Abstract
In pregnant women with type 1 diabetes, suboptimal glucose control in the first trimester is a strong predictor for giving birth to a large fetus. However, the mechanisms underlying this association are unknown. We hypothesized that transient hyperglycaemia in early pregnancy results in (1) increased placental growth and (2) an up-regulation of placental nutrient transport capacity, which leads to fetal overgrowth at term. In order to test this hypothesis, pregnant rats were given intraperitoneal injections of glucose (2 g kg−1, resulting in a 50–100% increase in blood glucose level during 90 min) or saline (control) in either early or late gestation using four different protocols: one single injection on gestational day (GD) 10 (n = 5), three injections on GD 10 (n = 8–9), six injections on GD 10 and 11 (n = 9–11) or three injections on GD 19 (n = 7–8). Multiple injections were given approximately 4 h apart. Subsequently, animals were studied on GD 21. Three glucose injections in early pregnancy significantly increased placental weight by 10%, whereas fetal weight was found to be increased at term in response to both three (9% increase in fetal weight, P < 0.05) and six glucose injections (7%, P = 0.05) in early gestation. A single glucose injection on GD 10 or three injections of glucose on GD 19 had no effect on placental or fetal growth. In groups where a change in feto-placental growth was observed, we measured placental system A and glucose transport activity in the awake animals on GD 21 and placental expression of the glucose and amino acid transporters GLUT1, GLUT3, SNAT2 (system A), LAT1 and LAT 2 (system L). Placental system A transport at term was down-regulated by six glucose injections in early pregnancy (by −33%, P < 0.05), whereas placental mRNA and protein levels were unchanged. No long-term alterations in maternal metabolic status were detected. In conclusion, we demonstrate that transient hyperglycaemia in early pregnancy is sufficient to increase fetal weight close to term. In contrast, brief hyperglycaemia in late pregnancy did not stimulate fetal growth. Increased fetal growth may be explained by a larger placenta, which would allow for more nutrients to be transferred to the fetus. These data suggest that maternal metabolic control in early pregnancy is an important determinant for feto-placental growth and placental function throughout the remainder of gestation. We speculate that maternal metabolism in early pregnancy represents a key environmental cue to which the placenta responds in order to match fetal growth rate with the available resources of the mother.
Increased fetal growth, resulting in the delivery of a large-for-gestational age (LGA) baby, is common in pregnancies complicated by maternal diabetes or obesity. Fetal overgrowth represents a risk factor for operative delivery, traumatic birth injury (Casey et al. 1997) and development of diabetes and obesity later in life (Pettitt et al. 1991, Pettitt 1993). However, the pathophysiological mechanisms underlying increased fetal growth remain to be fully established. The incidence of fetal overgrowth in pregnancies complicated by type 1 diabetes remains high despite strict glycaemic control with modern clinical management of these patients (Evers et al. 2002). Some observations indicate that maternal metabolism in early gestation may be important in determining fetal growth rate in these pregnancies. The level of glycosylated haemoglobin A1c (HbA1C) in the first trimester in diabetic pregnancies has been identified as a factor that predicts for fetal overgrowth (Rey et al. 1999). During the first trimester of pregnancy, mothers with type 1 diabetes often experience fluctuating glucose levels (Kerssen et al. 2003), and it has been suggested that brief periods of both hypo- and hyperglycaemia in early pregnancy may stimulate fetal growth (Greco et al. 2003). In addition, strict glucose control by insulin treatment during the first trimester has not been shown to reduce the number of overgrown fetuses in patients with type 1 diabetes (Persson & Hanson, 1996). These observations suggest that the mechanisms underlying fetal overgrowth in mothers with type 1 diabetes are complex and may not be solely due to increased substrate levels in the maternal circulation.
The primary factor determining fetal growth is nutrient supply, which affects growth-regulating physiological pathways in the fetus, in particular insulin secretion. A large body of evidence suggests that changes in placental transport functions constitute an important link between perturbations in the maternal compartment such as maternal diabetes, reduced placental blood flow and protein malnutrition, and altered fetal nutrient supply (for review see Sibley et al. 2005; Jansson & Powell, 2006). For example, down-regulation of placental amino acid transporters is likely to contribute directly to the decreased fetal nutrient supply in human intrauterine growth restriction (IUGR) (Sibley et al. 2005; Jansson & Powell, 2006) and in IUGR due to maternal protein malnutrition in the rodent (Jansson et al. 2006). Increased activity and expression of placental nutrient transporters has been suggested to be one mechanism underlying accelerated fetal growth in women with diabetes (Jansson & Powell, 2000). Indeed, glucose transporter activity and protein expression have been shown to be increased in the basal plasma membrane of the syncytiotrophoblast of the human placenta obtained from pregnancies complicated by type 1 diabetes and those resulting in LGA babies (Gaither et al. 1999; Jansson et al. 1999). In addition, we have reported an increased activity of the placental amino acid transporter system A in pregnancies complicated by type 1 diabetes and fetal overgrowth (Jansson et al. 2002), findings that are in contrast to those of a previous study in a different population of diabetic pregnancies (Kuruvilla et al. 1994).
Our population of pregnant women with type 1 diabetes is not only characterized by fetal overgrowth and increased placental nutrient transport capacity at term, but placental weights are also increased (Jansson et al. 1999, Jansson 2002,). Furthermore, these women had elevated HbA1c levels in the first trimester, but not in the second or third trimesters (Jansson et al. 1999, Jansson 2002). In the current series of experiments we used an animal model to directly test the hypothesis that transient hyperglycaemia in early pregnancy results in (1) increased placental growth and (2) an up-regulation of placental nutrient transport capacity, which leads to fetal overgrowth at term. Episodes of moderate hyperglycaemia were induced in the pregnant rat in early and late gestation, placental and fetal weights were examined and in vivo measurements of placental glucose and amino acid transport were carried out near term. Furthermore, mRNA and protein levels of the glucose transporters GLUT1 and GLUT3, and the amino acid transporter system A and L were analysed in placental homogenates.
Methods
Animals
All animal experiments were approved by the local ethical committee for animal research at Gothenburg University. In experiments in which vascular catheters were inserted, animals were anaesthetized with a mixture of xylazin (Rompun, 4.6 mg kg−1) and ketamine hydrochloride (Ketalar, 38.5 mg kg−1) i.p. and were given buprenorfin (Temgesic, 0.01 mg kg−1; s.c.) for analgesia. At the termination of experiments, animals were killed by an overdose of sodium pentobarbital administered i.v. (in animals with catheters) or i.p., followed by opening up the heart and thorax in the dam and decapitation of the fetuses.
Pregnant Sprague–Dawley rats (3–4 months of age) were obtained from B-K Universal, Sweden and delivered to the animal facilities. Day 1 of gestation was defined as the day after males had been present during the night. The pregnant rats were maintained on a 12 h light–dark cycle at 21°C with free access to food and water. As summarized in Table 1, the animals were divided into experimental groups, which were matched with regard to maternal weights. Animals were given either one, three or six injections of glucose (2 g kg−1) or saline (control) i.p. on gestational day (GD) 10 and 11 with approximately 4 h between injections, or three injections of glucose/saline i.p. on GD 19 every 4 h. Animals were allowed to return to their cages and remained undisturbed until they were killed at GD 21 (term, GD 23), using the protocol described above. Placental and fetal weights as well as maternal weight gain and litter size were recorded. Samples of placenta were homogenized on ice in buffer D containing (mm): Tris-Hepes 10, sucrose 250 and EDTA 1, and 1.6 μm antipain, 0.7 μm pepstatin and 0.5 μg ml−1 aprotinin, for Western blot analysis or placed in RNA STAT-60 (1 ml (g placenta)−1) for subsequent RNA extraction. All samples were snap-frozen in liquid nitrogen and stored at −80°C for subsequent expression analysis. In a subset of animals in which hyperglycaemia was induced in early pregnancy, animals were anaesthetized, as described above, on GD 18 and vascular catheters were implanted. Placental nutrient transport measurements were carried out on GD 21 in awake animals and maternal blood samples were collected for measurements of glucose and insulin concentrations. Placental and fetal weights as well as maternal weight gain and litter size were recorded in these animals. In order to monitor the degree of hyperglycaemia achieved with the treatments, a group of animals (n = 10) was anaesthetized (see above) and had vascular arterial catheters implanted on GD 7, and were given glucose (n = 7) or saline injections (n = 3) on GD 10. Blood glucose measurements were carried out continuously over a 3 h period. Animals were killed after the hyperglycaemic episode, using the procedure described above.
Table 1.
Characterization of the different experimental groups
| Experimental group | GD 7 | GD 10 | GD 11 | GD 18 | GD 19 | GD 21 |
|---|---|---|---|---|---|---|
| One injection early pregnancy | — | 1 × i.p. | — | — | — | Weights |
| Three injections early pregnancy | — | 3 × i.p. | — | — | — | Weights and expression analysis |
| — | 3 × i.p. | — | Catheters | — | Placental transport in vivo | |
| Six injections early pregnancy | — | 3 × i.p. | 3 × i.p. | — | — | Weights and expression analysis |
| — | 3 × i.p. | 3 × i.p. | Catheters | — | Placental transport in vivo | |
| Three injections late pregnancy | — | — | — | — | 3 × i.p. | Weights |
| Blood glucose measurements | Catheter | 1 × i.p. | — | — | — | — |
GD, gestational day, i.p., intraperitoneal injection of glucose or saline.
Glucose and insulin measurements
In studies to monitor the magnitude and duration of the induced hyperglycaemia, the effects of i.p. glucose injection on maternal blood glucose were measured in arterial blood samples (∼10 μl) obtained every 30 min starting with a measurement immediately before the injection and continued until blood glucose level was normalized (within ∼2 h). Blood glucose level was measured immediately (MediSense, Abbott Laboratories). In the animals subjected to six hyperglycaemic events on GD 10 and 11 and in which placental nutrient transport was being measured at term, maternal glucose concentrations were assayed in frozen plasma using the Biochemistry Analyser YSI 2700 SELECT, and maternal insulin was assayed using a sensitive rat insulin radioimmunoassay kit (Linco Research, St Charles, MS, USA).
Antibodies
Protein expression of glucose and amino acid transporters was studied using primary antibodies for GLUT1 (1 : 5000; Chemicon, CA, USA), GLUT3 (1 : 2500; Alpha Diagnostic, TX, USA) and the SNAT2 isoform of system A (1 : 4000; Ling et al. 2001). Secondary antibodies used were peroxidase-labelled anti-rabbit IgG (SNAT2, 1 : 1000; GLUT1, 1 : 7000; GLUT3, 1 : 2500; Vector Laboratories, Burlingame, CA, USA).
Western blot
Separation of proteins in rat placental homogenates was carried out by SDS-PAGE as previously described (Johansson et al. 2000), with minor changes. Protein concentrations were determined using the Bradford assay (Bradford, 1976) and samples were prepared with sample buffer containing dithiothreitol (GLUT1 and GLUT3) or Laemmli buffer (SNAT2) (Cannon-Carlson & Tang, 1997). A total of 30 μg protein was loaded onto 10% polyacrylamide gels for SNAT2 analysis and 10 μg protein for GLUT1 and GLUT 3. One control sample was used on each gel to normalize density readings between gels. The separated proteins were transferred onto a nitrocellulose transfer membrane over night at 30 V. The membranes were blocked in blotto (5% non-fat dry milk) buffer and then incubated with antibodies, and final detection was accomplished using enhanced chemiluminiscence (ECL, Amersham) to visualize signals on autoradiographic film (Hybond, Amersham). Relative density of the bands was evaluated by densitometry with Image Gauge software (version 3.45; Fuji film). Isolated microvillous plasma membrane (MVM) vesicles (GLUT1 and SNAT2) from human placenta and homogenate of rat brain (GLUT3) were used as positive controls. Pre-absorption blocking experiments were carried out by preincubating the SNAT2 antibody with its corresponding peptide antigen (300 times excess) in order to distinguish the SNAT2-specific signal.
Real-time RT-PCR
RNA was extracted from rat placental tissue using the RNA STAT-60 protocol (Tel.Test Inc., TX, USA) with a few modifications (Ericsson et al. 2005). Total RNA concentration and purity were determined by UV spectrophotometry at 260 and 280 nm. The reverse transcription reaction was carried out as previously described (Blomgren et al. 1999) with a Superscript RNase H- reverse transcriptase kit (Invitrogen, San Diego, CA, USA), random hexamer primers, and deoxy NTP (dNTP) (dATP, dCTP, dTTP and dGTP; Roche Diagnostics GmbH, Mannheim, Germany). For the detection of GLUT1, GLUT3, the SNAT2 isoform of system A and the LAT1 and 2 isoforms of the system L subunit the following primers were designed by Cybergene AB (Huddinge, Sweden): GLUT1 (forward) 5′-CCACCACACTCACCACACTC-3′ and (reverse) 5′-CACATACATGGGCACAAAGC-3′; GLUT3 (forward) 5′-CATCTCCGTTGTCCTCCAGT-3′ and (reverse) 5′-GCTCCAATCGTGGCATAGAT-3′; SNAT2 (forward) 5′-AACTACTCATACCCCACGAAG-3′ and (reverse) 5′-AAAGGTGCCATTCACCGTTTC-3′ (Ling et al. 2001); LAT1 (forward) 5′- GTACCAATCCAGCCTCCAAA-3′ and (reverse) 5′- TACAGAGCTGCCTAGCACGA-3′; LAT2 (forward) 5′- TTTCAGCTATGGGGATGAGC-3′ and (reverse) 5′- TCTTCATTTTGGCCCTTCAC-3′. Succinate dehydrogenase (SDHA; mitochondrial membrane-bound enzyme) served as a house keeping gene, which has previously been shown to be stable in placental tissue (Meller et al. 2005). The primers designed for SDHA were (forward) 5′-TCCTCCGATTAAGGCAAATG-3′ and (reverse) 5′-ATGGCTCTGCATCGACTTCT-3′. Real-time PCR reactions were carried out using the Roche Lightcycler, utilizing the SYBR Green I system according to the manufacturer's instructions. A total volume of 20 μl PCR mixture loaded in a capillary tube contained 2 μl cDNA (diluted 1 : 4) from the RT reaction, 2–3 mm MgCl2, 0.5 μm of each primer, and 2 μl of the reagent from an LC-FastStart DNA Master SYBR Green I kit (Roche Diagnostics). All samples were analysed in duplicate and water was used as negative control. A standard curve was obtained by 2-fold serial template dilutions of cDNA from 1 : 1 to 1 : 32 for each gene. To ensure that the correct product was amplified in the reaction, all samples were separated on a 1.5% agarose/0.5 × Tris borate EDTA gel containing ethidium bromide.
Transport measurements
Permanent vascular catheters were implanted on GD 18 and placental transport measurements were carried out on GD 21 in the awake animal as previously described in detail (Jansson et al. 2006). In brief, animals were anaesthetized using xylazin, ketamine and buprenorfin as described above. Local anaesthetics were given s.c. and a 2 cm long midline incision was performed, the right carotid artery and right jugular vein were localized and catheterized. Subsequently, catheters were tunnelled s.c. to the neck where they emerged through the skin, filled with heparinized saline and plugged. This surgical procedure lasted 20–30 min. After awakening from anaesthesia, animals were returned to the animal facilities where they were left for 3 days to fully recover; during this period the catheters were flushed daily with heparinized saline. Prior to the infusion of isotopes on GD 21, 1 ml arterial blood was withdrawn and Na2EDTA (final concentration, 1.2 mg ml−1) was added and the sample was centrifuged. Plasma was collected, snap-frozen and stored at −80°C for subsequent analysis of glucose and insulin levels. The isotopes 3-O-methyl-d-[3H]glucose (50 μCi kg−1) and [14C]methyl-aminoisobutyric acid (MeAIB, 10 μCi kg−1) were administered i.v. and the animal was allowed to move freely in a cage for 6 min. Then a sample of arterial blood (1 ml) was withdrawn and transferred to a vial containing Na2EDTA (final concentration, 1.2 mg ml−1) and plasma was collected after centrifugation for determination of radioactivity. The animal was killed by injection of 1 ml sodium pentobarbital (50 mg ml−1) i.v. exactly 7 min after isotope infusion. This time point was chosen based on preliminary experiments demonstrating low feto-maternal backflux of isotopes up to at least 7 min after isotope injection. Following the pentobarbital injection, the heart and thorax of the dam were opened and the fetuses were decapitated. All placentas and fetuses were weighed individually and then values for each were pooled from each litter and homogenized in three volumes of water. Homogenates were mixed with trichloroacetic (20%, 1 : 3) and the vials were centrifuged at 12 000 g for 10 min. Liquid scintillation fluid (12 ml; Aquasafe 300 plus, Zinsser Analytic) was added to 3 ml supernatant. Distilled water (3 ml) was added to 150 μl plasma samples followed by the addition of 12 ml scintillation fluid. Vials were shaken for 30 min prior to β-counting in a liquid scintillation counter.
Data presentation and statistics
Observations in individual fetuses and placentas of the same litter are not independent data points so an average was obtained for each litter in all measurements. Therefore, n = 1 represents averaged values in one litter and all data are presented as means ± s.e.m. Transport measurements are presented as placental uptake of isotope (placental disintegrations min−1 (d.p.m.) (g placenta)−1), placental transport of the isotope to the fetus (fetal d.p.m. (g fetus)−1) and the relative transport capacity of the placenta (fetal d.p.m. (g placenta)−1). Differences between control animals and those that were subjected to hyperglycaemic events in early or late pregnancy were evaluated statistically by Student's t test for unpaired observations. P < 0.05 was considered significant.
Results
Maternal blood glucose in response to I.P. glucose injections
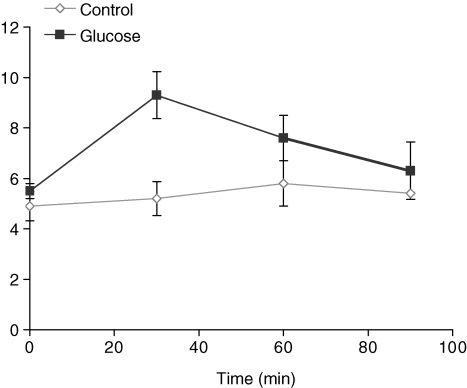
Administration of glucose i.p. to pregnant rats on GD 10 resulted in an approximately 2-fold increase in blood glucose level 30 min after the injection (Fig. 1). Subsequently, blood glucose concentrations declined and returned to baseline 90 min after the injection (Fig. 1).
Figure 1.
Blood glucose measurements in pregnant rats on GD 10 Animals were given a single i.p. injection of glucose (2 g kg−1, n = 5–8) or saline (control, n = 1–3).
Placental and fetal weights
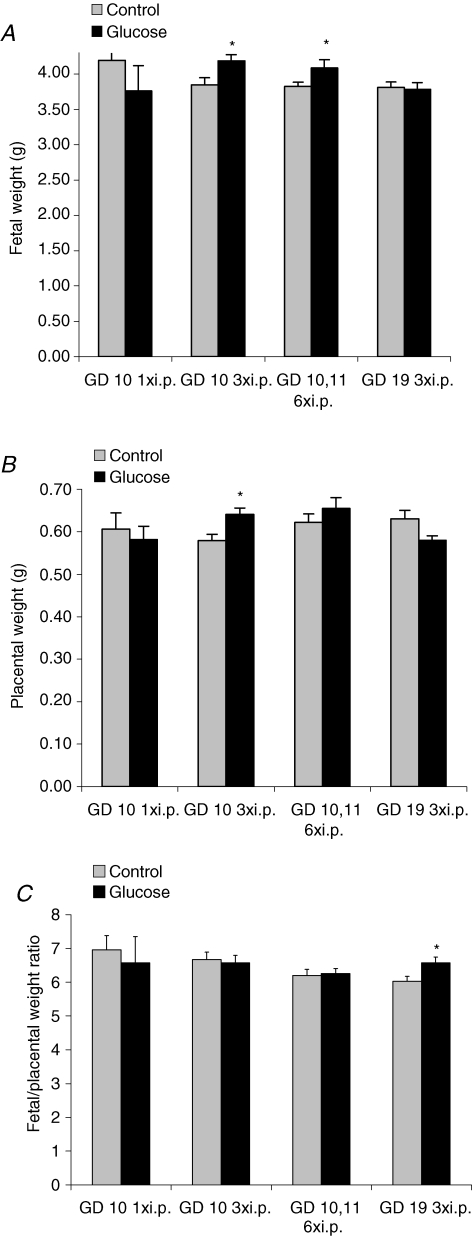
There was no significant difference in litter size between treatment and control animals in any of the groups (data not shown). One glucose injection in early pregnancy did not alter placental or fetal weight on GD 21 as compared to controls. By contrast, three glucose injections in early pregnancy caused an increase in fetal weight by 9% (4.19 ± 0.09 g, n = 9) compared to control animals (3.85 ± 0.10 g, n = 8, P < 0.05). Likewise, fetal weights at term were increased by 7% in rats receiving six glucose injections in early pregnancy (4.09 ± 0,12 g, n = 9) compared to controls (3.83 ± 0.06 g, n = 11, Fig. 2A, P = 0.05). Three glucose injections in late pregnancy (GD 19) did not affect fetal weights compared to the control group (Fig. 2A).
Figure 2.
Fetal and placental weights on GD 21 Fetal (A) and placental (B) weights and fetal/placental weight ratios (C) of rats given glucose or saline (control) i.p. once on GD 10 (n = 5), three times on GD 10 (n = 8–9), six times on GD 10 and 11 (n = 9–11) or three times on GD 19 (n = 7–8). *P < 0.05, versus control.
Placental weights were increased by 10% near term following three glucose injections in early pregnancy (0.64 ± 0.02 g, n = 9) compared to the control group (0.58 ± 0.02 g, n = 8, P < 0.05). A similar increase (+7%) in placental weight was observed in the animals that received six glucose injections in early pregnancy compared to control; however, this difference failed to reach statistical difference (Fig. 2B). As a result of the concomitant increases in fetal and placental weights in the groups given three and six glucose injections, respectively, in early pregnancy, the fetal/placental weight ratio was unaltered as compared to saline-injected controls (Fig. 2C). The fetal/placental weight ratio was significantly
(P < 0.05) increased after administration of three glucose injections on GD 19 (Fig. 2C).
Protein expression of placental transporters
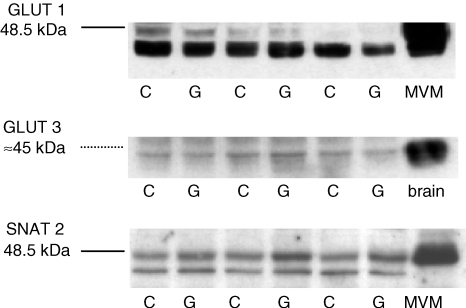
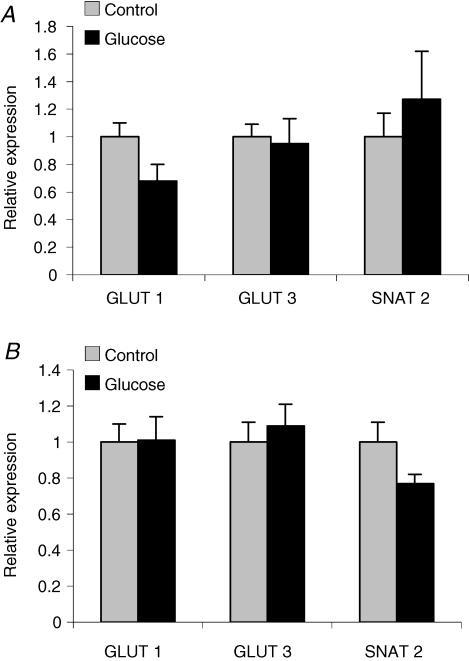
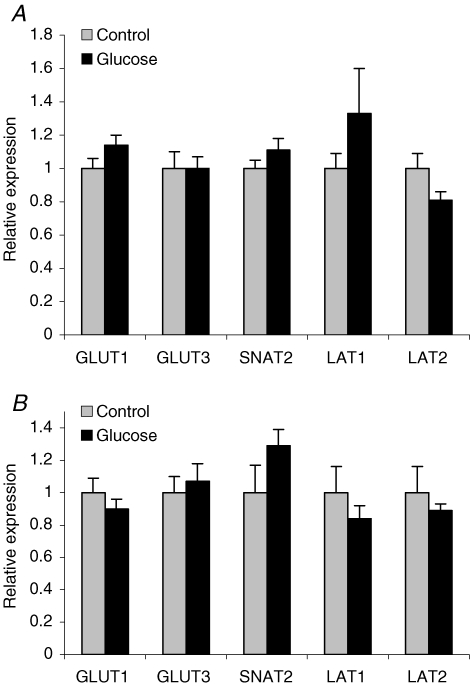
In order to identify alterations in placental nutrient transport, which could contribute to the increased fetal growth, we proceeded by measuring placental protein and gene expression of key nutrient transporters as well as placental nutrient transport in vivo at GD 21 in rats in which placental and fetal growth were elevated after glucose administration early in pregnancy (i.e. animals that received three or six glucose injections). GLUT1 and GLUT3 transporter proteins were identified at 45 kDa placental homogenates obtained at GD 21 (term) from animals that received three (n = 8–9) and six (n = 9–11) glucose injections in early pregnancy (representative Western blot shown in Fig. 3). Placental expression of GLUT1 and GLUT3 on GD 21 was unaffected by either treatment (Fig. 4A and B). The SNAT2 isoform of system A was detected at 48 kDa (Fig. 3) in placental homogenates at term. The specificity of the 48 kDa band was verified by pre-absorption of the SNAT2 antibody with the peptide antigen (data not shown). Placental SNAT 2 protein expression at GD 21 was not significantly changed in response to three (Fig. 4A) or six (Fig. 4B) glucose injections in early pregnancy.
Figure 3.
Representative Western blot of GLUT1, GLUT3 and SNAT2 protein in rat placental homogenates at GD 21 Isolated microvillous membranes (MVM) from human placenta and homogenate from rat brain were used as positive controls. G, placental homogenate obtained from glucose-treated animals; C, controls.
Figure 4.
The mean protein density of GLUT1, GLUT3 and SNAT2 as measured by Western blot analysis in rat placental homogenates Placental were collected from rats injected i.p. with glucose (2 g kg−1) or saline (control) three times on GD 10 (A, n = 8–9) and from rats injected a total of six times on GD 10 and 11 (B, n = 9–11). No significant changes were observed between glucose- and saline-treated groups. The mean density of the control group was assigned a value of 1 and the mean density of the hyperglycaemic group was calculated relative to the control group. Values are given as means ± s.e.m.
mRNA expression of placental transporters
mRNA of GLUT1, GLUT3, SNAT2, LAT1 and LAT2 were detected and amplified in placental tissue obtained on GD 21 in control rats and in animals subjected to three (n = 8) and six (n = 8–10) glucose injections in early pregnancy. The sizes of the PCR products were 260 bp for GLUT1, 130 bp for GLUT3, 705 bp for SNAT2, 202 bp for LAT1, 150 bp for LAT2 and 124 bp for SDHA; these were confirmed by gel electrophoresis. The quantity of GLUT1, GLUT3, SNAT2, LAT1 and LAT2 mRNA relative to the SDHA mRNA for each sample was unchanged in both treatment groups as compared to controls (Fig. 5A and B).
Figure 5.
The relative mRNA expression of GLUT1, GLUT3, SNAT2, LAT1 and LAT2 as measured by quantitative real time RT-PCR in rat placenta collected at GD 21 Animals given three i.p. injections of glucose or saline (control) on GD 10 are shown in A (n = 8) and animals receiving a total of six injections on GD 10 and 11 are shown in B (n = 8–10). No significant changes in mRNA levels were observed between controls and glucose-treated animals. Samples were normalized using the ratio of the target cDNA concentration to that of the housekeeping gene SDHA. The mean mRNA expression of the control group was assigned a value of 1 and the mean of the hyperglycaemic group was calculated relative to the control group for each gene. Values are given as means ± s.e.m.
Placental transport in vivo
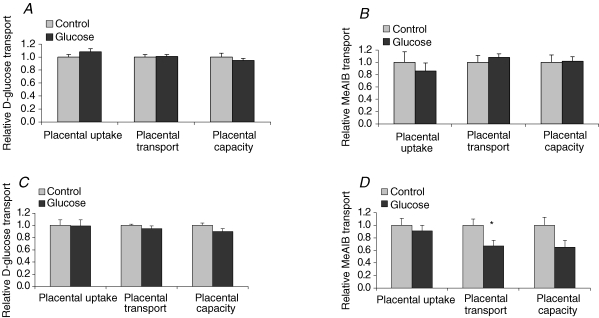
Placental transport measurements on GD 21 in animals subjected to three glucose injections in early pregnancy showed no significant differences in placental uptake (d.p.m. (g placenta)−1), placental transport (d.p.m. (g fetus)−1)or placental transport capacity (fetal d.p.m. (g placenta)−1) of 3-O-methyl-d-[3H]glucose (Fig. 6A) or MeAIB (Fig. 6B). A total of six glucose injections on GD 10 and 11 did not alter placental glucose transport at term (Fig. 6C). Likewise, no changes were observed in the placental uptake of MeAIB between groups (Fig. 6D). However, placental transport of MeAIB to the fetus at term in the animals that received six glucose injections in early pregnancy was significantly reduced by 33% (P < 0.05) and the placental capacity to transport MeAIB was 35% lower than in controls (P = 0.06, Fig. 6D).
Figure 6.
Placental transport in vivo on GD 21 Placental transport of 3-O-methyl-d-[3H]glucose and [14C]methyl-aminoisobutyric acid (MeAIB) in rats receiving glucose (n = 10) or saline (n = 9) three times on GD 10 (A) and in rats receiving glucose (n = 7) or saline (n = 8) a total of six times on GD 10 and 11. Placental d.p.m. (g placenta)−1 represents the placental uptake of isotope, fetal d.p.m. (g fetus)−1 shows the amount of isotope transported per gram fetus representing placental transport, and fetal d.p.m. (g placenta)−1 shows the amount of isotope transported per gram placenta (i.e. the relative transport capacity of the placenta). MeAIB transported to the fetus was significantly reduced (33%) in the group of animals that were treated with glucose six times in early pregnancy (B, P < 0.05) and placental transport capacity was also reduced (35%, P = 0.06). Transport data are presented as the glucose-treated group relative to the control group where control was assigned a value of 1. Values are given as means ± s.e.m.*P < 0.05, versus control.
Maternal circulating levels of glucose and insulin
In order to examine possible long-term alterations in maternal metabolic status after exposure to hyperglycaemia in early pregnancy we measured maternal glucose and insulin levels on GD 21 in animals subjected to a total of six i.p. injections on GD 10 and 11. Maternal plasma glucose concentration did not differ between the control group (6.65 ± 0.14 mm, n = 7) and the glucose-treated group (6.43 ± 0.06 mm, n = 6). Insulin levels on GD 21 were unchanged in the glucose-treated group of animals (2.29 ± 0.09 ng ml−1, n = 6) compared to control group (2.25 ± 0.08 ng ml−1, n = 7).
Discussion
The novel findings in this study are that (1) transient elevations of maternal blood glucose in early pregnancy, but not in late pregnancy, are sufficient to cause fetal overgrowth close to term in the pregnant rat, and (2) this effect could be explained by a larger placenta. These results suggest that the placental and fetal growth trajectory can be markedly affected by brief perturbations in maternal metabolism in early pregnancy, which may have implications for the clinical management of pregnancies complicated by type 1 diabetes.
The availability of nutrients determines fetal insulin secretion and therefore constitutes an important regulator of fetal growth. The delivery of nutrients to the fetus is dependent on factors such as maternal nutrient levels, placental blood flow and transport functions. In a now classical contribution to the research field, Pedersen (1954) proposed that increased maternal levels of nutrients, in particular hyperglycaemia, causes the increased fetal nutrient delivery and accelerated fetal growth in pregnancies complicated by type 1 diabetes. However, fetal overgrowth is still a major problem despite satisfactory glycaemic control in the pregnant woman with diabetes (Evers et al. 2002). One possible explanation is that complete normalization of glucose homeostasis may not be achievable in the pregnant woman with type 1 diabetes even with the most rigorous treatment regimens, and the tools used clinically to assess metabolic control may not be sensitive enough to detect it. Another possibility is that placental capacity to transport nutrients is altered by diabetes. In support of this hypothesis, placental glucose transporter expression and activity (Gaither et al. 1999; Jansson et al. 1999) and system A amino acid transporter activity (Jansson et al. 2002) are up-regulated at term in type 1 diabetes; these changes may result in increased fetal nutrient delivery even in situations with normal maternal nutrient levels.
The first trimester of pregnancy in women with type 1 diabetes is often associated with suboptimal glucose control. We have established an animal model where glucose injections (2 g kg−1, i.p.) to the early pregnant rat result in intermittent elevations of blood glucose by 50–100%. These changes are comparable to the blood glucose fluctuations often observed in the first trimester in pregnant women with type 1 diabetes (Kerssen et al. 2003). We have demonstrated that three or six transient hyperglycaemic episodes induced in early gestation (GD 10 and 11) were sufficient to increase fetal weights close to term. A single glucose injection at GD 10 or three glucose injections in late pregnancy (GD 19), however, did not alter fetal growth. Thus, there appears to be a fundamental difference in response to transient fluctuations in blood glucose between early and late pregnancy. These experiments demonstrate that even modest elevations of maternal blood glucose level in early pregnancy can affect the growth trajectory of the placenta and fetus, and establish a dose–response relationship between number of glucose injections and the effect. These data are in line with suggestions that in pregnancies in type 1 diabetic women, fluctuations in maternal glucose levels better explain accelerated fetal growth than glycosylated haemoglobin, which reflects average glucose levels over a longer time period (Greco et al. 2003). In addition, animal experiments have shown that the fetal insulin response is dependent on the duration and pattern of hyperglycaemia. In pregnant sheep, mild chronic hyperglycaemia in combination with three marked pulsatile hyperglycaemic episodes per day enhanced fetal insulin secretion more than chronic and more severe hyperglycaemia or hypoglycaemia, respectively (Carver et al. 1996).
In contrast to the situation in pregnant women with type 1 diabetes, endogenous insulin-secretion is not impaired in our rats and hyperglycaemia is accompanied by hyperinsulinaemia. Therefore, whether it is hyperglycaemia per se, increased plasma concentrations of insulin, or a combination of both that stimulate fetal growth in this model needs to be addressed in future studies. We hypothezised that transient hyperglycaemia in early pregnancy results in (1) increased placental growth and (2) an up-regulation of placental nutrient transport capacity. Placental weights were increased in response to three injections of glucose in early pregnancy (Fig. 2), suggesting that early placental growth is sensitive to glucose and/or insulin levels of the mother. Indeed, in most populations there is an intimate relationship between placental weight and birth weight both in normal pregnancies (Kloosterman, 1970) and in pregnancies complicated by restricted or accelerated fetal growth (Jansson et al. 1998, Jansson 2002). This suggests that changes in placental growth may in part mediate effects of reduced placental blood flow (as in many cases of IUGR) and altered maternal metabolism (as in maternal diabetes) on the growth of the fetus. Wallace et al. (2006) developed a model involving overnutrition of pregnant adolescent sheep and studied the effect of these nutritional manipulations on feto-placental growth and function. Overnourishing adolescent sheep results in placental and fetal growth restriction (Wallace et al. 2000). This is a distinctly different protocol compared to our rat model; nevertheless, a change in placental growth also appears to be an important cause of altered fetal growth in this adolescent sheep model. For example, switching maternal diets at the end of the first trimester affected the growth trajectory of the placenta, which altered fetal growth (Wallace et al. 1999), and by late pregnancy placental and fetal weights were changed in parallel and placental nutrient transport capacity, when expressed on a placental weight specific basis, remained unchanged (Wallace et al. 2003).
We did not find any experimental evidence to support our hypothesis that brief hyperglycaemia in early pregnancy stimulates fetal growth by up-regulation of placental nutrient transporters. Placental transport of glucose measured in vivo close to term was unaffected by hyperglycaemic episodes in early pregnancy. In line with these findings, placental expression of GLUT1 and GLUT3 was not altered in animals that had been subjected to three or six glucose injections in early pregnancy. However, specific changes in these placental nutrient transport systems prior to term were not investiged in this study and therefore cannot be excluded. Placental system A transport activity was shown to be down-regulated near term in response to six glucose injections, but unaffected by three glucose injections in early pregnancy. It is interesting that down-regulation of placental system A activity has been associated with increased fetal growth in a study of British women with diabetes and those giving birth to LGA babies (Kuruvilla et al. 1994). The down-regulation of placental system A in our study may be a mechanism to counteract the acceleration of fetal growth in response to an increased supply of other key nutrients. Thus, an increase of placental transport of nutrients other than those mediated by system A and glucose transporters may contribute to the increased fetal growth in response to hyperglycaemia in early pregnancy. For example, placental transport of free fatty acids or essential amino acids may be increased in analogy to the increased placental lipoprotein lipase activity in type 1 diabetes with fetal overgrowth (Magnusson et al. 2004) and the up-regulated placental leucine transport in women with gestational diabetes giving birth to LGA babies (Jansson et al. 2002).
We can only speculate on the physiological role of a mechanism where maternal metabolism and nutrient levels early in pregnancy has a marked influence on the growth trajectory of the fetus. Recently presented models for the intrauterine programming of adult disease have suggested that fetal metabolism and growth are adapted to the predicted postnatal metabolic environment, represented by the nutrient supply in utero (Gluckman & Hanson, 2004). We have recently proposed that it is the placenta that mediates this predictive adaptive response by sensing the ability of the maternal supply line to deliver nutrients, thus regulating placental growth and nutrient transporter expression/activity and, as a result, fetal nutrient supply (Jansson & Powell, 2006). We therefore speculate that maternal nutrition and metabolism in early pregnancy represent a key environmental cue to which the placenta responds in order to match fetal growth rate with the available resources of the mother.
In summary, we show for the first time in an experimental model that transient hyperglycaemia in early, but not late, pregnancy is sufficient to stimulate fetal growth. These findings may be relevant for pregnant women with type 1 diabetes, who often give birth to large babies despite excellent glucose control in the second and third trimester. In our rodent model, the accelerated fetal growth may be explained by the presence of a larger placenta allowing more nutrients to be transferred to the fetus. Indeed in most human pregnancies, placental weights are also elevated in cases of LGA births. These data suggest that metabolic control in early pregnancy is an important determinant for feto-placental growth throughout gestation.
Acknowledgments
We would like to thank AnnaLena Leverin at the Institute of Neuroscience and Physiology at Gothenburg University for technical assistance. This study was supported by grants from the Swedish Research Council (10838 and 14555), the Swedish Diabetes Association, Frimurare-Barnhus-direktionen, the Åhlens Foundation, the Sven Jerring Foundation, the Willhelm and Martina Lundgren Foundation and the Lars Hierta Foundation.
References
- Blomgren K, Hallin U, Andersson AL, Puka-Sundvall M, Bahr BA, McRae A, Saido TC, Kawashima S, Hagberg H. Calpastatin is up-regulated in response to hypoxia and is a suicide substrate to calpain after neonatal cerebral hypoxia-ischemia. J Biol Chem. 1999;274:14046–14052. doi: 10.1074/jbc.274.20.14046. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cannon-Carlson S, Tang J. Modification of the Laemmli sodium dodecyl sulfate-polyacrylamide gel electrophoresis procedure to eliminate artifacts on reducing and nonreducing gels. Anal Biochem. 1997;246:146–148. doi: 10.1006/abio.1997.2002. [DOI] [PubMed] [Google Scholar]
- Carver TD, Anderson SM, Aldoretta PW, Hay WW., Jr Effect of low-level basal plus marked ‘pulsatile’ hyperglycemia on insulin secretion in fetal sheep. Am J Physiol. 1996;271:E865–E871. doi: 10.1152/ajpendo.1996.271.5.E865. Endocrinol Metab. [DOI] [PubMed] [Google Scholar]
- Casey BM, Lucas MJ, McIntire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol. 1997;90:869–873. doi: 10.1016/s0029-7844(97)00542-5. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Hamark B, Powell TL, Jansson T. Glucose transporter isoform 4 is expressed in the syncytiotrophoblast of first trimester human placenta. Hum Reprod. 2005;20:521–530. doi: 10.1093/humrep/deh596. [DOI] [PubMed] [Google Scholar]
- Evers IM, de Valk HW, Mol BW, ter Braak EW, Visser GH. Macrosomia despite good glycaemic control in type I diabetic pregnancy; results of a nationwide study in the Netherlands. Diabetologia. 2002;45:1484–1489. doi: 10.1007/s00125-002-0958-7. [DOI] [PubMed] [Google Scholar]
- Gaither K, Quraishi AN, Illsley NP. Diabetes alters the expression and activity of the human placental GLUT1 glucose transporter. J Clin Endocrinol Metab. 1999;84:695–701. doi: 10.1210/jcem.84.2.5438. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Greco P, Vimercati A, Scioscia M, Rossi AC, Giorgino F, Selvaggi L. Timing of fetal growth acceleration in women with insulin-dependent diabetes. Fetal Diagn Ther. 2003;18:437–441. doi: 10.1159/000073139. [DOI] [PubMed] [Google Scholar]
- Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precede the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576:935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002;51:2214–2219. doi: 10.2337/diabetes.51.7.2214. [DOI] [PubMed] [Google Scholar]
- Jansson T, Powell TL. Placental nutrient transfer and fetal growth. Nutrition. 2000;16:500–502. doi: 10.1016/s0899-9007(00)00323-3. [DOI] [PubMed] [Google Scholar]
- Jansson T, Powell TL. IFPA 2005 Award in Placentology Lecture. Human placental transport in altered fetal growth: does the placenta function as a nutrient sensor?– a review. Placenta. 2006;27(Suppl. A):S91–S97. doi: 10.1016/j.placenta.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Jansson T, Wennergren M, Powell TL. Placental glucose transport and GLUT 1 expression in insulin-dependent diabetes. Am J Obstet Gynecol. 1999;180:163–168. doi: 10.1016/s0002-9378(99)70169-9. [DOI] [PubMed] [Google Scholar]
- Johansson M, Jansson T, Powell TL. Na+-K+-ATPase is distributed to microvillous and basal membrane of the syncytiotrophoblast in human placenta. Am J Physiol Regul Integr Comp Physiol. 2000;279:R287–R294. doi: 10.1152/ajpregu.2000.279.1.R287. [DOI] [PubMed] [Google Scholar]
- Kerssen A, Evers IM, de Valk HW, Visser GH. Poor glucose control in women with type 1 diabetes mellitus and ‘safe’ hemoglobin A1c values in the first trimester of pregnancy. J Matern Fetal Neonatal Med. 2003;13:309–313. doi: 10.1080/jmf.13.5.309.313. [DOI] [PubMed] [Google Scholar]
- Kloosterman GJ. On intrauterine growth, the significance of prenatal care. Int J Gynecol Obstet. 1970;8:985–989. [Google Scholar]
- Kuruvilla AG, D'Souza SW, Glazier JD, Mahendran D, Maresh MJ, Sibley CP. Altered activity of the system A amino acid transporter in microvillous membrane vesicles from placentas of macrosomic babies born to diabetic women. J Clin Invest. 1994;94:689–695. doi: 10.1172/JCI117386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling R, Bridges CC, Sugawara M, Fujita T, Leibach FH, Prasad PD, Ganapathy V. Involvement of transporter recruitment as well as gene expression in the substrate-induced adaptive regulation of amino acid transport system A. Biochim Biophys Acta. 2001;1512:15–21. doi: 10.1016/s0005-2736(01)00310-8. [DOI] [PubMed] [Google Scholar]
- Magnusson AL, Waterman IJ, Wennergren M, Jansson T, Powell TL. Triglyceride hydrolase activities and expression of fatty acid binding proteins in the human placenta in pregnancies complicated by intrauterine growth restriction and diabetes. J Clin Endocrinol Metab. 2004;89:4607–4614. doi: 10.1210/jc.2003-032234. [DOI] [PubMed] [Google Scholar]
- Meller M, Vadachkoria S, Luthy DA, Williams MA. Evaluation of housekeeping genes in placental comparative expression studies. Placenta. 2005;26:601–607. doi: 10.1016/j.placenta.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol (Copenh) 1954;16:1554–1562. doi: 10.1530/acta.0.0160330. [DOI] [PubMed] [Google Scholar]
- Persson B, Hanson U. Fetal size at birth in relation to quality of blood glucose control in pregnancies complicated by pregestational diabetes mellitus. Br J Obstet Gynaecol. 1996;103:427–433. doi: 10.1111/j.1471-0528.1996.tb09768.x. [DOI] [PubMed] [Google Scholar]
- Pettitt DJ, Bennett PH, Saad MF, Charles MA, Nelson RG, Knowler WC. Abnormal glucose tolerance during pregnancy in Pima Indian women. Long-term effects on offspring. Diabetes. 1991;40(Suppl. 2):126–130. doi: 10.2337/diab.40.2.s126. [DOI] [PubMed] [Google Scholar]
- Pettitt DJ, Nelson RG, Saad MF, Bennett PH, Knowler WC. Diabetes and obesity in the offspring of Pima Indian women with diabetes during pregnancy. Diabetes Care. 1993;16:310–314. doi: 10.2337/diacare.16.1.310. [DOI] [PubMed] [Google Scholar]
- Rey E, Attie C, Bonin A. The effects of first-trimester diabetes control on the incidence of macrosomia. Am J Obstet Gynecol. 1999;181:202–206. doi: 10.1016/s0002-9378(99)70460-6. [DOI] [PubMed] [Google Scholar]
- Sibley CP, Turner MA, Cetin I, Ayuk P, Boyd CA, D'Souza SW, Glazier JD, Greenwood SL, Jansson T, Powell T. Placental phenotypes of intrauterine growth. Pediatr Res. 2005;58:827–832. doi: 10.1203/01.PDR.0000181381.82856.23. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP, Cruickshank MA. Switching maternal dietary intake at the end of the first trimester has profound effects on placental development and fetal growth in adolescent ewes carrying singleton fetuses. Biol Reprod. 1999;61:101–110. doi: 10.1095/biolreprod61.1.101. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP, Milne JS, Hay WW., Jr Placental glucose transport in growth-restricted pregnancies induced by overnourishing adolescent sheep. J Physiol. 2003;547:85–94. doi: 10.1113/jphysiol.2002.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP, Palmer RM, Da Silva P, Cruickshank MA. Relationship between nutritionally-mediated placental growth restriction and fetal growth, body composition and endocrine status during late gestation in adolescent sheep. Placenta. 2000;21:100–108. doi: 10.1053/plac.1999.0440. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Luther JS, Milne JS, Aitken RP, Redmer DA, Reynolds LP, Hay WW., Jr Nutritional modulation of adolescent pregnancy outcome – a review. Placenta. 2006;27(Suppl. A):S61–S68. doi: 10.1016/j.placenta.2005.12.002. [DOI] [PubMed] [Google Scholar]