Abstract
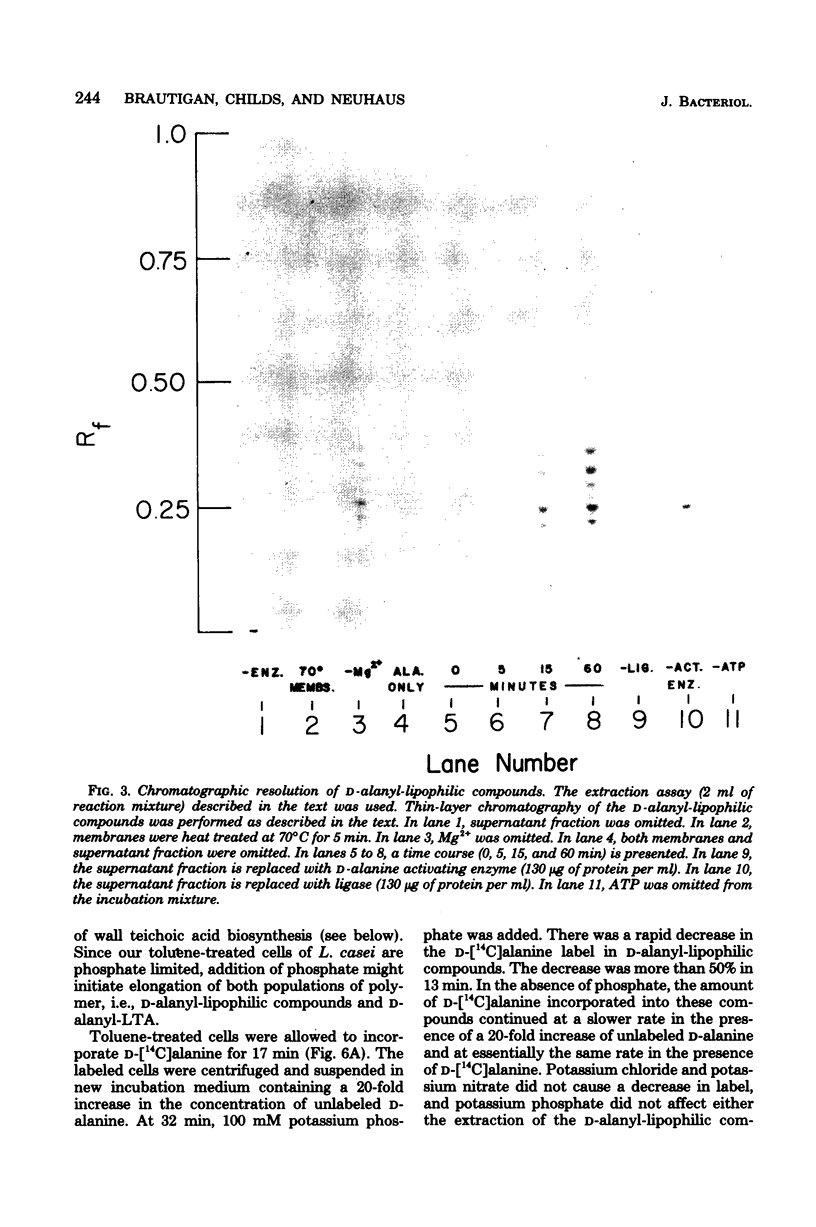
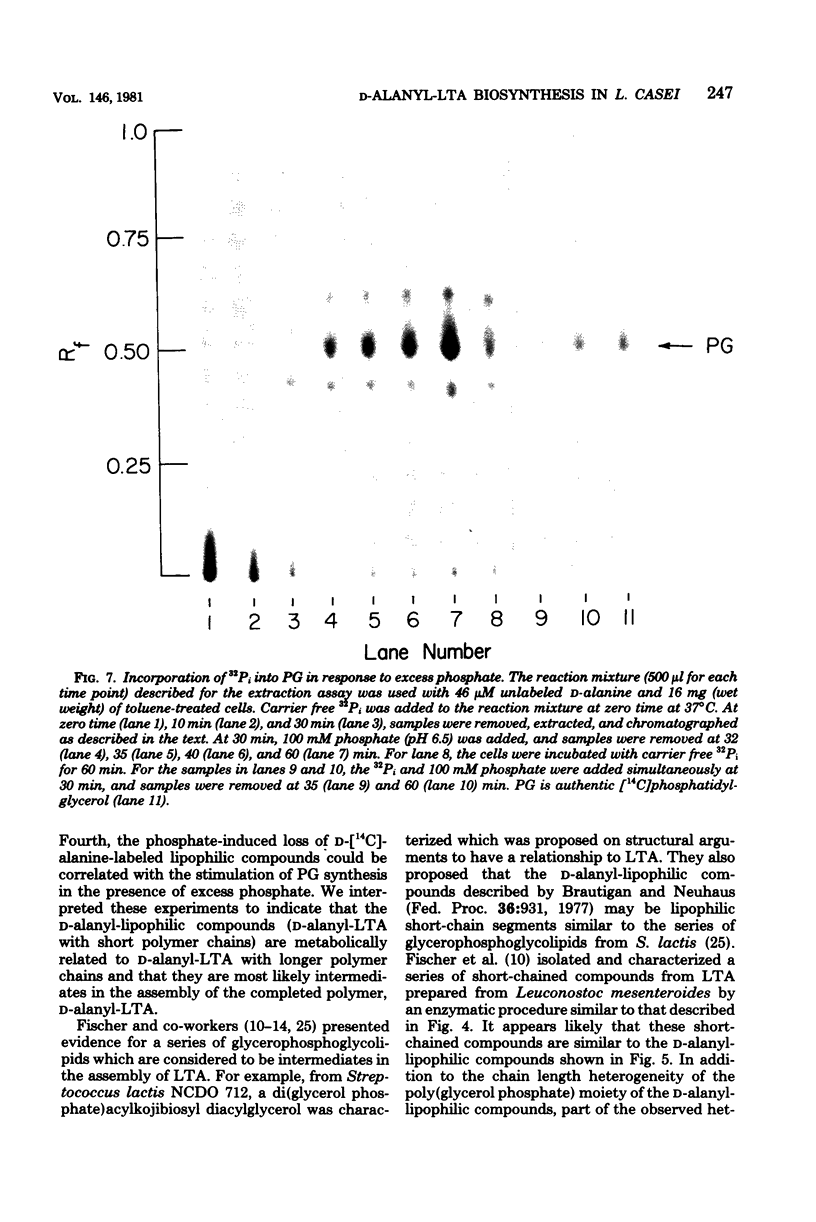
D-Alanyl-lipoteichoic acid (D-alanyl-LTA) from Lactobacillus casei contains a poly(glycerol phosphate) moiety that is selectively acylated with D-alanine ester residues. To characterize further the mechanism of D-alanine substitution, intermediates were sought that participate in the assembly of this LTA. From the incorporation system utilizing either toluene-treated cells or a combination of membrane fragments and supernatant fraction, a series of membrane-associated D-[14C]alanyl-lipophilic compounds was found. The assay of these compounds depended on their extractability into monophasic chloroform-methanol-water (0.8:3.2:1.0, vol/vol/vol) and subsequent partitioning into chloroform. Four lines of evidence suggested that the D-alanyl-lipophilic compounds are intermediates in the synthesis of D-alanyl-LTA. First, partial degradation of the poly(glycerol phosphate) moiety of D-alanyl-LTA by phosphodiesterase II/phosphatase from Aspergillus niger generated a series of D-alanyl-lipophilic compounds similar to those extracted from the toluene-treated cells during the incorporation of D-alanine. Second, enzymatic degradation of the D-alanyl-lipophilic compounds by the above procedure gave D-alanyl-glycerol, the same degradation product obtained from D-alanyl-LTA. Third, the incorporation of D-alanine into these compounds required the same components as the incorporation of D-alanine into membrane-associated D-alanyl-LTA. Fourth, the phosphate-induced loss of D-[14C]alanine-labeled lipophilic compounds could be correlated with the stimulation of phosphatidylglycerol synthesis in the presence of excess phosphate. We interpreted these experiments to indicate that the D-alanyl-lipophilic compounds are D-alanyl-LTA with short polymer chains and are most likely intermediates in the assembly of the completed polymer, D-alanyl-LTA.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADDILEY J., NEUHAUS F. C. The enzymic activation of D-alanine. Biochem J. 1960 Jun;75:579–587. doi: 10.1042/bj0750579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Ballesta J. P., De Garcia C. L., Schaechter M. Turnover of phosphatidylglycerol in Escherichia coli. J Bacteriol. 1973 Oct;116(1):210–214. doi: 10.1128/jb.116.1.210-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevers E. M., Singal S. A., Op den Kamp J. A., van Deenen L. L. Recognition of different pools of phosphatidylglycerol in intact cells and isolated membranes of Acholeplasma laidlawii by phospholipase A2. Biochemistry. 1977 Apr 5;16(7):1290–1295. doi: 10.1021/bi00626a008. [DOI] [PubMed] [Google Scholar]
- Childs W. C., 3rd, Neuhaus F. C. Biosynthesis of D-alanyl-lipoteichoic acid: characterization of ester-linked D-alanine in the in vitro-synthesized product. J Bacteriol. 1980 Jul;143(1):293–301. doi: 10.1128/jb.143.1.293-301.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdur L. I., Chiu T. H. Turnover of phosphatidylglycerol in Streptococcus sanguis. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1137–1144. doi: 10.1016/s0006-291x(74)80097-5. [DOI] [PubMed] [Google Scholar]
- Emdur L., Chiu T. The role of phosphatidylglycerol in the in vitro biosynthesis of teichoic acid and lipoteichoic acid. FEBS Lett. 1975 Jul 15;55(1):216–219. doi: 10.1016/0014-5793(75)80995-1. [DOI] [PubMed] [Google Scholar]
- Fischer W., Koch H. U., Rösel P., Fiedler F. Alanine ester-containing native lipoteichoic acids do not act as lipoteichoic acid carrier. Isolation, structural and functional characterization. J Biol Chem. 1980 May 25;255(10):4557–4562. [PubMed] [Google Scholar]
- Fischer W., Koch H. U., Rösel P., Fiedler F., Schmuck L. Structural requirements of lipoteichoic acid carrier for recognition by the poly(ribitol phosphate) polymerase from Staphylococcus aureus H. A study of various lipoteichoic acids, derivatives, and related compounds. J Biol Chem. 1980 May 25;255(10):4550–4556. [PubMed] [Google Scholar]
- Fischer W., Laine R. A., Nakano M. On the relationship between glycerophosphoglycolipids and lipoteichoic acids in Gram-positive bacteria. II. Structures of glycerophosphoglycolipids. Biochim Biophys Acta. 1978 Mar 30;528(3):298–308. doi: 10.1016/0005-2760(78)90019-x. [DOI] [PubMed] [Google Scholar]
- Fischer W., Schuster D., Laine R. A. Studies on the relationship between glycerophosphoglycolipids and lipoteichoic acids. IV. Trigalactosylglycerophospho-acylkojibiosyldiacylglycerol and related compounds from Streptococcus lactis Kiel 42172. Biochim Biophys Acta. 1979 Dec 18;575(3):389–398. doi: 10.1016/0005-2760(79)90108-5. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Wyrick P. B., Ward J. B., Rogers H. J. Effect of phosphate limitation on the morphology and wall composition of Bacillus licheniformis and its phosphoglucomutase-deficient mutants. J Bacteriol. 1973 Feb;113(2):969–984. doi: 10.1128/jb.113.2.969-984.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfield M. C., Pieringer R. A. The biosynthesis of nascent membrane lipoteichoic acid of Streptococcus faecium (S. faecalis ATCC 9790) from phosphatidylkojibiosyl diacylglycerol and phosphatidylglycerol. J Biol Chem. 1980 Jun 10;255(11):5164–5169. [PubMed] [Google Scholar]
- Glaser L., Lindsay B. The synthesis of lipoteichoic acid carrier. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1131–1136. doi: 10.1016/s0006-291x(74)80096-3. [DOI] [PubMed] [Google Scholar]
- Glaser L., Loewy A. Control of teichoic acid synthesis during phosphate limitation. J Bacteriol. 1979 Jan;137(1):327–331. doi: 10.1128/jb.137.1.327-331.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser L., Loewy A. Regulation of teichoic acid synthesis during phosphate limitation. J Biol Chem. 1979 Apr 10;254(7):2184–2186. [PubMed] [Google Scholar]
- Heptinstall S., Archibald A. R., Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970 Feb 7;225(5232):519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
- Hughes A. H., Hancock I. C., Baddiley J. The function of teichoic acids in cation control in bacterial membranes. Biochem J. 1973 Jan;132(1):83–93. doi: 10.1042/bj1320083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey H., Sueda S., Cheah S. C., Baddiley J. Control of teichoic acid synthesis in Bacillus licheniformis ATCC 9945. Eur J Biochem. 1978 Jan 2;82(1):169–174. doi: 10.1111/j.1432-1033.1978.tb12008.x. [DOI] [PubMed] [Google Scholar]
- IKAWA M. NATURE OF THE LIPIDS OF SOME LACTIC ACID BACTERIA. J Bacteriol. 1963 Apr;85:772–781. doi: 10.1128/jb.85.4.772-781.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koostra W. L., Smith P. F. D- and L-Alanylphosphatidylglycerols from Mycoplasma laidlawii, strain B. Biochemistry. 1969 Dec;8(12):4794–4806. doi: 10.1021/bi00840a022. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laine R. A., Fischer W. On the relationship between glycerophosphoglycolipids and lipoteichoic acids of gram-positive bacteria. III. Di(glycerophospho)-acylkojibiosyldiacylglycerol and related compounds from Streptococcus lactis NCDO 712. Biochim Biophys Acta. 1978 May 25;529(2):250–262. doi: 10.1016/0005-2760(78)90068-1. [DOI] [PubMed] [Google Scholar]
- Lambert P. A., Hancock I. C., Baddiley J. Influence of alanyl ester residues on the binding of magnesium ions to teichoic acids. Biochem J. 1975 Dec;151(3):671–676. doi: 10.1042/bj1510671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer R., Neuhaus F. C. Biosynthesis of membrane teichoic acid. A role of the D-alanine-activating enzyme. J Biol Chem. 1973 May 10;248(9):3196–3201. [PubMed] [Google Scholar]
- Lombardi F. J., Chen S. L., Fulco A. J. A rapidly metabolizing pool of phosphatidylglycerol as a precursor of phosphatidylethanolamine and diglyceride in Bacillus megaterium. J Bacteriol. 1980 Feb;141(2):626–634. doi: 10.1128/jb.141.2.626-634.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi F. J., Fulco A. J. Two distinct pools of membrane phosphatidylglycerol in Bacillus megaterium. J Bacteriol. 1980 Feb;141(2):618–625. doi: 10.1128/jb.141.2.618-625.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso D. J., Junker D. D., Hsu S. C., Chiu T. H. Biosynthesis of glycosylated glycerolphosphate polymers in Streptococcus sanguis. J Bacteriol. 1979 Nov;140(2):547–554. doi: 10.1128/jb.140.2.547-554.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnikin D. E., Abdolrahimzadeh H., Baddiley J. Variation of polar lipid composition of Bacillus subtilis (Marburg) with different growth conditions. FEBS Lett. 1972 Oct 15;27(1):16–18. doi: 10.1016/0014-5793(72)80398-3. [DOI] [PubMed] [Google Scholar]
- Nakano M., Fischer W. Trihexosyldiacylglycerol and acyltrihexosyldiacylglycerol as lipid anchors of the lipoteichoic acid of Lactobacillus casei DSM 20021. Hoppe Seylers Z Physiol Chem. 1978 Jan;359(1):1–11. doi: 10.1515/bchm.1978.359.1.1. [DOI] [PubMed] [Google Scholar]
- Neuhaus F. C., Linzer R., Reusch V. M., Jr Biosynthesis of membrane teichoic acid: role of the D-alanine-activating enzyme and D-alanine: membrane acceptor ligase. Ann N Y Acad Sci. 1974 May 10;235(0):502–518. doi: 10.1111/j.1749-6632.1974.tb43287.x. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Reusch V. M., Jr, Neuhaus F. C. D-Alanine: membrane acceptor ligase from Lactobacillus casei. J Biol Chem. 1971 Oct 25;246(20):6136–6143. [PubMed] [Google Scholar]
- Rosenberger R. F. Control of teichoic and teichuronic acid biosynthesis in Bacillus subtilis 168trp. Evidence for repression of enzyme synthesis and inhibition of enzyme activity. Biochim Biophys Acta. 1976 Apr 23;428(2):516–524. doi: 10.1016/0304-4165(76)90060-x. [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Kennedy E. P. A novel phosphodiesterase from Aspergillus niger and its application to the study of membrane-derived oligosaccharides and other glycerol-containing biopolymers. J Biol Chem. 1978 Nov 10;253(21):7738–7743. [PubMed] [Google Scholar]
- Short S. A., White D. C. Metabolism of phosphatidylglycerol, lysylphosphatidylglycerol, and cardiolipin of Staphylococcus aureus. J Bacteriol. 1971 Oct;108(1):219–226. doi: 10.1128/jb.108.1.219-226.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Detection of a rapidly metabolizing portion of the membrane cardiolipin in Haemophilus parainfluenzae. J Bacteriol. 1971 Dec;108(3):1058–1064. doi: 10.1128/jb.108.3.1058-1064.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]






