Abstract
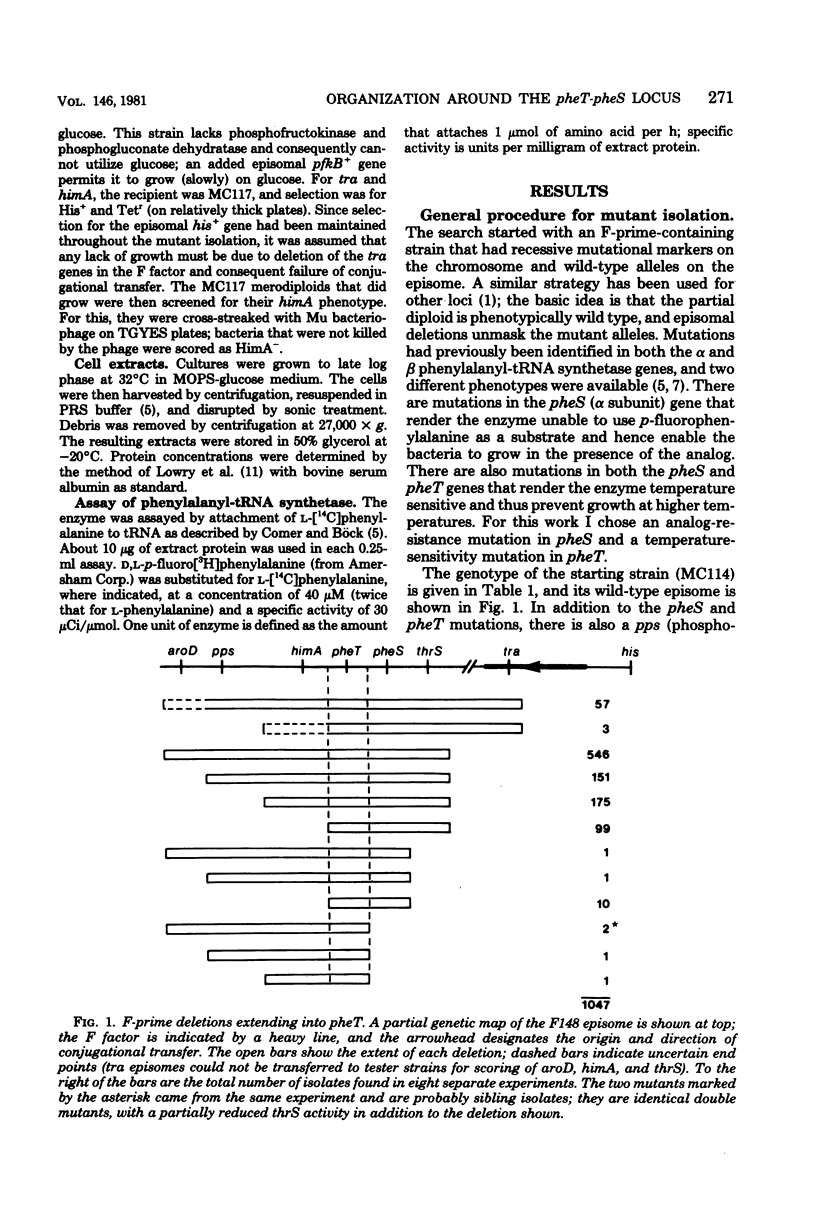
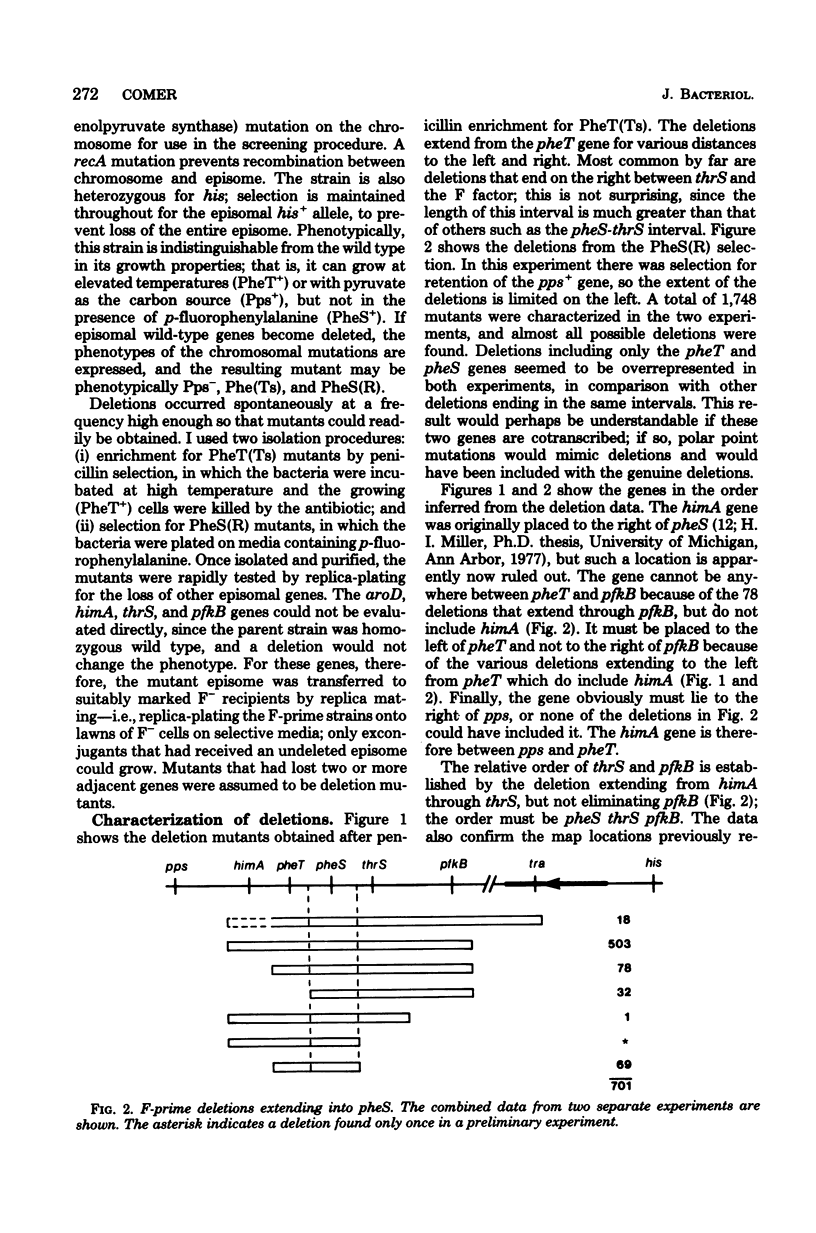
The organization of seven genes located at about 38 min on the genetic map of Escherichia coli was examined; these genes included pheS and pheT, which code for the alpha and beta subunits of phenylalanyl-transfer ribonucleic acid synthetase, and thrS, the structural gene for threonyl-transfer ribonucleic acid synthetase. Deletion mutants were isolated from an F-prime-containing merodiploid strain and were characterized genetically. Seventeen different kinds of deletions extending into pheS of pheT were identified. These deletions unambiguously defined the gene order as aroD pps himA pheT pheS thrS pfkB. Mutants with deletions covering either pheS or pheT, but not both, were analyzed further by assay of phenylalanyl-transfer ribonucleic acid synthetase. The phenotype of the mutants with a deletion from pfkB through pheS was anomalous; although the pheT gene was apparently still present, its product, the beta subunit, was much reduced in activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Comer M. M., Böck A. Genes for the alpha and beta subunits of the phenylalanyl-transfer ribonucleic acid synthetase of Escherichia coli. J Bacteriol. 1976 Aug;127(2):923–933. doi: 10.1128/jb.127.2.923-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H., Böck A. Altered alpha subunits in phenylalanyl-tRNA synthetases from p-fluorophenylalanine-resistant strains of Escherichis coli. Eur J Biochem. 1975 Jul 1;55(2):431–437. doi: 10.1111/j.1432-1033.1975.tb02179.x. [DOI] [PubMed] [Google Scholar]
- Hennecke H., Böck A., Thomale J., Nass G. Threonyl-transfer ribonucleic acid synthetase from Escherichia coli: subunit structure and genetic analysis of the structural gene by means of a mutated enzyme and of a specialized transducing lambda bacteriophage. J Bacteriol. 1977 Sep;131(3):943–950. doi: 10.1128/jb.131.3.943-950.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H. Use of mutant enzymes to demonstrate the presence of two active sites on phenylalanyl-tRNA synthetase from Eschericia coli. FEBS Lett. 1976 Dec 15;72(1):182–186. doi: 10.1016/0014-5793(76)80840-x. [DOI] [PubMed] [Google Scholar]
- Johnson E. J., Cohen G. N., Saint-Girons I. Threonyl-transfer ribonucleic acid synthetase and the regulation of the threonine operon in Escherichia coli. J Bacteriol. 1977 Jan;129(1):66–70. doi: 10.1128/jb.129.1.66-70.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. I., Kikuchi A., Nash H. A., Weisberg R. A., Friedman D. I. Site-specific recombination of bacteriophage lambda: the role of host gene products. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1121–1126. doi: 10.1101/sqb.1979.043.01.125. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Pedersen S., Reeh S. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J Bacteriol. 1977 Jan;129(1):378–387. doi: 10.1128/jb.129.1.378-387.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neihardt F. C., Parker J., McKeever W. G. Function and regulation of aminoacyl-tRNA synthetases in prokaryotic and eukaryotic cells. Annu Rev Microbiol. 1975;29:215–250. doi: 10.1146/annurev.mi.29.100175.001243. [DOI] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Girons I. New regulatory mutations affecting the expression of the threonine operon in Escherichia coli K-12. Mol Gen Genet. 1978 Jun 1;162(1):95–100. doi: 10.1007/BF00333855. [DOI] [PubMed] [Google Scholar]
- Springer M., Graffe M., Grunberg-Manago M. Genetic organization of the E. coli chromosome around the structural gene for initiation factor IF3 (infC). Mol Gen Genet. 1979 Feb 1;169(3):337–343. doi: 10.1007/BF00382279. [DOI] [PubMed] [Google Scholar]
- Springer M., Graffe M., Hennecke H. Specialized transducing phage for the initiation factor 3 gene in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3970–3974. doi: 10.1073/pnas.74.9.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinopal R. T., Fraenkel D. G. PfkB and pfkC loci of Escherichia coli. J Bacteriol. 1975 Jun;122(3):1153–1161. doi: 10.1128/jb.122.3.1153-1161.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



