Abstract
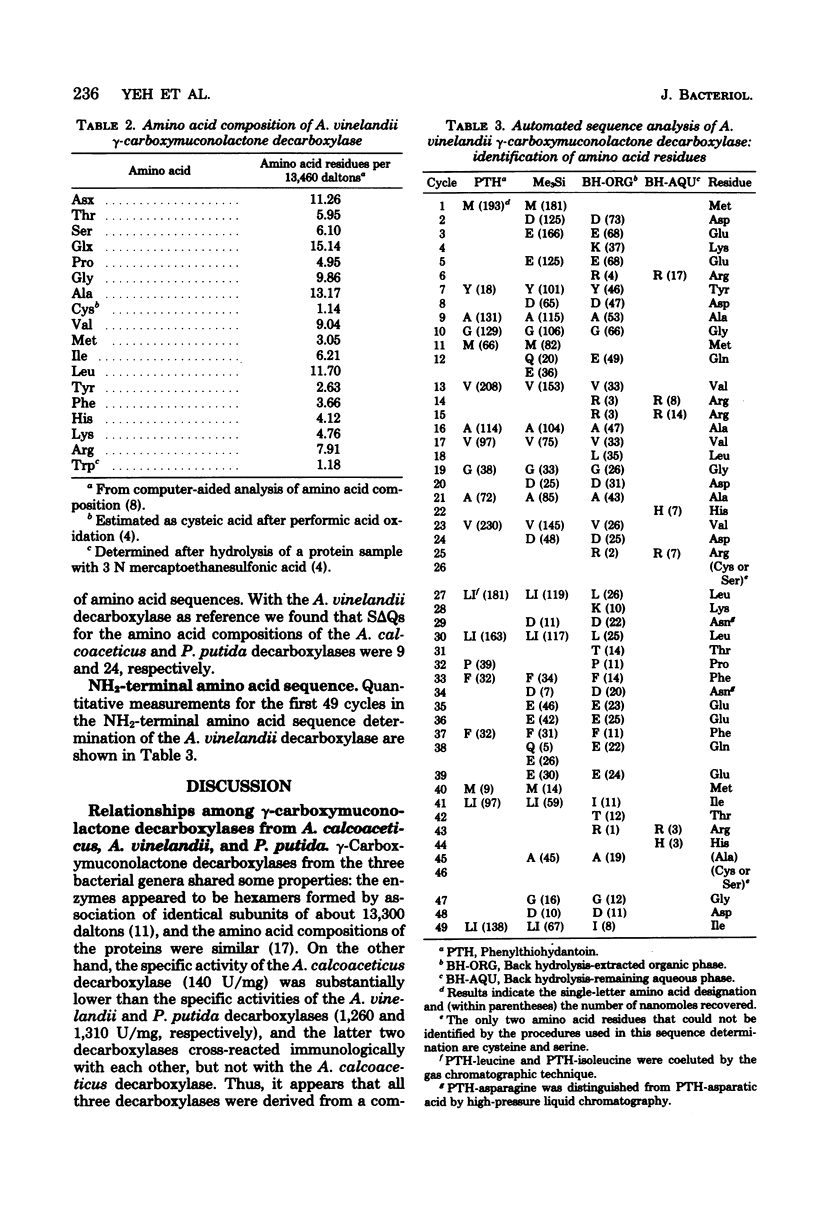
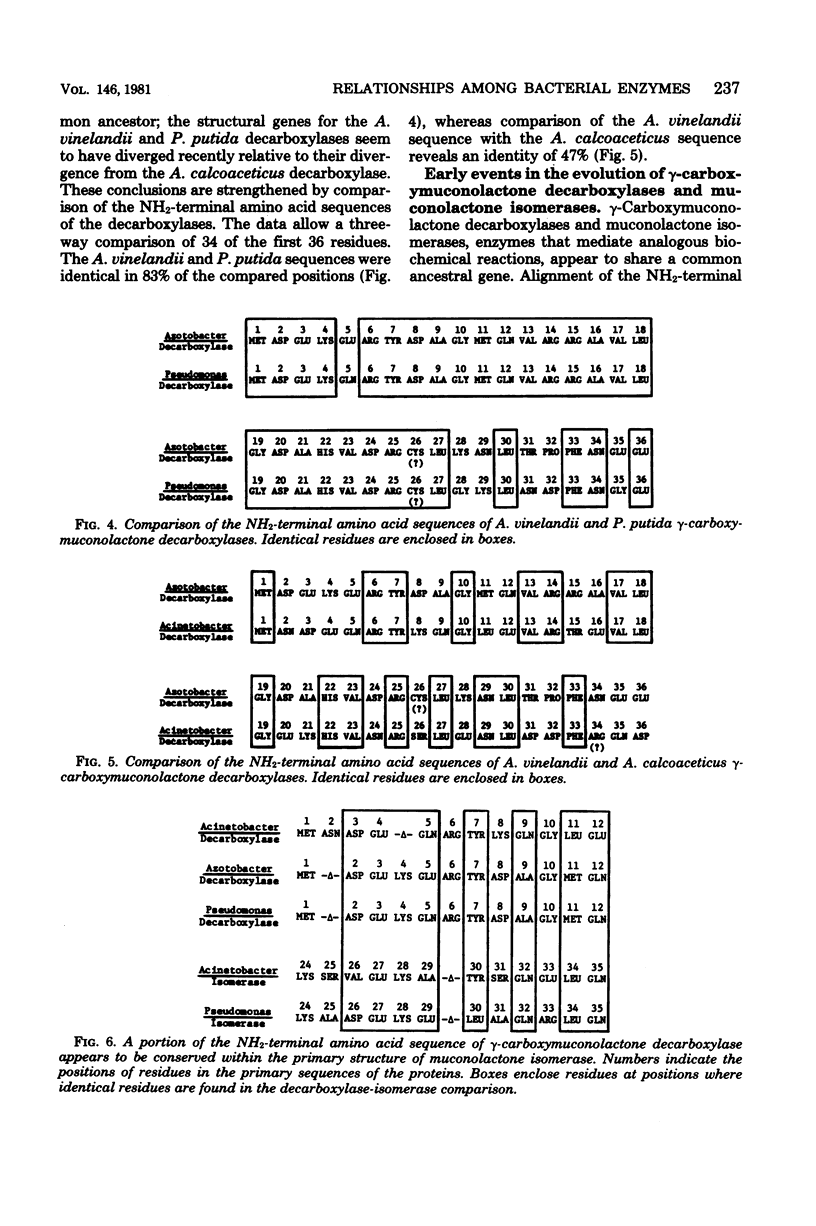
gamma-Carboxymuconolactone decarboxylase (EC 4.1.1.44) from Azotobacter vinelandii resembled the isofunctional enzymes from Acinetobacter calcoaceticus and Pseudomonas putida. All three decarboxylases appeared to be hexamers formed by association of identical subunits of about 13,300 daltons. The A. vinelandii and P. putida decarboxylases cross-reacted immunologically with each other, and the NH2-terminal amino acid sequences of the enzymes differed in no more than 7 of the first 36 residues. In contrast, the A. calcoaceticus decarboxylase did not cross-react with the decarboxylase from A. vinelandii or P. putida; the NH2-terminal amino acid sequences of these enzymes diverged about 50% from the NH2-terminal amino acid sequence of the A. calcoaceticus decarboxylase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Durham D. R., Ornston L. N. Homologous structural genes and similar induction patterns in Azotobacter spp. and Pseudomonas spp. J Bacteriol. 1980 Aug;143(2):834–840. doi: 10.1128/jb.143.2.834-840.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham D. R., Stirling L. A., Ornston L. N., Perry J. J. Intergeneric evolutionary homology revealed by the study of protocatechuate 3,4-dioxygenase from Azotobacter vinelandii. Biochemistry. 1980 Jan 8;19(1):149–155. doi: 10.1021/bi00542a023. [DOI] [PubMed] [Google Scholar]
- McCorkle G. M., Yeh W. K., Fletcher P., Ornston L. N. Repetitions in the NH2-terminal amino acid sequence of beta-ketoadipate enol-lactone hydrolase from Pseudomonas putida. J Biol Chem. 1980 Jul 10;255(13):6335–6341. [PubMed] [Google Scholar]
- OUCHTERLONY O. Antigen-antibody reactions in gels. IV. Types of reactions in coordinated systems of diffusion. Acta Pathol Microbiol Scand. 1953;32(2):230–240. [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ozawa K., Tanaka S. Computer-aided calculation of amino acid composition of proteins. Anal Biochem. 1968 Aug;24(2):270–280. doi: 10.1016/0003-2697(68)90180-2. [DOI] [PubMed] [Google Scholar]
- Parke D. Structural comparison of gamma-carboxymuconolactone decarboxylase and muconolactone isomerase from Pseudomonas putida. Biochim Biophys Acta. 1979 May 23;578(1):145–154. doi: 10.1016/0005-2795(79)90122-3. [DOI] [PubMed] [Google Scholar]
- Patel R. N., Meagher R. B., Ornston L. N. Relationships among enzymes of the beta-ketoadipate pathway. IV. Muconolactone isomerase from Acinetobacter calcoaceticus and Pseudomonas putida. J Biol Chem. 1974 Dec 10;249(23):7410–7419. [PubMed] [Google Scholar]
- Patel R. N., Orston L. N. Immunological comparison of enzymes of the beta-ketoadipate pathway. Arch Microbiol. 1976 Oct 11;110(1):27–36. doi: 10.1007/BF00416965. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Ornston L. N. The beta-ketoadipate pathway. Adv Microb Physiol. 1973;9(0):89–151. [PubMed] [Google Scholar]
- Stanier R. Y., Wachter D., Gasser C., Wilson A. C. Comparative immunological studies of two Pseudomonas enzymes. J Bacteriol. 1970 May;102(2):351–362. doi: 10.1128/jb.102.2.351-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yeh W. K., Davis G., Fletcher P., Ornston L. N. Homologous amino acid sequences in enzymes mediating sequential metabolic reactions. J Biol Chem. 1978 Jul 25;253(14):4920–4923. [PubMed] [Google Scholar]
- Yeh W. K., Fletcher P., Ornston N. Homologies in the NH2-terminal amino acid sequences of gamma-carboxymuconolactone decarboxylases and muconolactone isomerases. J Biol Chem. 1980 Jul 10;255(13):6347–6354. [PubMed] [Google Scholar]
- Yeh W. K., Ornston L. N. Origins of metabolic diversity: substitution of homologous sequences into genes for enzymes with different catalytic activities. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5365–5369. doi: 10.1073/pnas.77.9.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]