Abstract
During wakefulness, obstructive sleep apnoea patients appear to compensate for an anatomically narrow upper airway by increasing upper airway dilator muscle activity, e.g. genioglossus, at least partly via a negative-pressure reflex that may be diminished in sleep. Previous studies have assessed the negative-pressure reflex using multi-unit, rectified, moving-time-average EMG recordings during brief pulses of negative upper-airway pressure. However, moving-time averaging probably obscures the true time-related reflex morphology, potentially masking transient excitatory and inhibitory components. This study aimed to re-examine the genioglossus negative-pressure reflex in detail, without moving-time averaging. Bipolar fine-wire electrodes were inserted per orally into the genioglossus muscle in 17 healthy subjects. Two upper airway pressure catheters were inserted per nasally. Genioglossus EMG reflex responses were generated via negative-pressure stimuli (∼−10 cmH2O at the choanae, 250 ms duration) delivered during wakefulness and sleep. Ensemble-averaged, rectified, genioglossus EMG recordings demonstrated reflex activation (onset latency 26 ± 1 ms; peak amplitude 231 ± 29% of baseline) followed by a previously unreported suppression (peak latency 71 ± 4 ms; 67 ± 8% of baseline). Single-motor-unit activity, clearly identifiable in ∼10% of trials in six subjects, showed a concomitant increase in the interspike interval from baseline (26 ± 9 ms, P = 0.01). Genioglossus negative-pressure reflex morphology and amplitude of the initial peak were maintained in non-rapid eye movement (NREM) sleep but suppression amplitude was more pronounced during NREM and declined further during REM sleep compared to wakefulness. These data indicate there are both excitatory and inhibitory components to the genioglossus negative-pressure reflex which are differentially affected by state.
There are several protective respiratory reflexes that are activated during acute periods of increased respiratory load or airway occlusion. The genioglossus (gg) is the largest upper-airway dilator muscle and has been shown in animals and humans to be reflexively activated in response to negative upper-airway pressure to oppose upper-airway collapse (Mathew et al. 1982a,Mathew 1982b; Horner et al. 1991b). Our current understanding of the basic reflex derived from multi-unit recordings suggests that pressure-sensitive nerve endings in the pharyngeal airway respond to rapid changes in negative upper-airway pressure. Sensory information is relayed via the superior laryngeal nerve and arrives at the nucleus tractus solitarius. The motor output to the muscle originates at the nearby hypoglossal motor nucleus and is relayed to the muscle via the hypoglossal nerve (Mathew, 1984; Horner et al. 1991a; Ryan et al. 2001; White, 2005). In obstructive sleep apnoea, the pharyngeal airspace posterior to the gg is a common site for upper-airway collapse during sleep. Previous studies suggest that the gg negative-pressure reflex is largely attenuated during sleep, thereby contributing to the development of sleep-disordered breathing in individuals with an anatomically narrow upper airway (Wheatley et al. 1993; Horner et al. 1994; Shea et al. 1999). However, recent data demonstrating maintenance of, and in many individuals increased reflex activity to negative-pressure pulse stimuli in the supine position during non-rapid eye movement (NREM) sleep, suggest that gg reflex attenuation may be position dependent (Malhotra et al. 2004).
Early quantification of the gg negative-pressure reflex in humans was performed using bipolar intraoral surface electrodes (Horner et al. 1991b; Horner 1994). In these studies, data were expressed as a moving-time-average (10 ms) of the rectified, ensemble-averaged, electromyogram (EMG) to relatively few negative-pressure pulse stimuli (n = 6). Surface EMG recordings have a relatively poor signal-to-noise ratio compared with intramuscular recordings (Bouisset & Maton, 1972; Perry et al. 1981) and the use of sliding-window averages distorts the details of the electromyographic response such as the relationship between excitatory and inhibitory responses, and particularly their latencies. Thus, elucidation of the precise morphology of the gg negative-pressure reflex was not possible from these early studies.
Other studies of the gg negative-pressure reflex have used intramuscular electrodes and increased the number of trials to improve the signal-to-noise ratio (Wheatley et al. 1993; Shea et al. 1999; Shea et al. 2000; Malhotra et al. 2004). However, in these studies, reflex characteristics were examined using even longer moving-time averaging windows (50–100 ms) of the rectified EMG. While moving-time averaging techniques are convenient for quantifying tonic and phasic respiratory muscle activity under the relatively slow time-constant conditions of tidal breathing, they inevitably blunt and distort more rapid respiratory reflex responses. Transient short-latency excitatory and/or inhibitory components of the negative-pressure reflex are likely to be beyond the resolution of these techniques. Thus, this study aimed to investigate the characteristics of the gg negative-pressure reflex using more sensitive neurophysiological techniques.
Methods
Subject selection
Eighteen healthy, young, non-smoking males, with no history of respiratory disease, sleep-disordered breathing, or regular medication use, and with baseline forced expiratory volume in 1s (FEV1) and forced vital capacity (FVC) > 80% of predicted gave informed written consent to participate in the study. The study conformed to the standards set by the Declaration of Helsinki and was approved by the Daw Park Repatriation General Hospital and Adelaide University Human Research and Ethics Committees.
Measurements and equipment
Subjects were instrumented with electroencephalograms (C3 and C4 referenced to linked ears), left and right electrooculograms and submental EMG for sleep staging and arousal scoring. The nostrils were decongested with xylometazoline hydrochloride nasal spray (Otrivin, Novartis Australasia, Rowville, Victoria, Australia) and anaesthetized (2% lignocaine). Two custom-made air-perfused catheters (see Jordan et al. (2003) for further detail) were then inserted via the more patent nostril and attached to pressure transducers (MP45, Validyne Engineering, Northridge, CA, USA). One catheter was advanced 1–2 cm below the base of the tongue under direct visualization (Pepi) and the other to the level of the choanae (Pcho). Two fine-wire Teflon-coated intramuscular electrodes (316SS3T wire, Medwire, Mt Vernon, NY, USA) were inserted after surface anaesthesia (4% lignocaine) 4 mm either side of the frenulum to a depth of approximately 1–1.5 cm to measure gg EMG activity (EMGgg). These procedures were similar to those previously described (Mezzanotte et al. 1992; Eastwood et al. 2003). Subjects were fitted with a nasal mask (Gel mask, Respironics, Murrysville, PA, USA) with a two-way non-rebreathing valve attached (series 2600, Hans Rudolph, Kansas City, MO, USA). An additional pressure transducer was fitted to the mask to measure mask pressure (Pmask). A pneumotachograph (PT36, Erich Jaeger, Germany) in series was used to monitor inspiratory flow. Upper airway negative-pressure pulses (Pcho∼−10 cmH2O, 250 ms duration) were delivered during early inspiration via a computer-controlled rapid-actuating solenoid valve system (Iso star, SXE9575-A70-00, Norgren, Switzerland). One arm of the solenoid was connected to a negative-pressure reservoir evacuated to ∼−100 cmH2O via hospital suction, the other to air at atmospheric pressure. A pressure-limiting valve on the patient side of the solenoid valve was adjusted to achieve a Pcho pulse pressure of ∼ 10 cmH2O during wakefulness. The breathing circuit was similar to that previously described (Horner et al. 1991b). Negative-pressure pulse delivery was controlled via custom-written software that continuously monitored the inspiratory flow signal and triggered solenoid valve switching during early inspiration when flow reached 2 l min−1. Pulses were delivered at random during stable breathing every 2–10 breaths. Figure 1B displays an example tracing of the inspiratory flow profile during negative pressure pulse delivery.
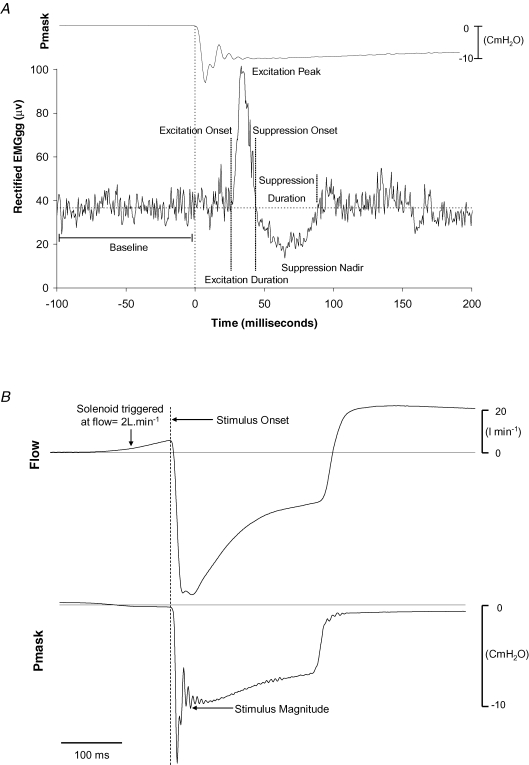
Figure 1.
EMGgg negative-Pressure retlex quantification procedures and stimulus characteristics. A, EMGgg reflex morphology and reflex quantification procedures. Ensemble-average of the rectified EMGgg, showing the reflex response of the genioglossus muscle and mask pressure tracing (Pmask) to 70 negative-pressure pulses in a representative subject. B, the ensemble-average flow and mask pressure tracings to negative pressure-pulse stimuli in a representative subject. The vertical dashed line at time zero corresponding to the first rapid dip in mask pressure (Pmask) was used to define stimulus onset. Note that the pressure pulse stimuli were delivered in early inspiration (flow tracing) and the point at which stimulus magnitude was quantified (Pmask tracing); refer to the text for further detail.
Data were acquired simultaneously on two separate recording systems. The first system (Compumedics E series, Abbotsford, Victoria, Australia) was used to determine sleep stage and to score arousals. All other data were acquired using the Windaq data acquisition system (DI-720 DATAQ Instruments Inc, OH, USA). In order to capture high-frequency reflex components and synchronize key stimulus magnitude parameters for event-related analysis, inspiratory flow, EMGgg and pressure channels were sampled at 2 kHz and filtered at 30–1000 Hz. The remaining channels that were not directly used for reflex-related synchronization purposes were sampled at 200 Hz. An event mark was simultaneously placed on both recording systems coincident with solenoid activation of each pulse, allowing both data acquisition systems to be accurately linked in time.
Protocol
Preliminary visit
Subjects attended a preliminary visit during the day for familiarization with the testing environment, recording equipment and staff, and to provide informed consent. Spirometry was performed to ensure normal lung function (JLab software version 4.53; Compactlab, Jaeger, Wuerzburg, Germany).
Main experimental visit
Subjects arrived at the laboratory 2.5 h before their usual bedtime after abstaining from alcohol and caffeine for at least 12 h. Once all the recording equipment was applied, several negative-pressure pulses were delivered to familiarize subjects with the experimental intervention, after which the lights were switched off and subjects were allowed to sleep. During the night, in the event that the subject became uncomfortable maintaining the supine posture, they were given the opportunity to stretch before returning to sleep on their back.
After at least 20 min of stable, stage 2 NREM sleep, upper-airway negative-pressure pulses were delivered every 2–10 breaths. In the event of an arousal from sleep, pulses were not resumed until there was at least 1 min of arousal-free sleep. After subjects awoke the following morning, a total of approximately 60 pulses were delivered every 2–10 breaths to elicit EMGgg reflex responses during wakefulness.
Data analysis
A single trained sleep technician, blinded to the experimental manipulations, defined the presence of arousals and sleep stage according to standard criteria (Rechtschaffen & Kales, 1968; American Sleep Disorders Association, 1992). Custom-designed software to detect the most rapid change in Pmask during pulse presentation was employed to align each individual pulse to an accurately identifiable and highly reproducible reference point for EMGgg event-related analyses. Briefly, on breaths preidentified as having had a negative-pressure pulse presented, the software identified the point in Pmask at which the change in pressure with respect to time (i.e. slope) was most negative. This point was then used to time-align all replicate pulses for ensemble averaging. Stimulus onset (time zero) was defined in the conventional manner as the last point preceding the sudden decrement in the ensemble-averaged Pmask following solenoid activation (Fig. 1). Negative-pressure pulse stimulus magnitude was calculated as the minimum pressure after the initial ‘ringing’ observed in the pressure channels (Fig. 1B) as previously described (Horner et al. 1994). Stimulus onset and rise times were quantified as the time from breath onset to the first sudden deflection in Pmask, and from this deflection point to the nadir of Pmask, respectively.
For each subject, all EMGgg trials free from movement artifact and arousal were grouped and ensemble-averaged according to sleep state. The various sleep states examined were (1) wakefulness; (2) stage 2 NREM sleep; (3) stages 3 and 4 NREM sleep combined (slow wave sleep (SWS)); (4) stages 2, 3 and 4 NREM sleep (combined NREM sleep); and (5) rapid eye movement sleep (REM). Raw EMGgg recordings were rectified without moving-time averaging for each subject. Individual subjects' ensemble-averaged, rectified EMGgg reflex responses were visually inspected to identify, using custom-designed semi-automated software, the presence, timing and amplitude of each positive and negative component of the EMGgg response. An example of a typical EMGgg reflex response and the criteria used to define the various reflex characteristics are displayed in Fig. 1. EMGgg reflex amplitude data were expressed as a percentage of baseline calculated as the average EMGgg activity for the 100 ms preceding pulse onset as previously described (Butler et al. 1997; Jeffery et al. 2006). Excitation onset was defined as the point at which the rectified EMGgg signal crossed baseline prior to the clearly defined positive EMGgg waveform. Suppression onset was defined as the first point at which the rectified EMGgg recording crossed the baseline level following the peak of the excitation response. The first point at which the rectified EMGgg returned to baseline levels after the suppression nadir was used to define the cessation of suppression.
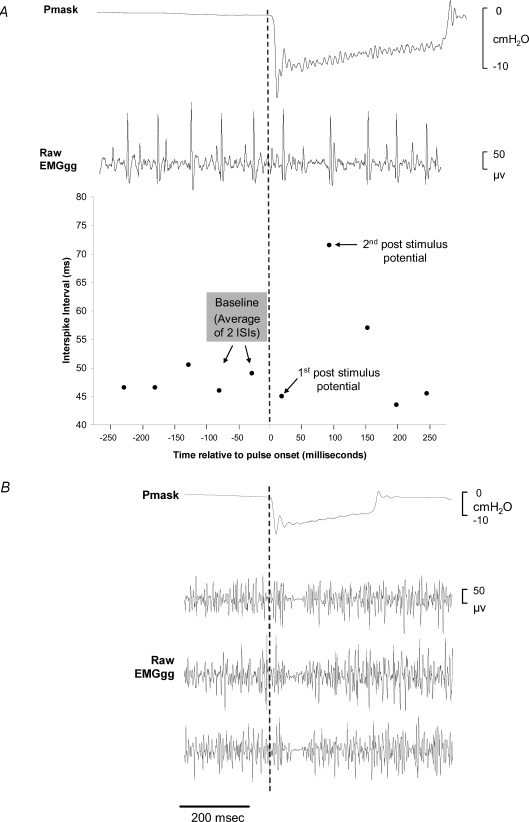
Where available, EMGgg single-motor unit discharges (e.g. Figure 2A) were examined throughout the negative-pressure pulse application. The baseline activity of a motor unit was defined as the average of two prestimulus interspike intervals (ISIs) calculated from adjacent motor-unit action potentials (i.e. three ISIs) (see Fig. 2A for further detail). The ISI of the unit for the first and second potential after pulse onset was also calculated. In order to perform group comparisons, baseline, first and second potential ISIs of six negative-pressure pulses selected at random were averaged in each subject in whom single-motor-unit EMGgg activity was clearly identifiable. The average ISI during the 100 ms baseline period (early inspiration) was also compared to the adjacent preceding expiration period (100 ms average between 400 and 500 ms prior to pulse delivery) for each pulse in order to assist with single-unit discharge pattern classification.
Figure 2.
EMGgg Single motor unit and raw EMGgg multi-unit responses to negative-pressure pulse stimuli. A, example of mask pressure (Pmask), raw EMGgg recording with a prominent single motor unit, and the interspike interval of the motor unit during a single pulse presentation in one subject. Note the clear increase in the interspike interval of the motor unit after the first post-stimulus potential. B, examples of the mean mask pressure (Pmask) and raw multi-unit EMGgg recordings to three separate negative-pressure pulse stimuli in one subject.
Statistical procedures
Repeated measures analysis of variance (ANOVA) was used to examine sleep state effects (awake, stage 2 NREM, SWS) for each EMGgg reflex peak amplitude component (i.e. the excitation and suppression peaks) and each EMGgg reflex latency component (i.e. excitation onset, excitation duration, suppression onset, suppression peak, suppression duration). Similarly, for comparisons incorporating REM data, repeated measures ANOVA was used to examine sleep state effects (awake, all NREM and REM) for EMGgg reflex inhibition amplitude and each EMGgg reflex latency component (i.e. inhibition onset, inhibition peak and inhibition duration). Repeated measures ANOVA was also used to examine the effect of negative-pressure pulse stimuli on EMGgg single-motor-unit discharges (baseline, first and second potentials). Where significant main effects were observed, post hoc comparisons were performed using Dunn–Sidak adjusted Student's paired t tests (Ludbrook, 1991). Statistical significance was inferred when P < 0.05. All data are reported as means ± s.e.m.
Results
Anthropometric characteristics and sleep architecture
Reflex data were collected in 17 of the 18 subjects during sleep and 16 subjects during wakefulness. One subject demonstrated significant sleep-disordered breathing early in the night, and the study was stopped at that point. Post hoc sleep-stage analysis revealed that one subject spent the majority of the wakefulness period drifting in and out of stage 1 sleep such that there were too few replicate trials to generate an ensemble-averaged reflex response during wakefulness in this subject. The mean age and body mass index of the 17 subjects were 24 ± 1 years and 24 ± 1 kg m−2, respectively. Subjects had normal lung function (Mean FEV1 107 ± 4% and FVC 108 ± 3% of predicted). Sleep architecture data are summarized in Table 1.
Table 1.
Sleep architecture data
| Characteristic | |
|---|---|
| Sleep-onset latency (min) | 17 ± 7 |
| Total sleep time (min) | 233 ± 12 |
| Sleep efficiency (%) | 68 ± 4 |
| Stage 1 (% total sleep time) | 15 ± 3 |
| Stage 2 (%total sleep time) | 52 ± 3 |
| SWS (%total sleep time) | 29 ± 4 |
| REM (%total sleep time) | 5 ± 1 |
| Arousal Index (arousals h−1) | 24 ± 3 |
Slow wave sleep (SWS), rapid eye movement sleep (REM). Values are means ± s.e.m. (n = 17 subjects).
Genioglossus negative-pressure reflex during wakefulness and NREM sleep
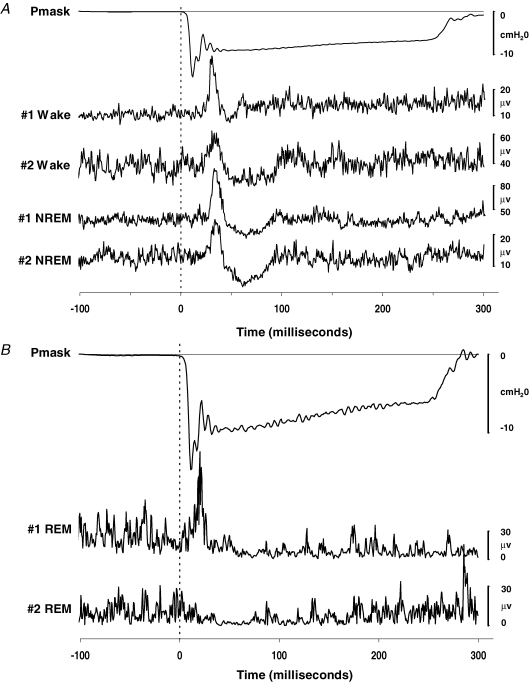
Inspiratory phasic EMGgg activity was observed in all subjects. There were no differences in the average EMGgg activity in the 100 ms prior to pulse onset during wakefulness compared to NREM sleep (19.7 ± 5.3 versus 20.2 ± 5.1 μV, P = 0.954). Negative-pressure pulse stimuli presented during wakefulness resulted in a short-latency peak followed by prolonged suppression of the rectified EMGgg in all subjects. The morphology of this reflex response was maintained during NREM sleep. Examples of the morphology of the EMGgg reflex response in two individual subjects during wakefulness and NREM sleep are displayed in Fig. 3A.
Figure 3.
Ensemble-averaged mask pressure (Pmask) and ensemble-averaged rectified EMGgg reflex responses to brief negative-pressure pulse stimuli in two subjects A, traces during wakefulness and NREM sleep, and B, traces during REM sleep.
The number of stimulus presentations, peak reflex amplitudes, timing and stimulus properties during wakefulness, stage 2 NREM and SWS are summarized in Table 2. There were no differences in the number of artifact-free stimulus presentations, EMGgg reflex component amplitudes, latencies or stimulus properties between stage 2 NREM and SWS. Negative-pressure pulse stimuli resulted in a greater percentage of instances in which arousal from sleep occurred during stage 2 NREM sleep compared to SWS (31 ± 7 versus 8 ± 3%, P = 0.004). The duration of the initial peak tended to be greater during wakefulness compared with stage 2 NREM and SWS (P = 0.050). After the initial peak phase, there was a suppression of EMGgg amplitude below baseline that was significantly greater during stage 2 NREM and SWS compared with wakefulness (Table 2). Suppression onset tended to be earlier during stage 2 NREM and SWS compared to wakefulness, but this difference was not statistically significant (P = 0.060). Mask and choanal pressure-stimulus magnitude was greater during stage 2 NREM and SWS compared to wakefulness (Table 2). However, the rise time from stimulus onset to Pmask minimum was not different between sleep states (Table 2). Epiglottic pressure catheters were prone to blockage and did not provide reliable recordings in most subjects. Of the limited data available in which reliable epiglottic recordings were available during wakefulness, stage 2 NREM and SWS, stimulus intensity at the level of the epiglottis was similar (−6.6 ± 1.1 versus −5.6 ± 1.6 versus −5.6 ± 1.4 cmH2O P = 0.267; n = 5 subjects). There were significantly fewer pulses presented during stage 2 NREM and SWS, respectively, compared with wakefulness (Table 2). However, for combined NREM sleep there were no differences in the number of artefact-free pulse presentations compared with wakefulness (68 ± 7 versus 53 ± 3 P = 0.089). There were no other differences in EMGgg reflex component amplitudes or latencies between stage 2, SWS and wakefulness, respectively. Negative-pressure pulse delivery produced inspiratory time prolongation compared to the preceding breath (2.4 ± 0.1 versus 2.0 ± 0.1 s, P < 0.01), but no change in subsequent expiratory time (2.6 ± 0.1 versus 2.5 ± 0.1 s, P = 0.38). Inspiratory time and expiratory time for the breath prior to pulse delivery compared to the breath immediately after pulse delivery was not different (2.0 ± 0.1 versus 2.0 ± 0.1 s, P = 0.999 and 2.6 ± 0.1 versus 2.8 ± 0.1 s, P = 0.072, respectively), indicating that pulse-related breath timing effects were short lasting.
Table 2.
Genioglossus reflex characteristics to negative-pressure pulse stimuli during wakefulness and NREM sleep
| Wake | Stage 2 | SWS | |
|---|---|---|---|
| Excitation phase | |||
| Onset latency (ms) | 26 ± 1 | 23 ± 1 | 25 ± 2 |
| Peak amplitude (% baseline) | 231 ± 29 | 249 ± 16 | 218 ± 11 |
| Peak latency (ms) | 38 ± 3 | 32 ± 2 | 34 ± 2 |
| Duration (ms) | 23 ± 2 | 16 ± 1 | 17 ± 2 |
| Suppression phase | |||
| Onset latency (ms) | 47 ± 2 | 39 ± 2 | 42 ± 2 |
| Nadir amplitude (% baseline) | 67 ± 8 | 39 ± 4* | 46 ± 4* |
| Nadir latency (ms) | 71 ± 4 | 63 ± 3 | 67 ± 2 |
| Duration (ms) | 42 ± 6 | 39 ± 4 | 40 ± 3 |
| Stimulus properties | |||
| Breath onset to pulse onset (ms) | 92 ± 3 | 93 ± 3 | 91 ± 3 |
| Pmask magnitude (cmH2O) | −9.5 ± 0.3 | −10.8 ± 0.3* | −10.7 ± 0.3* |
| Pmask rise time (ms) | 11.3 ± 0.2 | 10.9 ± 0.4 | 11.1 ± 0.4 |
| Pcho magnitude (cmH2O) | −8.3 ± 0.5 | −10.0 ± 0.4* | −9.8 ± 0.6* |
| Number of artefact-free pulse presentations | 53 ± 3 | 27 ± 5* | 40 ± 6* |
Slow wave sleep (SWS), mask (Pmask) and choanal pressures (Pcho). Reflex timing data are quoted in milliseconds (ms). Reflex amplitude data are quoted as percentage baseline of the average EMGgg activity 100 ms prior to pulse onset (% baseline).
Significant difference compared to wakefulness. Values are means ± s.e.m. (Data presented are from n = 16 subjects in whom complete data were available during wakefulness, stage 2 NREM and SWS).
Clearly identifiable single-motor-unit activity was observed in six subjects. An example of a prominent single EMGgg motor unit during and immediately prior to a single negative-pressure pulse and the ISI of the unit is displayed in Fig. 2A. The average ISI for the baseline, first and second potentials after stimulus onset for six separate pulses per subject and the motor-unit discharge patterns are displayed in Table 3. While detailed descriptive analyses as described by Saboisky et al. (2006) were not available in this study, of the 36 motor units examined all discharged during inspiration and expiration. Upon visual examination and according to the classification categories described by Saboisky et al. (2006), 18 appeared to increase their firing frequency with inspiration (inspiratory tonic), 12 did not appear to change their firing frequency throughout the respiratory cycle (tonic) and 6 units appeared to fire more rapidly during expiration (expiratory tonic). Accordingly, there was a decrease in the ISI during inspiration for motor units classified as inspiratory tonic (ΔISI −67 ± 25 ms, P = 0.016). The ISI during inspiration for motor units classified as expiratory tonic significantly increased (ΔISI 8 ± 3 ms, P = 0.032). The ISI for units classified as tonic did not differ between the expiratory period and the inspiratory period (45 ± 4 versus 47 ± 3 ms, P = 0.532). The ISI of the first potential after negative-pressure pulse onset did not change from baseline (P = 0.525), whereas the ISI of the second potential significantly increased (Table 3). In addition, a clear reflex-mediated reduction in EMGgg activity was, in many instances, observed during single negative-pressure pulse trials on the raw multi-unit EMGgg recording (Fig. 2B).
Table 3.
Genioglossus single-motor-unit interspike intervals during negative-pressure pulse stimuli
| Subject number and discharge pattern | Pre-stimulus baseline (ms) | First post-stimulus potential (ms) | Second post-stimulus potential (ms) |
|---|---|---|---|
| 1 (IT) | 57 ± 3 | 54 ± 5 | 79 ± 3*† |
| 2 (ET) | 51 ± 4 | 50 ± 3 | 60 ± 5* |
| 3 (T) | 55 ± 2 | 50 ± 5 | 84 ± 5*† |
| 4 (IT) | 99 ± 3 | 120 ± 23 | 112 ± 11* |
| 5 (IT) | 44 ± 2 | 45 ± 2 | 60 ± 3*† |
| 6 (T) | 40 ± 3 | 41 ± 4 | 108 ± 7*† |
| Mean | 58 ± 9 | 60 ± 12 | 84 ± 9*† |
The average interspike interval (ISI) of genioglossus single motor units during six pulse presentations per subject immediately prior to and the first and second potentials after pulse onset, respectively. (See Fig. 2A for an example tracing in one subject). Inspiratory tonic (IT), expiratory tonic (ET) and tonic (T) discharge patterns according the definitions described by Saboisky et al. (2006); see text for further details.
Significant difference compared to baseline
significant difference compared to first post-stimulus potential.
Genioglossus negative-pressure reflex during REM sleep
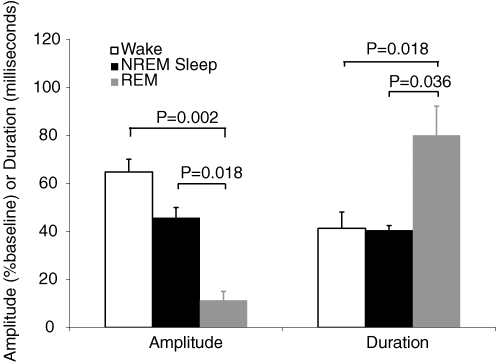
Sufficient REM sleep to present negative-pressure pulse stimuli and generate an ensemble-averaged reflex response was achieved in five subjects. There were no differences in the average EMGgg activity in the 100 ms prior to pulse onset during NREM compared to REM sleep in these subjects (12.6 ± 4.4 versus 17.9 ± 3.8 μV, P = 0.143). While replicate trials were limited (7 ± 2), the predominant reflex response of a prolonged period of suppression of muscle activity with (2/5) or without (3/5) any preceding excitation was still quite clear. Indeed, the suppression often approached complete silencing of the EMGgg. The rectified EMGgg reflex responses in two individual subjects during REM sleep are displayed in Fig. 3B. ANOVA for repeated measures exploring state (wake, NREM and REM) effects revealed significantly greater EMGgg amplitude suppression during NREM and REM sleep compared with wakefulness, with particularly marked suppression during REM sleep in these five subjects (Fig. 4). In addition, the duration of the suppression phase was significantly longer during REM sleep compared to wakefulness and NREM sleep. There were no significant state effects in reflex peak amplitudes or latencies for comparisons incorporating REM data.
Figure 4.
Ensemble-averaged, rectified EMGgg reflex suppression amplitude and suppression duration during REM sleep compared to wakefulness and NREM sleep Values are means ± s.e.m. (n = 5 subjects).
Discussion
The ensemble-averaged rectified EMGgg response to brief pulses of negative airway pressure revealed a previously unknown pattern, namely, an initial increase followed by a decrease from baseline EMGgg activity. This may not have been observed in earlier studies for several reasons. Previous studies of the gg negative-pressure reflex in humans have been conducted using a combination of surface and intramuscular electrodes for multiple-unit recordings (Horner et al. 1991a, Horner 1991b, Horner 1994; Wheatley et al. 1993; Shea et al. 1999, Shea 2000; Berry et al. 2003; Malhotra et al. 2004). The relatively poor signal-to-noise ratio arising from surface EMG electrodes and the small number of replicate trials (Horner et al. 1991a, Horner 1991b, Horner 1994) may have obscured these reflex characteristics in previous studies. Subsequent studies that utilized intramuscular electrodes with increased replicate trial numbers probably improved the signal-to-noise ratio characteristics (Wheatley et al. 1993; Shea et al. 1999, Shea 2000; Malhotra et al. 2004) but analysis using moving-time averaging may have obscured a short-latency inhibitory response.
Where a reflex response consisting of an excitation phase followed by suppression is present on the rectified EMG, it is possible that the suppression component is an epiphenomenon of motor-unit synchrony rather than reflex inhibition (Kasser & Cheney, 1985). Briefly, the excitation phase can cause otherwise out-of-phase motor-unit action potentials to become temporally aligned, as asynchronous units within the motor neuron population are near-simultaneously brought to firing threshold as a result of the stimulus. This temporal alignment can cause a subsequent suppression phase characterized by a relative lack of EMG activity where previously asynchronous motor units are refractory and do not discharge until their membrane potentials return to firing threshold and asynchronous firing patterns begin to return (Miles et al. 1989). In the current study, consistent with suppression due to reflex inhibition rather than purely a phenomenon of synchronous firing, there was a consistent marked increase in the ISI of single motor units in the second post-stimulus interval. Further, the inhibition phase was also evident on the raw multi-unit recordings during single trials.
While it is difficult to quantify definitively using the sampling frequencies and techniques in the current study, the lack of change from baseline in the ISI of the first post-stimulus discharge for the motor units examined suggests that the excitation phase of the gg negative-pressure reflex probably occurs primarily as a result of additional motor-unit recruitment rather than an increase in the firing frequency of individual motor units. Recent human studies in which detailed quantification of gg single-motor-unit activity was undertaken during normal respiration has highlighted the heterogeneous nature of the gg muscle and its neural inputs (Saboisky et al. 2006). To determine definitively the neural mechanisms mediating the initial peak of the gg negative-pressure reflex and the precise role of negative pressure in modulating gg activity would require a similar systematic exploration of the behaviour of single motor units within the muscle.
Functionally, these data suggest that the reflex response of the gg muscle to transient negative pressure is not simply an excitatory response resulting in tongue protrusion or perhaps stiffening as previously thought (Malhotra et al. 2000, Malhotra 2002; Akahoshi et al. 2001; Fogel et al. 2001; Malhotra & White, 2002). While moment-to-moment excitatory modulation of upper-airway gg activity may be an important protective mechanism in maintaining upper-airway patency during normal inspiration (Malhotra et al. 2000, Malhotra 2002; Akahoshi et al. 2001; Fogel et al. 2001; Malhotra & White, 2002), the findings of the present study raise the possibility that this response may be threshold dependent. For example, the response to relatively small negative pressures in the upper airway, as would occur during normal tidal breathing, may be largely excitatory whereas the predominant response to large negative pressures may be inhibitory. The relatively long stimulus duration in the present study (250 ms) combined with the substantial stimulus amplitude may be more akin to rapid airway occlusion than within-breath fluctuation in airway pressure to which gg reflexes may normally be able to respond effectively. Consequently it is possible that shorter-duration and less-negative airway pressure stimuli would show a more pronounced excitatory response compared with the dominant inhibitory response observed in this study.
The precise functional role of gg reflex inhibition is unclear and would appear to be counterproductive to respiratory homeostasis. However, it is possible that reflex inhibition in itself may be protective. The initial response appears to be excitatory, which would tend to dilate the upper airway. However, if this initial response was not sufficient to overcome the impediment to respiration (whether it be obstruction by the tongue or inhalation of a foreign object) it would be counterproductive, and perhaps deleterious to the maintenance of inspiratory flow (e.g. tending to cause worsening flow limitation), to continue making sustained inspiratory effort. Thus, the reflex inhibition observed may be a result of inhibition of the respiratory pattern-generator inputs to this reflex arc in the same manner that respiratory drive muscles have been shown to be inhibited by brief respiratory load stimuli (Davis & Sears, 1970; Butler et al. 1995, Butler 1996, Butler 1997; Jeffery et al. 2006). In support of this hypothesis, the latency of the onset of the gg reflex inhibition phase to negative-pressure pulse stimuli was remarkably similar to those reported in other respiratory drive muscles in these previous studies. Thus, reflex gg inhibition could simply reflect the upper-airway component of a more global inhibitory respiratory reflex serving to maintain an appropriate balance of upper-airway versus respiratory pump muscle drive to achieve non-flow-limited inspiratory airflow.
An alternate explanation is that the gg negative-pressure reflex to a negative-pressure pulse may inhibit normal respiratory inputs to the hypoglossal motor nucleus, and this inhibitory effect may outlast the direct excitatory effect and be manifest as gg suppression. A systematic exploration of single-motor-unit behaviour similar to that reported by Saboisky et al. (2006) would be helpful in delineating which types of motor units are involved in the excitatory and inhibitory reflex responses.
The effect of sleep on reflex responses to negative-pressure pulse stimuli
Earlier studies investigating the effect of sleep on gg reflex responsiveness to negative pressure either did not control for posture, or studied subjects in the lateral position (Wheatley et al. 1993; Horner et al. 1994). These studies found delayed latency and attenuation of the excitatory gg negative-pressure reflex response during NREM sleep. The present finding that the excitatory component of the gg negative-pressure reflex in the supine posture was unaffected by sleep is different, but is in agreement with the recent findings of Malhotra et al. (2004). This provides strong support for the concept that gg reflex activity to negative pressure is posture dependent.
In contrast to earlier studies (Wheatley et al. 1993; Horner et al. 1994), we found that the latencies of the various gg reflex characteristics were similar during NREM sleep compared to wakefulness. However, consistent with the general concept of more pronounced excitation during wakefulness, there was a trend for prolongation of the duration of excitation and delay of onset of suppression during wakefulness. While the precise reasons for the discrepancies between this and previous studies are unclear, they may relate to postural and other methodological differences. However, this is difficult to evaluate given that latency data were not reported in the only other study to examine systematically the gg negative-pressure reflex in the supine position (Malhotra et al. 2004).
The finding of marked reflex inhibition during REM sleep, often in the absence of any preceding excitation, is in agreement with previous gg negative-pressure reflex findings during REM sleep (Shea et al. 1999) and is consistent with earlier reports demonstrating reduced gg activation to occlusive stimuli during REM sleep (Kuna & Smickley, 1988; Okabe et al. 1994). The lack of excitatory responsiveness of the largest upper-airway dilating muscle to negative pressure during REM sleep is likely to be an important contributing factor to the increased severity of sleep-disordered breathing observed in this sleep stage.
In contrast to the previous concept that sleep caused a suppression of the excitatory response to negative-upper-airway pressure (Malhotra & White, 2002; White, 2005, White, 2006), thereby increasing the likelihood of upper-airway collapse, our data suggest that the secondary inhibition phase of the gg negative-pressure reflex may also play a key role. While the gg negative-pressure reflex has not been studied in this manner previously, it is possible that greater reflex inhibition of the gg negative-pressure reflex during sleep may be mediated by changes in sleep-specific neuromodulators such as serotonin (Jelev et al. 2001; Sood et al. 2005).
Possible relevance to sleep disordered breathing
A diminished excitatory gg negative-pressure reflex during sleep has been postulated to contribute to the development of obstructive sleep apnoea in patients with an anatomically narrow upper airway. The finding that the amplitude of the initial excitatory component of the reflex measured in the supine posture was not different between wake and NREM sleep does not support this hypothesis. However, more pronounced gg reflex inhibition following the initial excitation phase to negative pressure during NREM sleep could render the upper airway more prone to collapse. In the same way that inspiratory pump muscle reflex responses to respiratory occlusion are altered in obstructive sleep apnoea patients (Jeffery et al. 2006), it will be important to investigate if the inhibition component of the gg negative-pressure reflex is similarly affected in obstructive sleep apnoea patients.
Methodological considerations
There are several important methodological considerations with regard to this study. Firstly, we elected to study only male subjects due to the known influence of changes in respiratory stimulant hormones that occur throughout the menstrual cycle and their associated effects on ventilation and gg muscle activation (Popovic & White, 1998). Further, the prevalence of sleep-disordered breathing is greater in men than women (Young et al. 1993). While we believe that that the effects observed in this study in men would probably be similar in women if the stage of menstrual cycle was controlled, this has yet to be determined. Thus, future carefully designed studies are required to systematically explore this important unresolved issue.
Although we hypothesize that more pronounced gg reflex inhibition observed during NREM sleep compared to wakefulness occurs due to changes in sleep-specific neuromodulators, it is possible that this difference may be explained by the greater stimulus presented during NREM sleep. However, we feel that this is unlikely, given that differences in stimulus magnitude were small (∼1–2 cmH2O) and other components of the reflex were not different between states. In addition, the precise stimuli required to activate the gg negative-pressure reflex are not known. It may be that the rate of change in negative pressure, which was not different between states, may be more important than the absolute change in pressure in eliciting the gg negative-pressure reflex. However, the possibility that differences in stimulus magnitude between NREM sleep and wakefulness may contribute to the greater reflex inhibition observed in NREM sleep can not be dismissed.
Another potential limitation of the study is that negative pressure was applied nasally rather than being generated distal to the upper airway as occurs in obstructive sleep apnoea. While this approach may stimulate additional receptor systems not implicated in upper-airway collapse in obstructive sleep apnoea, available evidence suggests nasal and laryngeal receptors respond in a similar manner (Horner et al. 1991a; Malhotra et al. 2004). While the present findings suggest that this is unlikely, it is possible that this type of stimulus stimulates additional proprioceptive afferents that are known to cause suppression of gg activity in animal models (Sauerland & Mizuno, 1970).
Finally, subjects were heavily instrumented during the study and it is possible that reflex responses may have been altered as a result. The inspiratory circuit added a small (∼2 cmH2O l−1 s) resistance to inspiration, and all subjects breathed nasally. However, given that all subjects experienced the same conditions, these factors are unlikely to account for the sleep-state-related change observed in this within-subjects study design.
Summary
This study describes a previously unreported reflex suppression of EMGgg in response to brief pulses of negative upper-airway pressure that is consistent with reflex inhibition of the gg muscle. This reflex inhibition was more pronounced during NREM and particularly during REM sleep compared to wakefulness. Greater gg reflex inhibition to negative-pressure stimuli during sleep raises the possibility that this mechanism may contribute to upper-airway collapse in individuals with an anatomically narrow airway.
Acknowledgments
This work was funded by the National Health and Medical Research Council of Australia. The authors are extremely grateful to Samantha Windler for her valuable assistance in scoring arousals and staging the sleep studies. Professor Timothy Miles provided valuable advice on reflex morphology, single-motor-unit analyses and helpful feedback on the manuscript. David Schembri and the Respiratory Function Unit staff, Repatriation General Hospital provided helpful assistance with lung function measurements.
References
- Akahoshi T, White DP, Edwards JK, Beauregard J, Shea SA. Phasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humans. J Physiol. 2001;531:677–691. doi: 10.1111/j.1469-7793.2001.0677h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- Berry RB, White DP, Roper J, Pillar G, Fogel RB, Stanchina M, Malhotra A. Awake negative pressure reflex response of the genioglossus in OSA patients and normal subjects. J Appl Physiol. 2003;94:1875–1882. doi: 10.1152/japplphysiol.00324.2002. [DOI] [PubMed] [Google Scholar]
- Bouisset S, Maton B. Quantitative relationship between surface EMG and intramuscular electromyographic activity in voluntary movement. Am J Phys Med. 1972;51:285–295. [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Crawford MR, Gandevia SC. Role of airway receptors in the reflex responses of human inspiratory muscles to airway occlusion. J Physiol. 1995;487:273–281. doi: 10.1113/jphysiol.1995.sp020878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Gandevia SC. Impaired reflex responses to airway occlusion in the inspiratory muscles of asthmatic subjects. Thorax. 1996;51:490–495. doi: 10.1136/thx.51.5.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Glanville AR, Gandevia SC. Pulmonary afferents are not necessary for the reflex inhibition of human inspiratory muscles produced by airway occlusion. J Neurophysiol. 1997;78:170–176. doi: 10.1152/jn.1997.78.1.170. [DOI] [PubMed] [Google Scholar]
- Davis JN, Sears TA. The proprioceptive reflex control of the intercostal muscles during their voluntary activation. J Physiol. 1970;209:711–738. doi: 10.1113/jphysiol.1970.sp009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood PR, Allison GT, Shepherd KL, Szollosi I, Hillman DR. Heterogeneous activity of the human genioglossus muscle assessed by multiple bipolar fine-wire electrodes. J Appl Physiol. 2003;94:1849–1858. doi: 10.1152/japplphysiol.01017.2002. [DOI] [PubMed] [Google Scholar]
- Fogel RB, Malhotra A, Pillar G, Edwards JK, Beauregard J, Shea SA, White DP. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am J Respir Crit Care Med. 2001;164:2025–2030. doi: 10.1164/ajrccm.164.11.2102048. [DOI] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol. 1991a;436:31–44. doi: 10.1113/jphysiol.1991.sp018537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Morrell MJ, Shea SA, Guz A. The effect of sleep on reflex genioglossus muscle activation by stimuli of negative airway pressure in humans. J Physiol. 1994;476:141–151. [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol. 1991b;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery S, Butler JE, McKenzie DK, Wang L, Gandevia SC. Brief airway occlusion produces prolonged reflex inhibition of inspiratory muscles in obstructive sleep apnea. Sleep. 2006;29:321–328. doi: 10.1093/sleep/29.3.321. [DOI] [PubMed] [Google Scholar]
- Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol. 2001;532:467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan AS, Eckert DJ, Catcheside PG, McEvoy RD. Ventilatory response to brief arousal from non-rapid eye movement sleep is greater in men than in women. Am J Respir Crit Care Med. 2003;168:1512–1519. doi: 10.1164/rccm.200302-150OC. [DOI] [PubMed] [Google Scholar]
- Kasser RJ, Cheney PD. Characteristics of corticomotoneuronal postspike facilitation and reciprocal suppression of EMG activity in the monkey. J Neurophysiol. 1985;53:959–978. doi: 10.1152/jn.1985.53.4.959. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Smickley J. Response of genioglossus muscle activity to nasal airway occlusion in normal sleeping adults. J Appl Physiol. 1988;64:347–353. doi: 10.1152/jappl.1988.64.1.347. [DOI] [PubMed] [Google Scholar]
- Ludbrook J. On making multiple comparisons in clinical and experimental pharmacology and physiology. Clin Exp Pharmacol Physiol. 1991;18:379–392. doi: 10.1111/j.1440-1681.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Pillar G, Fogel RB, Beauregard J, Edwards JK, Slamowitz DI, Shea SA, White DP. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med. 2000;162:1058–1062. doi: 10.1164/ajrccm.162.3.9912067. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Pillar G, Fogel RB, Edwards JK, Ayas N, Akahoshi T, Hess D, White DP. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med. 2002;165:71–77. doi: 10.1164/ajrccm.165.1.2011065. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Trinder J, Fogel R, Stanchina M, Patel SR, Schory K, Kleverlaan D, White DP. Postural effects on pharyngeal protective reflex mechanisms. Sleep. 2004;27:1105–1112. doi: 10.1093/sleep/27.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- Mathew OP. Upper airway negative-pressure effects on respiratory activity of upper airway muscles. J Appl Physiol. 1984;56:500–505. doi: 10.1152/jappl.1984.56.2.500. [DOI] [PubMed] [Google Scholar]
- Mathew OP, Abu-Osba YK, Thach BT. Genioglossus muscle responses to upper airway pressure changes: afferent pathways. J Appl Physiol. 1982a;52:445–450. doi: 10.1152/jappl.1982.52.2.445. [DOI] [PubMed] [Google Scholar]
- Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus muscle respiratory activity. J Appl Physiol. 1982b;52:438–444. doi: 10.1152/jappl.1982.52.2.438. [DOI] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles TS, Turker KS, Le TH. Ia reflexes and EPSPs in human soleus motor neurones. Exp Brain Res. 1989;77:628–636. doi: 10.1007/BF00249616. [DOI] [PubMed] [Google Scholar]
- Okabe S, Hida W, Kikuchi Y, Taguchi O, Takishima T, Shirato K. Upper airway muscle activity during REM and non-REM sleep of patients with obstructive apnea. Chest. 1994;106:767–773. doi: 10.1378/chest.106.3.767. [DOI] [PubMed] [Google Scholar]
- Perry J, Easterday CS, Antonelli DJ. Surface versus intramuscular electrodes for electromyography of superficial and deep muscles. Phys Ther. 1981;61:7–15. doi: 10.1093/ptj/61.1.7. [DOI] [PubMed] [Google Scholar]
- Popovic RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol. 1998;84:1055–1062. doi: 10.1152/jappl.1998.84.3.1055. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, kales A. A Manual of Standardized terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. UCLA, Los Angeles: Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- Ryan S, McNicholas WT, O'Regan RG, Nolan P. Reflex respiratory response to changes in upper airway pressure in the anaesthetized rat. J Physiol. 2001;537:251–265. doi: 10.1111/j.1469-7793.2001.0251k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol. 2006;95:2213–2221. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Mizuno N. A protective mechanism for the tongue: suppression of genioglossal activity induced by stimulation of trigeminal proprioceptive afferents. Experientia. 1970;26:1226–1227. doi: 10.1007/BF01897980. [DOI] [PubMed] [Google Scholar]
- Shea SA, Akahoshi T, Edwards JK, White DP. Influence of chemoreceptor stimuli on genioglossal response to negative pressure in humans. Am J Respir Crit Care Med. 2000;162:559–565. doi: 10.1164/ajrccm.162.2.9908111. [DOI] [PubMed] [Google Scholar]
- Shea SA, Edwards JK, White DP. Effect of wake-sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J Physiol. 1999;520:897–908. doi: 10.1111/j.1469-7793.1999.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172:1338–1347. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- Wheatley JR, Mezzanotte WS, Tangel DJ, White DP. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis. 1993;148:597–605. doi: 10.1164/ajrccm/148.3.597. [DOI] [PubMed] [Google Scholar]
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- White DP. The pathogenesis of obstructive sleep apnea: advances in the past 100 years. Am J Respir Cell Mol Biol. 2006;34:1–6. doi: 10.1165/rcmb.2005-0317OE. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]