Abstract
Nitric oxide (NO) and endothelin-1 (ET-1) are known to play a major role in renal and vascular pathophysiology and exhibit a close interaction with ET-1, stimulating NO production; NO in turn inhibits ET-1 expression. Our objectives were (1) to establish a novel transgenic mouse model facilitating ET-1 expression assessment in vivo, (2) to validate this model by assessing prepro-ET-1 promoter activity in mice embryos by means of our novel model and comparing expression sites to well-established data on ET-1 in fetal development and (3) to investigate renal ET–NO interaction by assessing prepro-ET-1 promoter activity in different structures of the renal cortex in the setting of blocked NO synthases via l-NAME administration. We established transgenic mice carrying a lacZ reporter gene under control of the human prepro-ET-1 gene promoter sequence (8 kb of 5′ sequences). Bluo-Gal staining of tissue sections revealed intracellular blue particles as indicators of prepro-ET-1 promoter activity. In mouse embryos, we detected high prepro-ET-1 promoter activity in the craniofacial region, as well as in bone and cartilage consistent with the literature. In order to investigate the interaction of ET-1 and NO in the kidney in vivo, transgenic mice at the age of 3–4 months were treated with a single dose of the NO synthase inhibitor l-NAME (25 mg (kg bw)−1i.p.) 12 h before kidney removal. Bluo-Gal staining of kidney sections revealed intracellular blue particles as indicators of prepro-ET-1 promoter activity in tubular and vascular endothelium and glomerular cells. Particle count was closely correlated to kidney tissue ET-1 content (R = 0.918, P < 0.001). Comparison of counts revealed an increase by 135 ± 53% in l-NAME treated (n = 12) compared to non-treated mice (n = 10, P = 0.001). Cell-type specific evaluation revealed an increase of 136 ± 51% in tubular (P = 0.001) and 105 ± 41% in glomerular cells (P = 0.046), but no significant increase in vascular endothelium. In conclusion, our study revealed a close interaction of renal endothelin and the NO system in a cell-type specific manner. Our new transgenic model provides a unique opportunity to analyse regulation of the ET system on a cellular level in vivo.
Endothelin (ET-1) is one of the most potent endogenous vasoconstrictors acting via two subtypes of G protein-coupled heptahelical receptors, termed ETA and ETB (Sakurai et al. 1990; Arai et al. 1990). The vascular ETA receptors are located on smooth muscle cells mediating sustained vasoconstriction. ETB receptors are present on endothelial cells and mediate the production of nitric oxide (NO) and vasodilator prostanoids (de Nucci et al. 1988), but they are also present on some vascular smooth muscle where they mediate vasoconstriction (Haynes et al. 1995).
Within the kidney, the main actions of ET-1 are the paracrine and autocrine regulation of blood flow and glomerular haemodynamics (Yokokawa et al. 1989), sodium and water balance (Hocher et al. 2001), and acid–base homeostasis (Wesson, 2000). ET-1 is produced by glomerular epithelial, mesangial, renal tubular and medullary collecting duct cells (Kohan, 1997). The role of ET-1 in the kidney may even be dissociated from circulating ET-1 as plasma endothelin levels do not account for urinary ET-1 content, which is therefore assumed to be predominantly of renal origin (Serneri et al. 1995). Moreover, the endothelin system has both in animal models and in human studies been shown to play a major role in kidney pathophysiology, e.g. in diabetic kidney disease (Lee et al. 1994; Minchenko et al. 2003;Pfab et al. 2006), polycystic kidney disease (Hocher et al. 2003; Reiterova et al. 2006), contrast-induced nephropathy (Wang et al. 2000) and kidney fibrosis (Hocher et al. 1997).
The human mRNA for prepro-ET-1 is encoded in five exons distributed over 6836 base pairs (Inoue et al. 1989). Two cis-acting elements, the GATA and AP-1 sites, which are located upstream of the transcription start site, are essential to maintain high promoter activity in endothelial cells (Lee et al. 1990). GATA-2 binds to the GATA site, and c-fos and c-jun bind to the AP-1 site (Lee et al. 1991; Kawana et al. 1995). The primary translation product of the human ET-1 gene is the preproendothelin-1 (prepro-ET-1) peptide, containing 212 amino acids.
The regulation of ET-1 release occurs mainly at the level of gene transcription (Inoue et al. 1989; Miyauchi & Masaki, 1999). Various stimuli have been shown to increase ET-synthesis including vasoactive hormones such as angiotensin II and vasopressin. In contrast, the NO–cGMP system (Boulanger & Luscher, 1990), prostacyclin, and the natriuretic peptides have been shown to inhibit ET-1 expression (Giannessi et al. 2001) thus creating a negative feedback loop with ET-1 stimulating production of NO, and this in turn inhibiting ET-1 production. This ET-1–NO interaction is of major pathophysiological impact as we recently demonstrated that in the case of an activated ET system additional lack of NO leads to significant further up-regulation of the ET system followed by increased hypertension (Quaschning et al. 2007).
Investigation of ET-1 expression and its modulation by NO in different structures in vivo is technically demanding and resource consuming. On the other hand, extrapolation from cell-culture studies with various cell types to the situation in vivo is limited to a certain extent.
Thus the purpose of our present study was threefold. First we established a transgenic mouse model harbouring a reporter gene (lacZ) under control of the prepro-ET-1 promoter to facilitate in vivo assessment of ET-1 gene expression. Second, we tested the validity of our model by assessing prepro-ET-1 promoter activity in mice embryos by means of our novel model and comparing the detected expression sites to well-established data on ET-1 in fetal development. Third, we used our model to investigate renal ET–NO interaction by assessing prepro-ET-1 promoter activity in different structures of the renal cortex in the setting of blocked NO-synthases via l-NAME administration.
Methods
Prepro-ET-1 lacZ transgenic mice
All animal experiments were conducted in accordance with state laws governing the use of experimental animals. For detection of the activity of the prepro-ET-1 promoter and its spatial expression pattern, transgenic mice were generated carrying a reporter gene construct of the bacterial β-galactosidase gene (lacZ) driven by the human prepro-ET-1 promoter sequence (Inoue et al. 1989; Kawana et al. 1995). A construct harbouring 8.0 kb of genomic 5′ sequences of the human prepro-ET-1 gene (kindly provided by Dr Thomas Quertermous, Stanford University, USA) fused to the lacZ gene was microinjected into pronuclei of NMRI fertilized mouse oocytes, as previously described (Theuring et al. 1990; Aguzzi & Theuring, 1994). Transgenic animals were identified by Southern Blot and PCR. Initially, two lines of transgenic mice were created. As in preliminary investigations there were no differences detectable between the lines thus we randomly selected one line for further studies. Male and female heterozygous prepro-ET-1 lacZ-transgenic mice were used for further experiments. In a separate series of experiments, we also analysed prepro-ET-1 promoter activity during embryonic development. Mouse embryos were obtained by caesarean section at various embryonic days (E13.5, 14.5, and 16.5).
l-NAME treatment
l-NAME was given i.p. 12 h before sacrifice in a dose of 25 mg (kg body weight)−1 as described in the literature (Brandes et al. 2000; Kaminski et al. 2006). For comparison of β-galactosidase activity we used 12 l-NAME treated prepro-ET-1 lacZ transgenic mice and 12 non-treated prepro-ET-1 lacZ transgenic animals as a control. All mice were 3–4 months of age. Mice were sacrificed by rapid neck dislocation without general anaesthesia, and kidneys were removed and stained with Bluo-Gal as described below except for a 3 mm piece from the upper pole of the left kidney, which was snap frozen in liquid nitrogen for ET-1 ELISA. For assessment of the effect of l-NAME on arterial blood pressure we measured blood pressure as described below in prepro-ET-1 lacZ transgenic mice directly before and 12 h after l-NAME administration.
Detection of β-galactosidase activity
β-Galactosidase activity was detected by 5-bromo-3-indolyl-β-d-galactopyranoside (Bluo-Gal) staining (Aguzzi & Theuring, 1994). Frozen kidney sections and embryo sections of 2–3 μm were prefixed in PBS (1.9 mm NaH2PO4, 8.1 mm Na2HPO4, 154 mm NaCl) containing 1% (v/v) formalin, 0.2% glutaraldehyde, and 0.02% Nonidet P40 for 1 h at 4°C, washed twice in PBS and stained for 24 h at 31°C in a solution of PBS containing 3.1 mm K3Fe(CN)6, 3.1 mm K4Fe(CN)6, 1 mm MgCl2 and 0.05 m Bluo-Gal. The kidney sections and whole embryo sections were then washed twice in PBS and fixed in PBS containing 4% (v/v) formalin. Fixed kidney sections were embedded in paraffin and 5 μm sections were counterstained with Sirius Red. Reporter gene activity in kidney sections was quantified by counting blue precipitates in one whole section of both kidneys per mouse under a light microscope (×400 magnification) without prior knowledge of the investigator to which group the sample belonged. The amounts of particles were counted for each tubule, intrarenal artery and glomerulus in the renal cortex and a mean value for these counts was calculated for each animal per square millimetre of section surface. For further analysis we used the Image 1.6 software (shareware from the NIH). Whole sagittal kidney sections were scanned and the image colour was divided into three channels (red–green–blue). Afterwards, the percentage of the blue coloured area of the kidney section was calculated.
Invasive blood pressure measurement
Arterial blood pressure was measured in anaesthetized (100 mg (kg body weight)−1 ketamine and 10 mg (kg body weight)−1 xylazine i.p.) mice. Mice were placed on a heating table to maintain body temperature. After preparation, the right carotid artery was cannulated with a Teflon tube (i.d. 0.3 mm; o.d. 0.6 mm) connected to a Transpac blood pressure transducer, Abbott Ireland, Sligo, Ireland and a PowerLab/4sp system, ADInstruments, Hastings, UK. The blood pressure curves were recorded and further analysed with Chart 4.0 for Windows. From the pressure curves systolic and diastolic blood pressure, as well as the heart rate were calculated with the automated functions of the program.
Clinical chemistry and peripheral blood cell count
Blood was drawn from the abdominal v. cava of anaesthetized prepro-ET-1 lacZ transgenic mice and non-transgenic mice from the same strain. Serum concentrations of sodium, potassium, protein, creatinine and urea were determined using the appropriate commercial kits in a Hitachi 717 automatic analyser (Boehringer Mannheim, Germany). Peripheral blood cell counts were performed in a Sysmex K1000 blood cell counter (Sysmex Corp., Kobe, Japan). All analyses were performed in the department of clinical biochemistry and laboratory medicine of the Charité university hospital, Berlin, Germany.
Histological evaluation
Histological evaluation was performed as previously described (Hocher et al. 2001). For pathohistological evaluation all samples were embedded in paraffin. Kidney sections of 3 μm were submitted to haematoxylin–eosin, periodic acid–Schiff (PAS), or Sirius Red staining. Glomerulosclerosis was defined by the presence of increased amounts of PAS positive material within the glomeruli. To consider differences in the degree of glomerulosclerosis, a semiquantitative score was used. A minimum of 80 glomeruli in each specimen was examined and each lesion was graded from 0 to 4 according to the percentage of glomerular involvement. Thus a grade 1 lesion represented an involvement of 25% of the glomerulus, while a grade 4 lesion indicated that 100% of the glomerulus was PAS positive. All tissue samples were independently evaluated by two investigators without prior knowledge of the group to which the sample belonged. The severity of interstitial matrix deposition was likewise evaluated by semiquantitative scoring after Sirius Red staining.
ET-1 ELISA
Analysis of kidney tissue ET-1 concentrations was performed as recently described (Hocher et al. 1998). Frozen kidney sections were powdered in the presence of liquid nitrogen. The powder was suspended and subsequently homogenized using a motor-driven pestle homogenizer in 2 ml of 0.14 mol l−1 NaCl; 2.6 mmol l−1 KCl; 8 mmol l−1 Na2HPO4; 1.4 mmol l−1 KH2PO4; 1% Triton X-100; at pH 7.4. The homogenates were centrifuged at 4°C for 60 min at 100 000 g and the supernatants retained for determination of ET-1 and big ET-1 by ELISA performed according to the instructions given by the manufacturer (Biomedica, Vienna, Austria). Cross reactivity for ET-1 was as follows: ET-1 (1–21): 100%; ET-2 (1–21): 100%; ET-3 (1–21): < 5%; big ET-1 (1–38): < 1%; big ET-1 (22–38): < 1%. Tissue ET-1 content was calculated as content per mg protein in the tissue.
Statistical analysis
Values are expressed as means ± s.d. Comparison between groups for significant differences were performed by Mann–Whitney U-testing in SPSS 12.0.1 (SPSS Inc., Chicago, IL, USA). Statistical significance was assumed with a probability error P < 0.05.
Results
Phenotypic characterization of Prepro-ET-1 lacZ transgenic mice
In order to rule out potential alteration of the phenotype related to insertion of the transgene we performed extensive phenotypic assessment including histological evaluation of kidney sections, analysis of clinical chemistry, peripheral blood count and measurement of arterial blood pressure in prepro-ET-1 lacZ transgenic mice compared to non-transgenic mice from the same strain. Histological evaluation of kidney sections showed no differences between prepro-ET-1 lacZ transgenic and non-transgenic mice. In each group 10 mice were evaluated. The semiquantitative score for glomerulosclerosis was 2.21 ± 0.29 in prepro-ET-1 lacZ transgenic and 2.16 ± 0.23 in non-transgenic mice, P = 0.631. The scores for interstitial fibrosis were 1.76 ± 0.14 in prepro-ET-1 lacZ transgenic and 1.72 ± 0.09 in non-transgenic mice, P = 0.529. In basic clinical chemistry and peripheral blood count there were no differences between the groups (see Table 1). The mean ± s.d. of systolic blood pressure (SBP) was 124.3 ± 7.7 mmHg, diastolic blood pressure (DBP) was 90.7 ± 7.5 mmHg, and heart rate (HR) was 640 ± 23 min−1 in prepro-ET-1 lacZ transgenic mice. In non-transgenic mice from the same strain blood pressure was similar (SBP: 126.3 ± 6.5 mmHg, DBP: 91.8 ± 8.1 mmHg, HR: 628 ± 38 min−1; n = 5 in each group).
Table 1.
Basic characteristics of the transgenic model versus wildtype control
| lacZ transgenic | non-transgenic | |
|---|---|---|
| Blood cell count | ||
| Ery (x 106 mm−3) | 9.475 ± 0.72 | 9.115 ± 0.68 |
| Leuc (x 103 mm−3) | 6.915 ± 0.66 | 6.605 ± 0.62 |
| Hb (g l−1) | 8.435 ± 0.74 | 8.35 ± 0.59 |
| Ht (%) | 0.46 ± 0.05 | 0.435 ± 0.06 |
| MCV (fl) | 48.78 ± 2.78 | 47.93 ± 3.01 |
| MCHC (%) | 4.52 ± 0.33 | 4.77 ± 0.52 |
| Thromb (x 104 mm−3) | 111.60 ± 55.01 | 122.35 ± 74.45 |
| Clinical chemistry | ||
| Creatinine (mmol l−1) | 34.40 ± 7.11 | 35.25 ± 9.34 |
| Sodium (mmol l−1) | 148.00 ± 2.58 | 147.25 ± 6.37 |
| Urea (mmol l−1) | 16.40 ± 4.51 | 15.04 ± 5.77 |
| Protein (mg dl−1) | 64.05 ± 7.48 | 63.25 ± 4.66 |
| Histological evaluation | ||
| Interstitial fibrosis | 1.76 ± 0.14 | 1.72 ± 0.09 |
| Glomerulosclerosis | 2.21 ± 0.29 | 2.16 ± 0.23 |
Basic characterization of our transgenic model versus wild-type control: clinical chemistry, peripheral blood count and kidney histology. No differences between prepro-ET-1 lacZ transgenic and non-transgenic mice were detectable. Ery = erythrocytes, Leuc = leucocytes, Hb = haemoglobin, Ht = haematocrit, MCV = mean corpuscular volume, MCHC, mean corpuscular haemoglobin concentration, Thromb = thrombocytes.
Prepro-ET-1 promoter activity during embryonic development
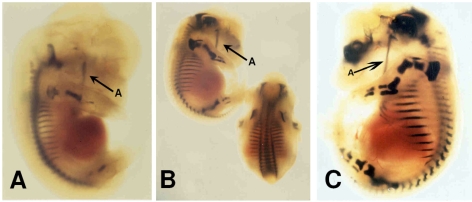
By employing a Bluo-Gal staining protocol, a clear staining was detected at various stages during embryogenesis by analysing whole mount embryos and sagittal sections thereof (Fig. 1).
Figure 1.
Typical whole-mount embryos of prepro-ET-1 lacZ transgenic mice Whole-mount pattern of 8.0 kb hET-1-lacZ expression in transgenic mice. Embryo at E13.5 (A), E14.5 (B) and E16.5 (C) exhibiting high lacZ labelling, i.e. dark blue staining in chondrification centres (e.g. the mandible, arrow A).
Expression of the reporter gene correlates with the occurrence of chondrification centres during embryonic development, the process of forming cartilage by the secretion of a homogeneous matrix between the more primitive mesodermal cells. Serial sections obtained from these embryos revealed that proliferating chondrocytes displayed reporter gene activity.
Arterial blood pressure after l-NAME dosing
Twelve hours after l-NAME dosing of adult prepro-ET-1 lacZ transgenic mice systolic and diastolic blood pressure increased significantly (P = 0.025) versus non-treated prepro-ET-1 lacZ transgenic mice (SBP: 175.5 ± 14.4 mmHg, DBP: 110.7 ± 11.9 mmHg versus SBP: 124.3 ± 7.7 mmHg and DBP: 90.7 ± 7.5 mmHg, n = 5 in each group), whereas HR did not change significantly (HR: 640 ± 23 versus 628 ± 38 min−1).
Prepro-ET-1 promoter activity in kidney sections
Kidney sections of non-transgenic mice from the same strain were stained with Bluo-Gal according to the standard protocol. In those kidney sections only a very small amount of blue particles was detected due to weak unspecific β-galactosidase expression in the kidney.
In adult prepro-ET-1 lacZ transgenic mice, prominent blue particles were detected in renal tubular epithelial, glomerular, as well as vascular endothelial cells. Almost all particles were located to the intracellular space despite a very small number of particles (< 1% of the total count) that were not clearly associated to the intracellular space. We suggest that those particles are artificial and left the intracellular space during the procedure of staining. Therefore extracellular particles were excluded from analysis.
The amount of blue particles after Bluo-Gal staining of prepro-ET-1 lacZ transgenic mice was quantified in order to assess the activity of the prepro-ET-1 promoter. After subdividing the image colour of scans of whole kidney sections the percentage of blue of the whole image colour in kidney sections was 58.6 ± 9.6% in controls and 67.8 ± 7.5% in l-NAME treated mice, P = 0.002 for n = 10 in each group.
For further analysis we counted blue particles as indicators for Prepro-ET-1 promoter activity in kidney sections (Fig. 2). Comparison of the overall blue particle counts of the kidney cortex revealed an increase by 135 ± 53% in l-NAME treated (n = 12) compared to non-treated mice (n = 10, P = 0.001) (Fig. 3A). Cell-type specific analysis revealed significantly higher particle counts in both renal tubular and glomerular cells of l-NAME treated mice, whereas in vascular endothelium no significant difference versus non-treated mice was detected: The increase was 136 ± 51% in tubular (P = 0.001) and 105 ± 41% in glomerular cells (P = 0.046) (Fig. 3B).
Figure 2.
Typical kidney sections of prepro-ET-1 lacZ transgenic mice in Bluo-Gal-staining The Bluo-Gal reaction product precipitates in the form of small needle-shaped blue crystals. To access the activity of the prepro-ET-1 promoter the amount of blue particles after Bluo-Gal staining of prepro-ET-1 lacZ transgenic mice was quantified in Sirius Red counterstained kidney sections. After l-NAME treatment (A), the amount of blue particles was higher in renal tubules compared to non-treated prepro-ET-1 lacZ transgenic mice (B).
Figure 3.
Overall and cell-type specific assessment of Bluo-Gal-positive particles in the kidney cortex A, Bluo-Gal-positive particles in kidney cortex. Counts of Bluo-Gal-positive particles in the cortex of whole kidney sections of non-treated prepro-ET-1 lacZ transgenic mice (n = 10) versusl-NAME-treated transgenic mice (n = 12). All values are given as means ± s.d.; *P < 0.05. B, relative increase of Bluo-Gal-positive particles in different renal tissues after l-NAME treatment. Relative increase of Bluo-Gal positive particles in tubular (tub), glomerular (glom) and vascular (vasc) tissue after l-NAME treatment. All values (mean ± s.d.) are given as percentage in l-NAME-treated prepro-ET-1 lacZ mice normalized to untreated controls (100%). ns: no significant increase due to l-NAME treatment.
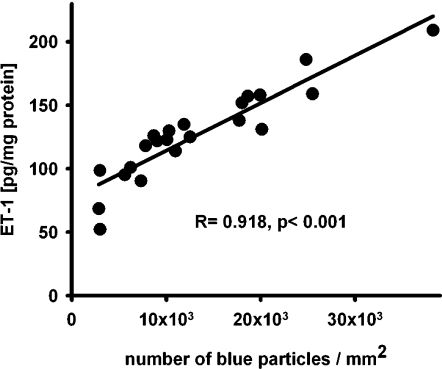
Moreover, regression analysis showed very close and significant correlation (R = 0.918, P < 0.001) between renal tissue concentration of ET-1 and the amount of blue particles in the kidney sections of each mouse (Fig. 4).
Figure 4.
Regression analysis of ET-1 contents and Bluo-Gal-positive particles in the kidney Regression analysis of renal ET-1 tissue content and Bluo-Gal-positive particles in kidneys from prepro-ET-1 lacZ mice.
Discussion
The ET system is involved in kidney pathophysiology such as diabetic nephropathy and polycystic kidney disease. Moreover the ET system has a strong and complex interaction with the NO system (Quaschning et al. 2007). Thus our objective was to investigate renal cell-type specific ET-1–NO interaction on the promoter level in vivo. We established a transgenic mouse model carrying a lacZ reporter gene construct under control of the human prepro-ET-1 gene promoter sequence (8 kb of 5′ sequences). In order to validate our new model, we demonstrated high prepro-ET-1 promoter activity in the craniofacial region, as well as in the bone and cartilage during fetal development consistent with the literature. In adult mice our study furthermore revealed a close interaction of the renal endothelin and nitric oxide system in a cell-type specific manner, which is especially displayed in tubular cells and to a lesser extent in glomerular cells. Therefore our study highlights the dual functional role of ET-1 of being an important mediator of bone formation during fetal development and a major player in renal physiology during adulthood.
The insertion of the reporter gene construct into the genome of the mice led to no phenotypic changes, as shown by extensive phenotype assessment including blood pressure measurements, clinical chemistry, peripheral blood count and histology of kidney sections.
The Bluo-Gal staining of whole mount embryos at E16.5 showed high prepro-ET-1 promoter activity in the craniofacial region, as well as in the bone and cartilage. This pattern of expression is in accordance with the severe craniofacial deformities of mice deficient for ET-1 (Kurihara et al. 1994; Kurihara et al. 1995) or ETA (Clouthier et al. 1998) thus serving as a plausibility control by indicating that our novel model indeed monitors prepro-ET-1 promoter activity in vivo. Furthermore, our observations indicating a close relationship between bone formation and the endothelin system are in good agreement with literature describing that tumour-produced ET-1 stimulates new bone formation in vitro and osteoblastic metastases in vivo via the ETA receptor (Yin et al. 2003).
The almost strict localization of blue particles after Bluo-Gal staining to the intracellular space, as well as the absence of particles in stained kidney sections of non-transgenic mice gives evidence for a causal relationship between intracellular transcription of the reporter transgene and the presence of blue particles. Furthermore, the correlation between particle count and tissue ET-1 content was strong. Besides evidence for similar regulation of the reporter gene promoter and the original mouse prepro-ET-1 promoter this gives further evidence for the regulation of ET-1 synthesis mainly on the transcriptional level, as likewise shown by other investigators (Inoue et al. 1989; Miyauchi & Masaki, 1999). The correlation was not absolute, most probably due to imprecision of ELISA measurements and particle counts. Additionally, post-transcriptional regulation of ET-1 synthesis has also been described (Reimunde et al. 2005).
Prepro-ET-1 promoter activity in the kidney increased after blockade of NO synthesis by l-NAME in spite of an increase in systemic blood pressure. Our study demonstrated that the l-NAME induced increase of prepro-ET-1 promoter activity was highest in the tubular epithelium and to a lesser extent in glomerular cells, whereas prepro-ET-1 promoter activity was unaffected in vascular endothelial cells. We thus conclude the sensitivity of the negative feedback of nitric oxide and its second messenger cGMP on ET-1 promoter activity to be cell-type specific. Our data are in agreement with recent in situ hybridization studies demonstrating that prepro-ET-1 mRNA levels are strongly detectable even under baseline conditions in all tubular segments of the rat kidney (Moridaira et al. 2003) and that l-NAME-treated SHR showed an increased grain density versus placebo-treated SHR in glomeruli, but not in renal or mesenteric arteries (Sventek et al. 1996; Deng & Schiffrin, 1998). Furthermore, Northern blot analysis in cultured endothelial cells revealed no significant effects of l-NAME on ET-1 promoter activity (Kahler et al. 2000). Tissue and cell-type specific gene expression is a well-described phenomenon, for example in other vasoactive pathways like the renin–angiotensin system (de Gasparo et al. 2000), as well as in the endothelin system itself, where the ECE-1a isoform is expressed cell-type specifically (Funke-Kaiser et al. 1998; Valdenaire et al. 1999), whereas the ECE-1c isoform promoter is less prone to specific stimuli, thereby exhibiting housekeeping properties (Funke-Kaiser et al. 2003).
In tubular epithelium the main action of ET-1 is most probably the promotion of natriuresis by tonic inhibition of the amiloride-sensitive epithelial Na+ channel (eNaC) via the ETB receptor (Gariepy et al. 2000; Hocher et al. 2001). In contrast to the vascular endothelium, where ET-1 and NO act as counterparts in regulation of vascular tone the effect of NO in the renal tubular epithelium is similar to the effect of ET-1. Several studies have reported that NO decreases net active sodium transport in renal epithelia and in turn inhibitors of endogenous NO production in the kidney decreased water and sodium excretion (Lahera et al. 1993; Stoos et al. 1994; Stoos et al. 1995; Garcia et al. 1999; Mattson & Wu, 2000). In cultured renal epithelial cells the NO donor drug NONOate decreased ENaC open probability (Helms, 2005). In our experiment, the inhibition of NO synthase leads to an increase of prepro-ET-1 promoter activity in tubular epithelium, physiologically followed by an increase in the amount of ET-1 peptide. This negative feedback is observed despite the similar effect on natriuresis and eNaC.
In summary, we established a transgenic mouse model which provides a unique opportunity to analyse the regulation of the prepro-ET-1 promoter activity pattern on a cellular level in vivo. We validated our new model by demonstrating in fetal development high prepro-ET-1 promoter activity in the craniofacial region as well as in bone and cartilage consistent with literature. Our study furthermore revealed a very close interaction of the renal endothelin and nitric oxide system in a cell-type specific manner.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG) to Dr Berthold Hocher (grant-No. Ho 1665/5-1 and Ho 1665/5-2) and Schering AG, Berlin to Dr F. Theuring. The work of Dr P Kalk and K. Relle was supported by a grant from the DFG (PE 388/20-1); the former was also supported by a grant from the Dr Werner-Jackstaedt-Stiftung. The generation of the transgenic mice was undertaken when Fred Schmager and Dr Franz Theuring were with Schering AG, Berlin. The technical assistance of Norma Schulz and Sylvia Chotzen is highly acknowledged.
References
- Aguzzi A, Theuring F. Improved in situ β-galactosidase staining for histological analysis of transgenic mice. Histochemistry. 1994;102:477–481. doi: 10.1007/BF00269579. [DOI] [PubMed] [Google Scholar]
- Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Boulanger C, Luscher TF. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes RP, Schmitz-Winnenthal FH, Feletou M, Godecke A, Huang PL, Vanhoutte PM, Fleming I, Busse R. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc Natl Acad Sci U S A. 2000;97:9747–9752. doi: 10.1073/pnas.97.17.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- Deng LY, Schiffrin EL. Endothelin-1 gene expression in blood vessels and kidney of spontaneously hypertensive rats (SHR), L-NAME-treated SHR, and renovascular hypertensive rats. J Cardiovasc Pharmacol. 1998;31(Suppl. 1):S380–S383. doi: 10.1097/00005344-199800001-00108. [DOI] [PubMed] [Google Scholar]
- de Nucci G, Thomas R, D'Orleans-Juste P, Antunes E, Walder C, Warner TD, Vane JR. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988;85:9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke-Kaiser H, Orzechowski HD, Richter M, Paul M. Human endothelin-converting enzyme-1 beta mRNA expression is regulated by an alternative promoter. J Cardiovasc Pharmacol. 1998;31(Suppl. 1):S7–S9. doi: 10.1097/00005344-199800001-00004. [DOI] [PubMed] [Google Scholar]
- Funke-Kaiser H, Thomas A, Bremer J, Kovacevic SD, Scheuch K, Bolbrinker J, Theis S, Lemmer J, Zimmermann A, Zollmann FS, Herrmann SM, Paul M, Orzechowski HD. Regulation of the major isoform of human endothelin-converting enzyme-1 by a strong housekeeping promoter modulated by polymorphic microsatellites. J Hypertens. 2003;21:2111–2124. doi: 10.1097/00004872-200311000-00021. [DOI] [PubMed] [Google Scholar]
- Garcia NH, Plato CF, Stoos BA, Garvin JL. Nitric oxide-induced inhibition of transport by thick ascending limbs from Dahl salt-sensitive rats. Hypertension. 1999;34:508–513. doi: 10.1161/01.hyp.34.3.508. [DOI] [PubMed] [Google Scholar]
- Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest. 2000;105:925–933. doi: 10.1172/JCI8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannessi D, Del Ry S, Vitale RL. The role of endothelins and their receptors in heart failure. Pharmacol Res. 2001;43:111–126. doi: 10.1006/phrs.2000.0758. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Strachan FE, Webb DJ. Endothelin ETA and ETB receptors cause vasoconstriction of human resistance and capacitance vessels in vivo. Circulation. 1995;92:357–363. doi: 10.1161/01.cir.92.3.357. [DOI] [PubMed] [Google Scholar]
- Helms MN, Yu L, Malik B, Kleinhenz DJ, Hart CM, Eaton DC. Role of SGK1 in nitric oxide inhibition of ENaC in Na+-transporting epithelia. Am J Physiol Cell Physiol. 2005;289:C717–C726. doi: 10.1152/ajpcell.00006.2005. [DOI] [PubMed] [Google Scholar]
- Hocher B, Dembowski C, Slowinski T, Friese ST, Schwarz A, Siren AL, Neumayer HH, Thone-Reineke C, Bauer C, Nafz B, Ehrenreich H. Impaired sodium excretion, decreased glomerular filtration rate and elevated blood pressure in endothelin receptor type B deficient rats. J Mol Med. 2001;78:633–641. doi: 10.1007/s001090000158. [DOI] [PubMed] [Google Scholar]
- Hocher B, Kalk P, Slowinski T, Godes M, Mach A, Herzfeld S, Wiesner D, Arck PC, Neumayer HH, Nafz B. ETA receptor blockade induces tubular cell proliferation and cyst growth in rats with polycystic kidney disease. J Am Soc Nephrol. 2003;14:367–376. doi: 10.1097/01.asn.0000042165.63601.65. [DOI] [PubMed] [Google Scholar]
- Hocher B, Thone-Reineke C, Rohmeiss P, Schmager F, Slowinski T, Burst V, Siegmund F, Quertermous T, Bauer C, Neumayer HH, Schleuning WD, Theuring F. Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J Clin Invest. 1997;99:1380–1389. doi: 10.1172/JCI119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocher B, Zart R, Schwarz A, Vogt V, Braun C, Thone-Reineke C, Braun N, Neumayer HH, Koppenhagen K, Bauer C, Rohmeiss P. Renal endothelin system in polycystic kidney disease. J Am Soc Nephrol. 1998;9:1169–1177. doi: 10.1681/ASN.V971169. [DOI] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Takuwa Y, Mitsui Y, Kobayashi M, Masaki T. The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression. J Biol Chem. 1989;264:14954–14959. [PubMed] [Google Scholar]
- Kahler J, Mendel S, Weckmuller J, Orzechowski HD, Mittmann C, Koster R, Paul M, Meinertz T, Munzel T. Oxidative stress increases synthesis of big endothelin-1 by activation of the endothelin-1 promoter. J Mol Cell Cardiol. 2000;32:1429–1437. doi: 10.1006/jmcc.2000.1178. [DOI] [PubMed] [Google Scholar]
- Kaminski A, Kasch C, Zhang L, Kumar S, Sponholz C, Choi YH, Ma N, Liebold A, Ladilov Y, Steinhoff G, Stamm C. Endothelial nitric oxide synthase mediates protective effects of hypoxic preconditioning in lungs. Respir Physiol Neurobiol. 2006;155:280–285. doi: 10.1016/j.resp.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Kawana M, Lee ME, Quertermous EE, Quertermous T. Cooperative interaction of GATA-2 and AP1 regulates transcription of the endothelin-1 gene. Mol Cell Biol. 1995;15:4225–4231. doi: 10.1128/mcb.15.8.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan DE. Endothelins in the normal and diseased kidney. Am J Kidney Dis. 1997;29:2–26. doi: 10.1016/s0272-6386(97)90004-4. [DOI] [PubMed] [Google Scholar]
- Kurihara Y, Kurihara H, Oda H, Maemura K, Nagai R, Ishikawa T, Yazaki Y. Aortic arch malformations and ventricular septal defect in mice deficient in endothelin-1. J Clin Invest. 1995;96:293–300. doi: 10.1172/JCI118033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara Y, Kurihara H, Suzuki H, Kodama T, Maemura K, Nagai R, Oda H, Kuwaki T, Cao WH, Kamada N. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368:703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- Lahera V, Navarro J, Biondi ML, Ruilope LM, Romero JC. Exogenous cGMP prevents decrease in diuresis and natriuresis induced by inhibition of NO synthesis. Am J Physiol Renal Physiol. 1993;264:F344–F347. doi: 10.1152/ajprenal.1993.264.2.F344. [DOI] [PubMed] [Google Scholar]
- Lee ME, Bloch KD, Clifford JA, Quertermous T. Functional analysis of the endothelin-1 gene promoter. Evidence for an endothelial cell-specific cis-acting sequence. J Biol Chem. 1990;265:10446–10450. [PubMed] [Google Scholar]
- Lee ME, Dhadly MS, Temizer DH, Clifford JA, Yoshizumi M, Quertermous T. Regulation of endothelin-1 gene expression by Fos and Jun. J Biol Chem. 1991;266:19034–19039. [PubMed] [Google Scholar]
- Lee YJ, Shin SJ, Tsai JH. Increased urinary endothelin-1-like immunoreactivity excretion in NIDDM patients with albuminuria. Diabetes Care. 1994;17:263–266. doi: 10.2337/diacare.17.4.263. [DOI] [PubMed] [Google Scholar]
- Mattson DL, Wu F. Control of arterial blood pressure and renal sodium excretion by nitric oxide synthase in the renal medulla. Acta Physiol Scand. 2000;168:149–154. doi: 10.1046/j.1365-201x.2000.00647.x. [DOI] [PubMed] [Google Scholar]
- Minchenko AG, Stevens MJ, White L, Abatan OI, Komjati K, Pacher P, Szabo C, Obrosova IG. Diabetes-induced overexpression of endothelin-1 and endothelin receptors in the rat renal cortex is mediated via poly (ADP-ribose) polymerase activation. FASEB J. 2003;17:1514–1516. doi: 10.1096/fj.03-0013fje. [DOI] [PubMed] [Google Scholar]
- Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annu Rev Physiol. 1999;61:391–415. doi: 10.1146/annurev.physiol.61.1.391. [DOI] [PubMed] [Google Scholar]
- Moridaira K, Nodera M, Sato G, Yanagisawa H. Detection of prepro-ET-1 mRNA in normal rat kidney by in situ RT-PCR. Nephron Exp Nephrol. 2003;95:e55–e61. doi: 10.1159/000073672. [DOI] [PubMed] [Google Scholar]
- Pfab T, Thone-Reineke C, Theilig F, Lange I, Witt H, Maser-Gluth C, Bader M, Stasch JP, Ruiz P, Bachmann S, Yanagisawa M, Hocher B. Diabetic endothelin B receptor-deficient rats develop severe hypertension and progressive renal failure. J Am Soc Nephrol. 2006;17:1082–1089. doi: 10.1681/ASN.2005080833. [DOI] [PubMed] [Google Scholar]
- Quaschning T, Voss F, Relle K, Kalk P, Vignon-Zellweger N, Pfab T, Bauer C, Theilig F, Bachmann S, Kraemer-Guth A, Wanner C, Theuring F, Galle J, Hocher B. Lack of endothelial nitric oxide synthase promotes endothelin-induced hypertension: Lessons from endothelin-1 transgenic/endothelial nitric oxide synthase knockout mice. J Am Soc Nephrol. 2007;18:730–740. doi: 10.1681/ASN.2006050541. [DOI] [PubMed] [Google Scholar]
- Reimunde FM, Castanares C, Redondo-Horcajo M, Lamas S, Rodriguez-Pascual F. Endothelin-1 expression is strongly repressed by AU-rich elements in the 3′-untranslated region of the gene. Biochem J. 2005;387:763–772. doi: 10.1042/BJ20041687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiterova J, Merta M, Stekrova J, Cabartova Z, Cibulka R, Maixnerova D, Rysava R, Rihova Z, Tesar V, Motan J. Influence of endothelin-1 gene polymorphisms on the progression of autosomal dominant polycystic kidney disease. Kidney Blood Press Res. 2006;29:182–188. doi: 10.1159/000095504. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Serneri GG, Modesti PA, Cecioni I, Biagini D, Migliorini A, Costoli A, Colella A, Naldoni A, Paoletti P. Plasma endothelin and renal endothelin are two distinct systems involved in volume homeostasis. Am J Physiol Heart Circ Physiol. 1995;268:H1829–H1837. doi: 10.1152/ajpheart.1995.268.5.H1829. [DOI] [PubMed] [Google Scholar]
- Stoos BA, Carretero OA, Garvin JL. Endothelial-derived nitric oxide inhibits sodium transport by affecting apical membrane channels in cultured collecting duct cells. J Am Soc Nephrol. 1994;4:1855–1860. doi: 10.1681/ASN.V4111855. [DOI] [PubMed] [Google Scholar]
- Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol. 1995;6:89–94. doi: 10.1681/ASN.V6189. [DOI] [PubMed] [Google Scholar]
- Sventek P, Turgeon A, Garcia R, Schiffrin EL. Vascular and cardiac overexpression of endothelin-1 gene in one-kidney, one clip Goldblatt hypertensive rats but only in the late phase of two-kidney one clip Goldblatt hypertension. J Hypertens. 1996;14:57–64. [PubMed] [Google Scholar]
- Theuring F, Gotz W, Balling R, Korf HW, Schulze F, Herken R, Gruss P. Tumorigenesis and eye abnormalities in transgenic mice expressing MSV-SV40 large T-antigen. Oncogene. 1990;5:225–232. [PubMed] [Google Scholar]
- Valdenaire O, Lepailleur-Enouf D, Egidy G, Thouard A, Barret A, Vranckx R, Tougard C, Michel JB. A fourth isoform of endothelin-converting enzyme (ECE-1) is generated from an additional promoter molecular cloning and characterization. Eur J Biochem. 1999;264:341–349. doi: 10.1046/j.1432-1327.1999.00602.x. [DOI] [PubMed] [Google Scholar]
- Wang A, Holcslaw T, Bashore TM, Freed MI, Miller D, Rudnick MR, Szerlip H, Thames MD, Davidson CJ, Shusterman N, Schwab SJ. Exacerbation of radiocontrast nephrotoxicity by endothelin receptor antagonism. Kidney Int. 2000;57:1675–1680. doi: 10.1046/j.1523-1755.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- Wesson DE. Physiologic and pathophysiologic renal consequences of H+-stimulated endothelin secretion. Am J Kidney Dis. 2000;35:LII–LIV. doi: 10.1016/s0272-6386(00)70188-0. [DOI] [PubMed] [Google Scholar]
- Yin JJ, Mohammad KS, Kakonen SM, Harris S, Wu-Wong JR, Wessale JL, Padley RJ, Garrett IR, Chirgwin JM, Guise TA. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci U S A. 2003;100:10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokokawa K, Kohno M, Murakawa K, Yasunari K, Horio T, Inoue T, Takeda T. Acute effects of endothelin on renal hemodynamics and blood pressure in anesthetized rats. Am J Hypertens. 1989;2:715–717. doi: 10.1093/ajh/2.9.715. [DOI] [PubMed] [Google Scholar]