Abstract
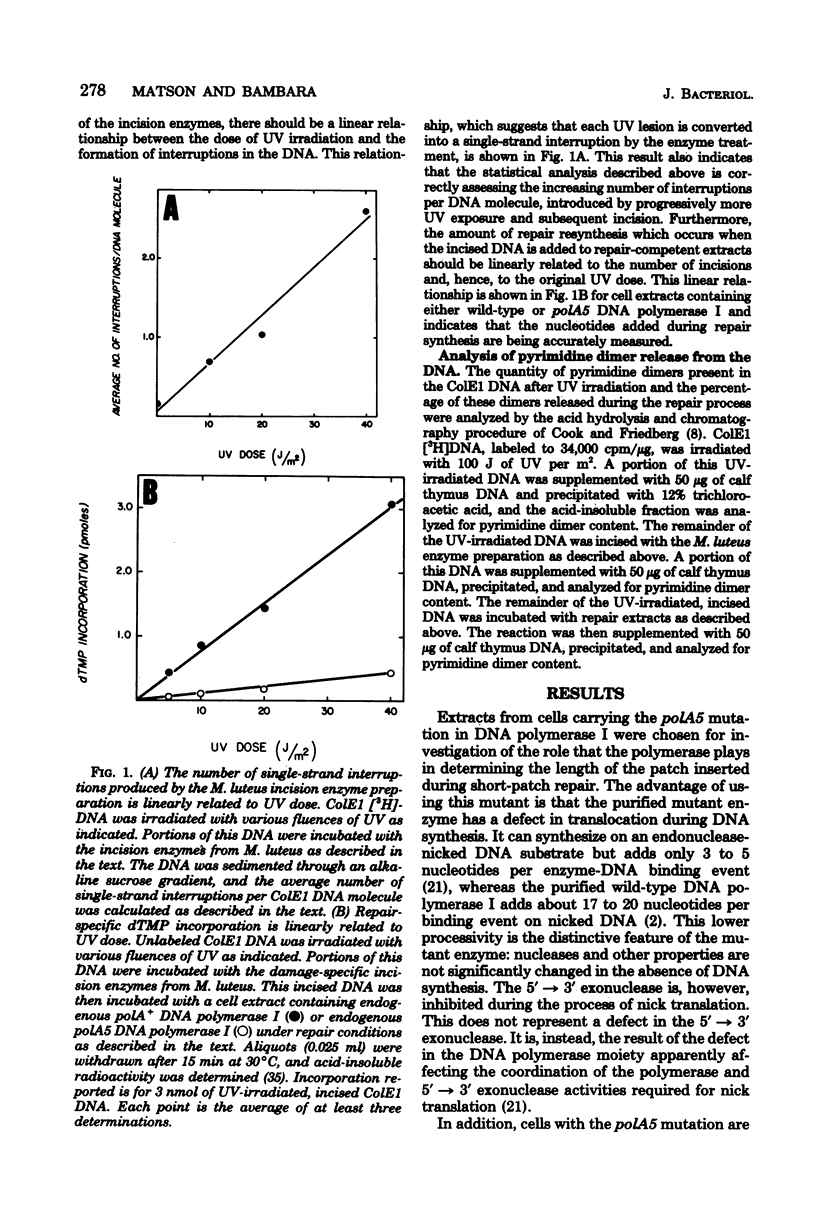
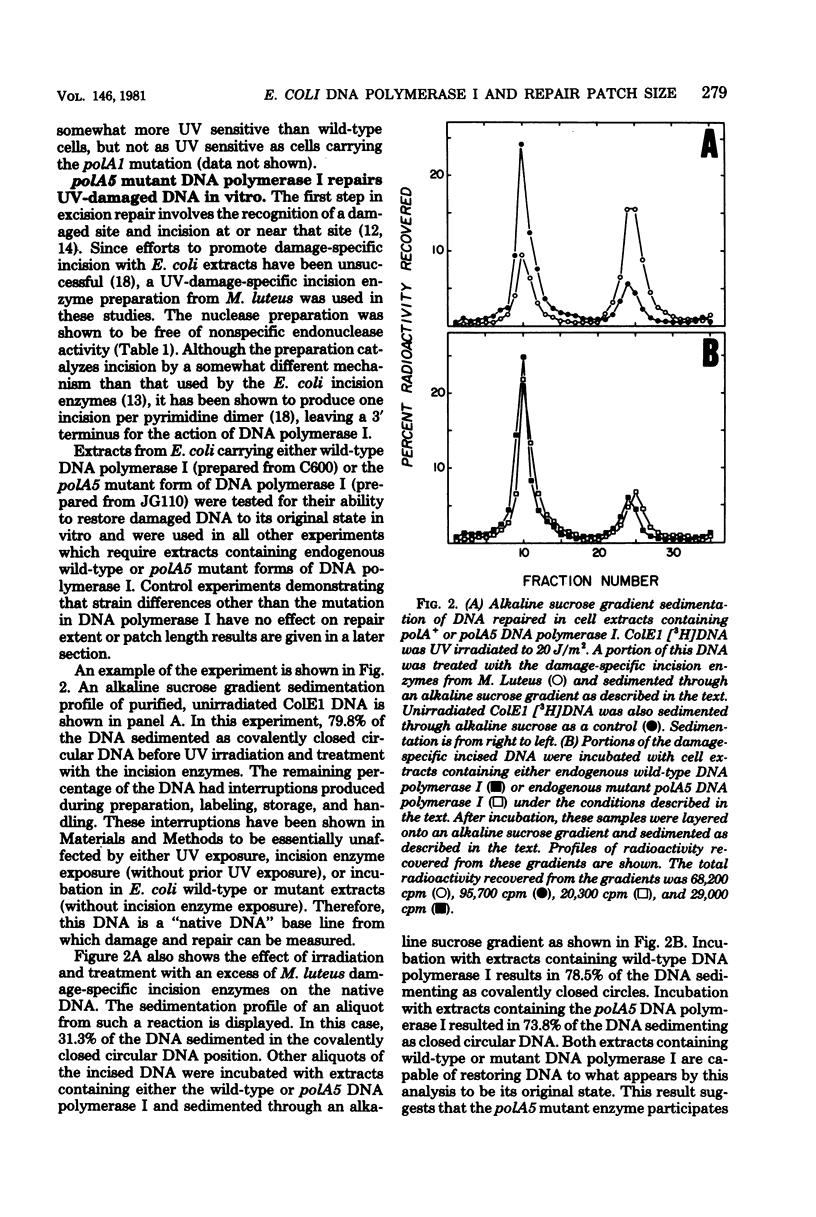
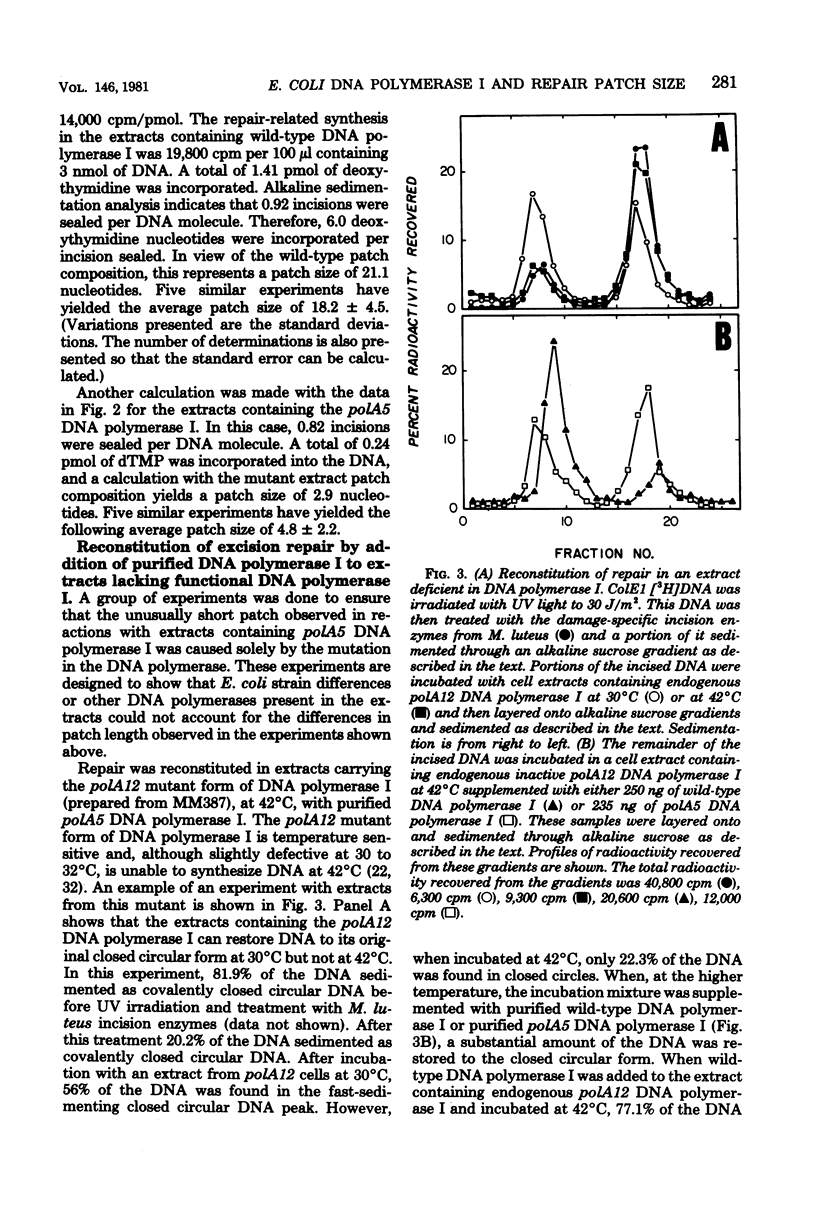
The lengths of ultraviolet irradiation-induced repair resynthesis patches were measured in repair-competent extracts of Escherichia coli. Extracts containing wild-type deoxyribonucleic acid (DNA) polymerase I introduced a patch 15 to 20 nucleotides in length during repair of ColE1 plasmid DNA; extracts containing the polA5 mutant form of DNA polymerase I introduced a patch only about 5 nucleotides in length in a similar reaction. The repair patch length in the presence of either DNA polymerase corresponded to the processivity of that polymerase (the average number of nucleotides added per enzyme-DNA binding event) as determined with purified enzymes and DNA treated with a nonspecific endonuclease. The base composition of the repair patch inserted by the wild-type DNA polymerase was similar to that of the bacterial genome, whereas the patch inserted by the mutant enzyme was skewed toward greater pyrimidine incorporation. This skewing is expected, considering the predominance of pyrimidine incorporation occurring at the ultraviolet lesion and the short patch made by the mutant enzyme. Since the defect in the polA5 DNA polymerase which causes premature dissociation from DNA is reflected exactly in the repair patch length, the processive mechanism of the polymerase must be a central determinant of patch length.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambara R. A., Uyemura D., Choi T. On the processive mechanism of Escherichia coli DNA polymerase I. Quantitative assessment of processivity. J Biol Chem. 1978 Jan 25;253(2):413–423. [PubMed] [Google Scholar]
- Ben-Ishai R., Sharon R. Patch size and base composition of ultraviolet light-induced repair synthesis in toluenized Escherichia coli. J Mol Biol. 1978 Apr 15;120(3):423–432. doi: 10.1016/0022-2836(78)90428-x. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Sherratt D. J., Clewell D. B., Helinski D. R. Isolation of supercoiled colicinogenic factor E 1 DNA sensitive to ribonuclease and alkali. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2518–2522. doi: 10.1073/pnas.69.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Endonuclease from Micrococcus luteus which has activity toward ultraviolet-irradiated deoxyribonucleic acid: purification and properties. J Bacteriol. 1970 Apr;102(1):178–186. doi: 10.1128/jb.102.1.178-186.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. H., Friedberg E. C. Measurement of thymine dimers in DNA by thin-layer chromatography. II. The use of one-dimensional systems. Anal Biochem. 1976 Jun;73(2):411–418. doi: 10.1016/0003-2697(76)90188-3. [DOI] [PubMed] [Google Scholar]
- Cooper P. K., Hanawalt P. C. Heterogeneity of patch size in repair replicated DNA in Escherichia coli. J Mol Biol. 1972 Jun 14;67(1):1–10. doi: 10.1016/0022-2836(72)90381-6. [DOI] [PubMed] [Google Scholar]
- Cooper P. K., Hanawalt P. C. Role of DNA polymerase I and the rec system in excision-repair in Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1156–1160. doi: 10.1073/pnas.69.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch W. A., Dorson J. W., Moses R. E. Excision of pyrimidine dimers in toluene-treated Escherichia coli. J Bacteriol. 1976 Jan;125(1):220–224. doi: 10.1128/jb.125.1.220-224.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman L., Braun A., Feldberg R., Mahler I. Enzymatic repair of DNA. Annu Rev Biochem. 1975;44:19–43. doi: 10.1146/annurev.bi.44.070175.000315. [DOI] [PubMed] [Google Scholar]
- Grossman L., Riazuddin S., Haseltine W. A., Lindan C. Nucleotide excision repair of damaged DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):947–955. doi: 10.1101/sqb.1979.043.01.104. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Ganesan A. K., Smith C. A. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- Ley R. D., Setlow R. B. Repair replication in Escherichia coli as measured by the photolysis of bromodeoxyuridine. Biophys J. 1972 Apr;12(4):420–431. doi: 10.1016/S0006-3495(72)86094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W. E. Deoxyribonucleic acid repair in vitro by extracts of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1415–1423. doi: 10.1128/jb.129.3.1415-1423.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W. E., Hanawalt P. C. Ultraviolet-stimulated DNA synthesis in toluenzied Escherichia coli deficient in DNA polymerase I. Proc Natl Acad Sci U S A. 1973 Jan;70(1):129–133. doi: 10.1073/pnas.70.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W. E. The ATP dependence of the incision and resynthesis steps of excision repair. Biochim Biophys Acta. 1976 Aug 18;442(2):162–173. doi: 10.1016/0005-2787(76)90487-1. [DOI] [PubMed] [Google Scholar]
- Masker W., Hanawalt P., Shizuya H. Role of DNA polymerase II in repair replication in Escherichia coli. Nat New Biol. 1973 Aug 22;244(138):242–243. doi: 10.1038/newbio244242a0. [DOI] [PubMed] [Google Scholar]
- Matson S. W., Capaldo-Kimball F. N., Bambara R. A. On the processive mechanism of Escherichia coli DNA Polymerase I. The polA5 mutation. J Biol Chem. 1978 Nov 10;253(21):7851–7856. [PubMed] [Google Scholar]
- Monk M., Kinross J. Conditional lethality of recA and recB derivatives of a strain of Escherichia coli K-12 with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1972 Mar;109(3):971–978. doi: 10.1128/jb.109.3.971-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses R. E., Moody E. E. DNA repair synthesis dependent on the uvrA,B gene products in toluene-treated cells. J Biol Chem. 1975 Oct 25;250(20):8055–8061. [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Tomizawa J. I. Replication of colicin E1 plasmid DNA in cell extracts. Proc Natl Acad Sci U S A. 1974 Mar;71(3):802–806. doi: 10.1073/pnas.71.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Strike P. Excision repair of ultraviolet-irradiated deoxyribonucleic acid in plasmolyzed cells of Escherichia coli. J Bacteriol. 1976 Mar;125(3):787–795. doi: 10.1128/jb.125.3.787-795.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L., Bollum F. J. Pyrimidine dimers in UV-irradiated poly dI:dC. Proc Natl Acad Sci U S A. 1965 May;53(5):1111–1118. doi: 10.1073/pnas.53.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon R., Miller C., Ben-Ishai R. Two modes of excision repair in toluene-treated Escherichia coli. J Bacteriol. 1975 Sep;123(3):1107–1114. doi: 10.1128/jb.123.3.1107-1114.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait R. C., Harris A. L., Smith D. W. DNA repair in Escherichia coli mutants deficient in DNA polymerases I, II and-or 3. Proc Natl Acad Sci U S A. 1974 Mar;71(3):675–679. doi: 10.1073/pnas.71.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglini E. C., Mildvan A. S., Loeb L. A. Kinetic analysis of Escherichia coli deoxyribonucleic acid polymerase I. J Biol Chem. 1975 Nov 25;250(22):8647–8656. [PubMed] [Google Scholar]
- Uyemura D., Lehman I. R. Biochemical characterization of mutant forms of DNA polymerase I from Escherichia coli. I. The polA12 mutation. J Biol Chem. 1976 Jul 10;251(13):4078–4084. [PubMed] [Google Scholar]
- Waldstein E. A., Sharon R., Ben-Ishai R. Role of ATP in excision repair of ultraviolet radiation damage in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2651–2654. doi: 10.1073/pnas.71.7.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Padmanabhan R., Bambara R. Nucleotide sequence analysis of bacteriophage DNA. Methods Enzymol. 1974;29:231–253. doi: 10.1016/0076-6879(74)29025-6. [DOI] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Evidence for the control by exrA and polA genes of two branches of the uvr gene-dependent excision repair pathway in Escherichia coli K-12. J Bacteriol. 1973 Oct;116(1):175–182. doi: 10.1128/jb.116.1.175-182.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Involvement of DNA polymerase 3 in excision repair after ultraviolet irradiation. Nat New Biol. 1973 Aug 22;244(138):240–241. doi: 10.1038/newbio244240a0. [DOI] [PubMed] [Google Scholar]



