Abstract
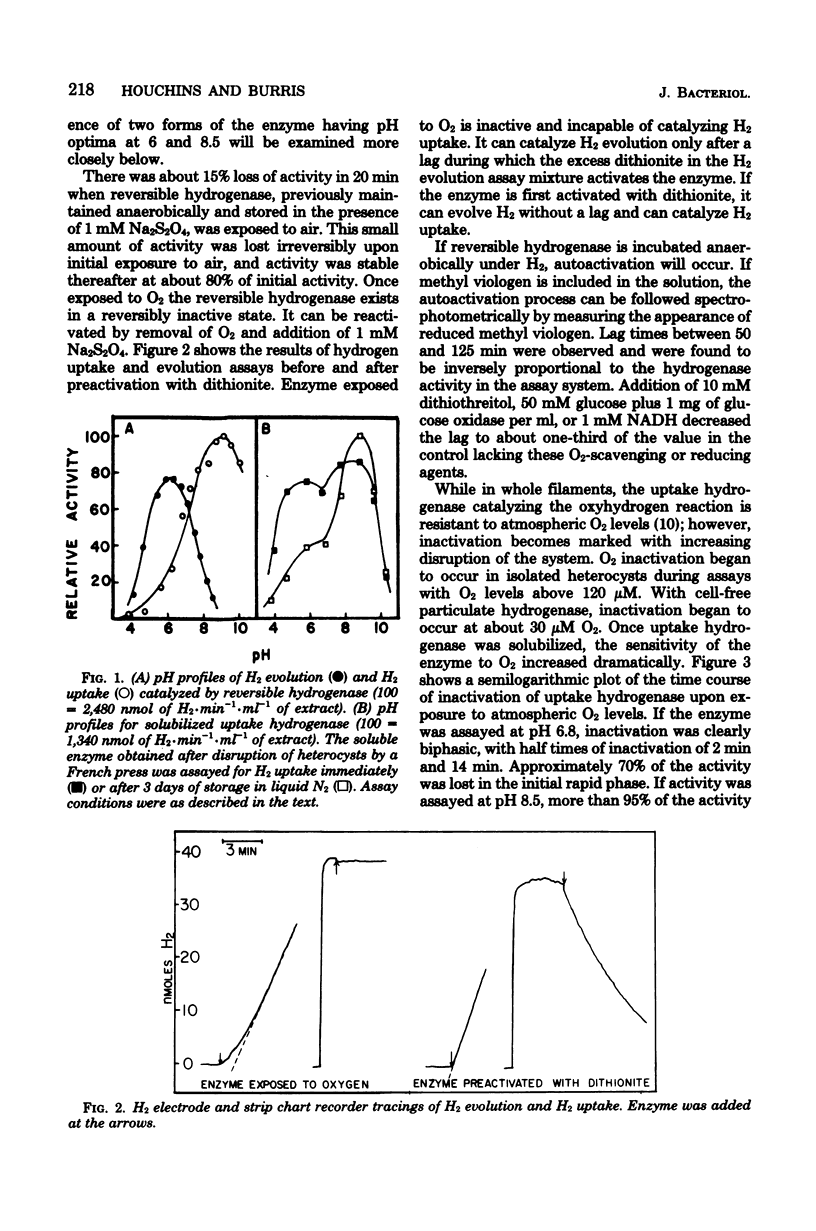
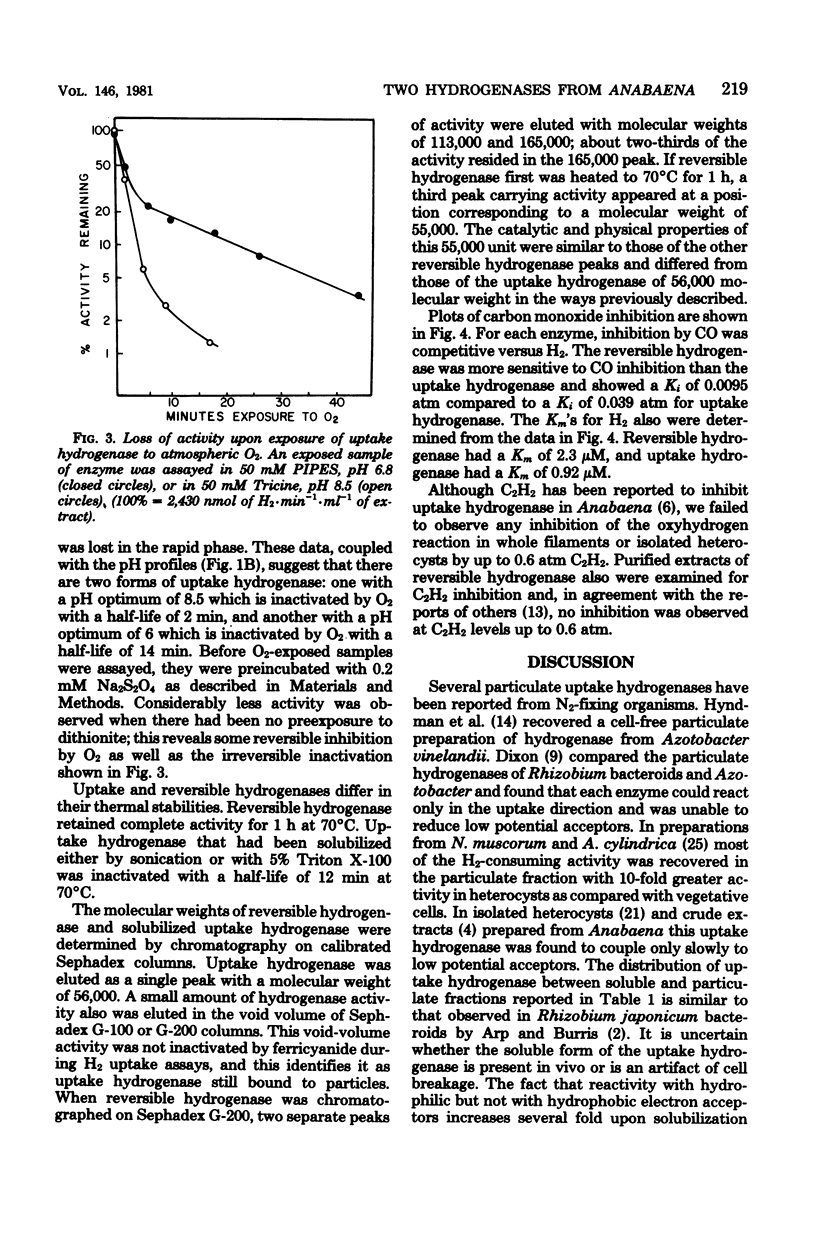
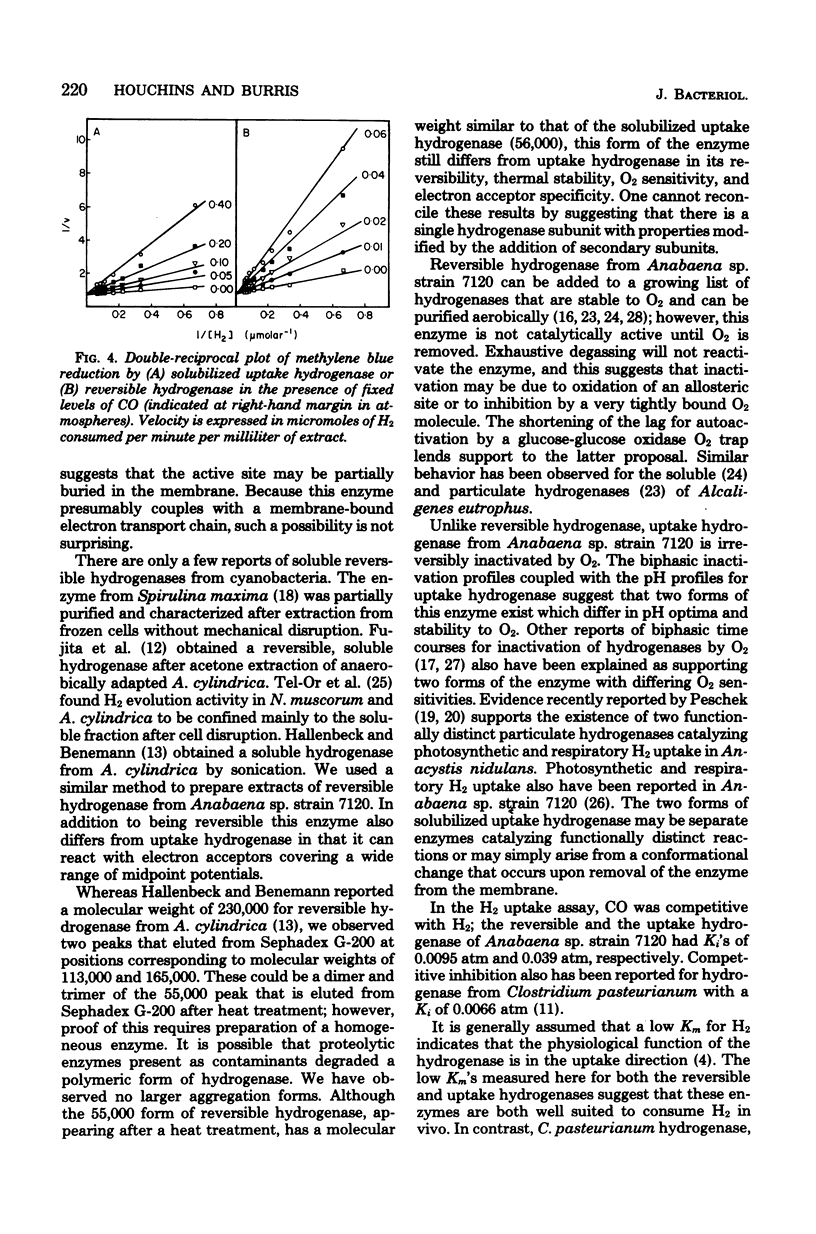
Two distinct hydrogenases, hereafter referred to as "uptake" and "reversible" hydrogenase, were extracted from Anabaena sp. strain 7120 and partially purified. The properties of the two enzymes were compared in cell-free extracts. Uptake hydrogenase was largely particulate, and although membrane bound, it could catalyze an oxyhydrogen reaction. Particulate and solubilized uptake hydrogenase could catalyze H2 uptake with a variety of artificial electron acceptors which had midpoint potentials above 0 mV. Reversible hydrogenase was soluble, could donate electrons rapidly to electron acceptors of both positive and negative midpoint potential, and could evolve H2 rapidly when provided with reduced methyl viologen. Uptake hydrogenase was irreversibly inactivated by O2, whereas reversible hydrogenase was reversibly inactivated and could be reactivated by exposure to dithionite or H2. Reversible hydrogenase was stable to heating at 70 degrees C, but uptake hydrogenase was inactivated with a half-life of 12 min at this temperature. Uptake hydrogenase was eluted from Sephadex G-200 in a single peak of molecular weight 56,000, whereas reversible hydrogenase was eluted in two peaks with molecular weights of 165,000 and 113,000. CO was competitive with H2 for each enzyme; the Ki's for CO were 0.0095 atm for reversible hydrogenase and 0.039 atm for uptake hydrogenase. The pH optima for H2 evolution and H2 uptake by reversible hydrogenase were 6 and 9, respectively. Uptake hydrogenase existed in two forms with pH optima of 6 and 8.5. Both enzymes had very low Km's for H2, and neither was inhibited by C2H2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. B., Arnon D. I. Studies on Nitrogen-Fixing Blue-Green Algae. I. Growth and Nitrogen Fixation by Anabaena Cylindrica Lemm. Plant Physiol. 1955 Jul;30(4):366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arp D. J., Burris R. H. Purification and properties of the particulate hydrogenase from the bacteroids of soybean root nodules. Biochim Biophys Acta. 1979 Oct 11;570(2):221–230. doi: 10.1016/0005-2744(79)90142-6. [DOI] [PubMed] [Google Scholar]
- Bothe H., Distler E., Eisbrenner G. Hydrogen metabolism in blue-green algae. Biochimie. 1978;60(3):277–289. doi: 10.1016/s0300-9084(78)80824-4. [DOI] [PubMed] [Google Scholar]
- Bothe H., Tennigkeit J., Eisbrenner G. The utilization of molecular hydrogen by the blue-green alga Anabaena cylindrica. Arch Microbiol. 1977 Jul 26;114(1):43–49. doi: 10.1007/BF00429628. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Daday A., Lambert G. R., Smith G. D. Measurement in vivo of hydrogenase-catalysed hydrogen evolution in the presence of nitrogenase enzyme in cyanobacteria. Biochem J. 1979 Jan 1;177(1):139–144. doi: 10.1042/bj1770139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Erbes D. L., Burris R. H. The kinetics of methyl viologen oxidation and reduction by the hydrogenase from Clostridium pasteurianum. Biochim Biophys Acta. 1978 Jul 7;525(1):45–54. doi: 10.1016/0005-2744(78)90198-5. [DOI] [PubMed] [Google Scholar]
- HYNDMAN L. A., BURRIS R. H., WILSON P. W. Properties of hydrogenase from Azotobacter vinelandii. J Bacteriol. 1953 May;65(5):522–531. doi: 10.1128/jb.65.5.522-531.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. W., Bishop N. I. Simultaneous measurement of oxygen and hydrogen exchange from the blue-green alga anabaena. Plant Physiol. 1976 Apr;57(4):659–665. doi: 10.1104/pp.57.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuno T., Kaplan N. O., Kamen M. D. Chromatium hydrogenase. Proc Natl Acad Sci U S A. 1977 Mar;74(3):861–863. doi: 10.1073/pnas.74.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappi D. A., Stolzenbach F. E., Kaplan N. O., Kamen M. D. Immobilization of hydrogenase on glass beads. Biochem Biophys Res Commun. 1976 Apr 19;69(4):878–884. doi: 10.1016/0006-291x(76)90455-1. [DOI] [PubMed] [Google Scholar]
- Llama M. J., Serra J. L., Rao K. K., Hall D. O. Isolation and characterization of the hydrogenase activity from the non-heterocystous cyanobacterium Spirulina maxima. FEBS Lett. 1979 Feb 15;98(2):342–346. doi: 10.1016/0014-5793(79)80213-6. [DOI] [PubMed] [Google Scholar]
- Peschek G. A. Aerobic hydrogenase activity in Anacystis nidulans. The oxyhydrogen reaction. Biochim Biophys Acta. 1979 Nov 8;548(2):203–215. doi: 10.1016/0005-2728(79)90129-4. [DOI] [PubMed] [Google Scholar]
- Peschek G. A. Anaerobic hydrogenase activity in Anacystis nidulans. H2-dependent photoreduction and related reactions. Biochim Biophys Acta. 1979 Nov 8;548(2):187–202. doi: 10.1016/0005-2728(79)90128-2. [DOI] [PubMed] [Google Scholar]
- Peterson R. B., Wolk C. P. High recovery of nitrogenase activity and of Fe-labeled nitrogenase in heterocysts isolated from Anabaena variabilis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6271–6275. doi: 10.1073/pnas.75.12.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. The membrane-bound hydrogenase of Alcaligenes eutrophus. I. Solubilization, purification, and biochemical properties. Biochim Biophys Acta. 1979 Apr 12;567(2):315–324. doi: 10.1016/0005-2744(79)90117-7. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Purification and properties of soluble hydrogenase from Alcaligenes eutrophus H 16. Biochim Biophys Acta. 1976 Nov 8;452(1):66–80. doi: 10.1016/0005-2744(76)90058-9. [DOI] [PubMed] [Google Scholar]
- Tel-Or E., Luijk L. W., Packer L. Hydrogenase in N2-fixing cyanobacteria. Arch Biochem Biophys. 1978 Jan 15;185(1):185–194. doi: 10.1016/0003-9861(78)90158-3. [DOI] [PubMed] [Google Scholar]
- Tetley R. M., Bishop N. I. The differential action of metronidazole on nitrogen fixation, hydrogen metabolism, photosynthesis and respiration in Anabaena and Scenedesmus. Biochim Biophys Acta. 1979 Apr 11;546(1):43–53. doi: 10.1016/0005-2728(79)90168-3. [DOI] [PubMed] [Google Scholar]
- van der Westen H. M., Mayhew S. G., Veeger C. Separation of hydrogenase from intact cells of Desulfovibrio vulgaris. Purification and properties. FEBS Lett. 1978 Feb 1;86(1):122–126. doi: 10.1016/0014-5793(78)80112-4. [DOI] [PubMed] [Google Scholar]



