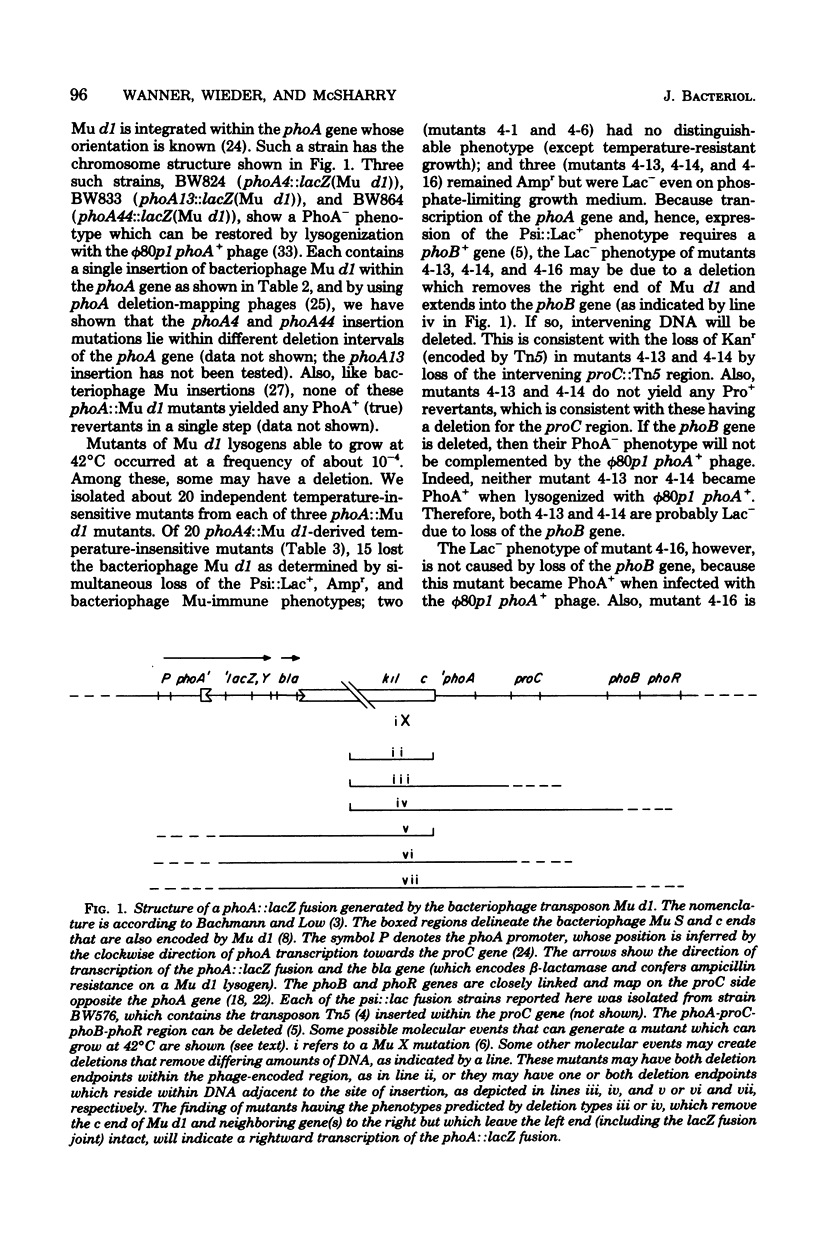
We have previously used the bacteriophage transposon Mu d1 (which encodes the lacZY structural genes but without their promoter) to construct strains that have lacZY fused to phosphate-regulated promoters in Escherichia coli K-12. Among 18 identified phosphate-starvation-inducible (psi) genes, three (the phoA and two new genes: psiF and psiG) are closely linked to the proC region. The gene order (clockwise) is phoA psiF proC psiG phoB phoR. Using these mutants containing Mu d1 insertions, we devised and tested a new method to determine their orientation. In this procedure, mutants with deletions that are selectable by their ability to grow at 42 degrees C are tested for the presence of Mu d1 and of neighboring genes. Some difficulties arose during analysis of suspected deletion-containing strains derived from Mu d1 lysogens (which also contained a Tn5 element) that were caused by Mu d1 and transposon transpositions and other possible genome rearrangements. Nevertheless, we have shown that the phoA and psiF genes are transcribed clockwise and the psiG gene is transcribed counterclockwise towards proC. Because phoA, but not psiF, gene expression requires the phoB+ (positive regulator) gene product, the phoA and psiF genes do not constitute an operon. On the other hand, the psiG:lacZ fusion-bearing strain may have a fusion to the promoter-distal end of the phoB gene. This implies that phoB expression is phosphate regulated. We believe that this method may be useful in general to elucidate the direction of gene transcription.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argast M., Boos W. Co-regulation in Escherichia coli of a novel transport system for sn-glycerol-3-phosphate and outer membrane protein Ic (e, E) with alkaline phosphatase and phosphate-binding protein. J Bacteriol. 1980 Jul;143(1):142–150. doi: 10.1128/jb.143.1.142-150.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and phi80 transducing phages. J Mol Biol. 1975 Aug 5;96(2):307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I. Reversal of mutator phage Mu integration. J Mol Biol. 1975 Jul 25;96(1):87–99. doi: 10.1016/0022-2836(75)90183-7. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Jacob F. Genetic mapping of the regulator and operator genes of the lac operon. J Mol Biol. 1968 Sep 28;36(3):413–417. doi: 10.1016/0022-2836(68)90165-4. [DOI] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- Faelen M., Toussaint A. Inversion induced by temperature bacteriophage mu-1 in the chromosome of Escherichia coli K-12. J Bacteriol. 1980 May;142(2):391–399. doi: 10.1128/jb.142.2.391-399.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faelen M., Toussaint A. Stimulation of deletions in the Escherichia coli chromosome by partially induced Mucts62 prophages. J Bacteriol. 1978 Nov;136(2):477–483. doi: 10.1128/jb.136.2.477-483.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., ECHOLS H. Genetic control of induction of alkaline phosphatase synthesis in E. coli. Proc Natl Acad Sci U S A. 1962 Aug;48:1398–1402. doi: 10.1073/pnas.48.8.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe M. A., Foster T. J. Transposon 10 promoted deletions and inversions in the transfer genes of R100-1. Mol Gen Genet. 1979 Oct 2;176(1):113–120. doi: 10.1007/BF00334302. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Reichardt K., Botstein D. Inversions and deletions of the Salmonella chromosome generated by the translocatable tetracycline resistance element Tn10. J Mol Biol. 1979 Jan 5;127(1):89–115. doi: 10.1016/0022-2836(79)90461-3. [DOI] [PubMed] [Google Scholar]
- Kreuzer K., Pratt C., Torriani A. Genetic analysis of regulatory mutants of alkaline phosphatase of E. coli. Genetics. 1975 Nov;81(3):459–468. doi: 10.1093/genetics/81.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. H., Reznikoff W. S., Beckwith J. Genetic fusions that help define a transcription termination region in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):441–457. doi: 10.1016/0022-2836(76)90239-4. [DOI] [PubMed] [Google Scholar]
- Morris H., Schlesinger M. J., Bracha M., Yagil E. Pleiotropic effects of mutations involved in the regulation of Escherichia coli K-12 alkaline phosphatase. J Bacteriol. 1974 Aug;119(2):583–592. doi: 10.1128/jb.119.2.583-592.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata A., Peterson G. R., Brooks E. L., Rothman F. G. Location and orientation of the phoA locus on the Escherichia coli K-12 linkage map. J Bacteriol. 1971 Sep;107(3):683–689. doi: 10.1128/jb.107.3.683-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D. G., Swan J., Kleckner N. Physical structures of Tn10-promoted deletions and inversions: role of 1400 bp inverted repetitions. Cell. 1979 Apr;16(4):721–731. doi: 10.1016/0092-8674(79)90088-6. [DOI] [PubMed] [Google Scholar]
- Rothstein D. M., Pahel G., Tyler B., Magasanik B. Regulation of expression from the glnA promoter of Escherichia coli in the absence of glutamine synthetase. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7372–7376. doi: 10.1073/pnas.77.12.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy A., Michaelis S., Beckwith J. Deletion map of the Escherichia coli structural gene for alkaline phosphatase, phoA. J Bacteriol. 1981 Jan;145(1):288–292. doi: 10.1128/jb.145.1.288-292.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy A., Michaelis S., Beckwith J. Use of gene fusions to determine the orientation of gene phoA on the Escherichia coli chromosome. J Bacteriol. 1981 Jan;145(1):293–298. doi: 10.1128/jb.145.1.293-298.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvanen M. Tn903 induces inverted duplications in the chromosome of bacteriophage lambda. J Mol Biol. 1980 May 5;139(1):1–17. doi: 10.1016/0022-2836(80)90112-6. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Outer membrane protein e of Escherichia coli K-12 is co-regulated with alkaline phosphatase. J Bacteriol. 1980 Jul;143(1):151–157. doi: 10.1128/jb.143.1.151-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Moreno F., Schwartz M. Pleiotropic mutations rendering Escherichia coli K-12 resistant to bacteriophage TP1. J Bacteriol. 1980 Sep;143(3):1374–1383. doi: 10.1128/jb.143.3.1374-1383.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Kodaira R., Neidhardt F. C. Physiological regulation of a decontrolled lac operon. J Bacteriol. 1977 Apr;130(1):212–222. doi: 10.1128/jb.130.1.212-222.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Sarthy A., Beckwith J. Escherichia coli pleiotropic mutant that reduces amounts of several periplasmic and outer membrane proteins. J Bacteriol. 1979 Oct;140(1):229–239. doi: 10.1128/jb.140.1.229-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., Bennett R. L., Malamy M. H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973 Feb;113(2):529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., Malamy M. H. Control of the synthesis of alkaline phosphatase and the phosphate-binding protein in Escherichia coli. J Bacteriol. 1976 Jul;127(1):595–609. doi: 10.1128/jb.127.1.595-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


