Abstract
Although thin fibre muscle afferents possess acid sensing ion channels (ASICs), their contribution to the exercise pressor reflex is not known. This lack of information is partly attributable to the fact that there is no known selective in vivo antagonist for ASICs. Although amiloride has been shown to antagonize ASICs, it also has been shown to antagonize voltage-gated sodium channels, thereby impairing impulse conduction in sensory nerves. Our aim was to test the hypothesis that lactic acid accumulation in exercising muscle acted on ASICs located on thin fibre muscle afferents to evoke the metabolic component of the exercise pressor reflex. To test this hypothesis, we determined in decerebrate cats if amiloride attenuated the pressor and cardioaccelerator responses to static contraction, to tendon stretch and to arterial injections of lactic acid and capsaicin. We found a dose of amiloride (0.5 μg kg−1; i.a.) that attenuated the pressor and cardioaccelerator responses to both contraction and lactic acid injection, but had no effect on the responses to stretch and capsaicin. A higher dose of amiloride (5 μg kg−1, i.a.) not only blocked the pressor and cardioaccelerator responses to lactic acid and contraction, but also attenuated the responses to stretch and to capsaicin, manoeuvers in which ASICs probably play no significant role. In addition, we found that the low dose of amiloride (0.5 μg kg−1) had no effect on the responses of muscle spindles to tendon stretch and to succinylcholine, whereas the high dose (5 μg kg−1) attenuated the responses to both. Our data suggest the low dose of amiloride used in our experiments selectively blocked ASICs, whereas the high dose blocked ASICs and impulse conduction in muscle afferents. We conclude that ASICs play a role in the metabolic component of the exercise pressor reflex.
The exercise pressor reflex is one of the neural mechanisms evoking the cardiovascular and ventilatory responses to exercise (Mitchell et al. 1983). The afferent arm of the exercise pressor reflex is comprised of group III and IV muscle afferents. Both mechanical and metabolic stimuli are believed to stimulate these thin fibre afferents during muscular contraction. Group III afferents are thought to be responsive to mechanical stimuli arising in the contracting muscles, whereas group IV afferents are thought to be responsive to metabolic stimuli. Nevertheless, exposure of group III afferent endings to metabolic products of contraction has been shown to increase their sensitivity to mechanical stimuli (Rotto et al. 1990; Middlekauff & Chiu, 2004; Hayes et al. 2006).
Lactic acid is one of the metabolic products of contraction that has been suggested to be a metabolic stimulus to thin fibre muscle afferents (Victor et al. 1988; Sinoway et al. 1989; Pryor et al. 1990; MacLean et al. 1998; Fadel et al. 2003). For example, injection of lactic acid into the arterial supply of skeletal muscle has been shown to evoke reflex increases in arterial pressure, heart rate and ventilation that approximate those occurring during static exercise (Rotto et al. 1989). In contrast, injection of sodium lactate at a neutral pH failed to evoke any reflex cardiovascular effects (Rotto et al. 1989). Moreover, injection of lactic acid into the arterial supply of skeletal muscle has been shown to increase the discharge of group III and IV muscle afferents as well as to make group III afferents more sensitive to mechanical stimuli (Rotto & Kaufman, 1988; Sinoway et al. 1993).
To demonstrate that a particular metabolite contributes to the elicitation of the exercise pressor reflex, investigators need to show that blockade of a specific receptor to the metabolite prevents or attenuates the expression of the reflex. This demonstration has been difficult to achieve for lactic acid because its receptor on group III and IV afferents was not clearly identified. In particular, lactic acid was thought to activate both the transient receptor potential vanilloid 1 (TRPV 1) receptor as well as the acid sensing ion channel (ASIC). Recently, the TRPV 1 receptor was shown to play little if any role in evoking the metabolic component of the exercise pressor reflex (Kindig et al. 2005). This finding has shifted our attention to acid sensing ion channels in general and to the ASIC3 in particular for two reasons. First, the pH required to activate the ASIC1 and 2 channels is at or below 5.0, a level which is not physiologically relevant in vivo (Alvarez de la Rosa et al. 2002). Second, the ASIC3 is much more sensitive to lactic acid than other acids when compared with the other ASIC channels (Immke & McCleskey, 2001). Although this finding pointed towards the ASIC 3 receptor, there was no known specific antagonist for it.
One possible candidate is amiloride, which has been shown to block ASICs (Waldmann et al. 1997). Unfortunately, amiloride has also been shown to antagonize voltage-gated sodium channels, rendering it able to block impulse conduction in excitable membranes. As a consequence, amiloride may prevent or attenuate the metabolic component of the exercise pressor reflex by blocking impulse conduction in group III and IV muscle afferents as well as by blocking the acid-sensitive ion channel on their endings in the interstitium. In the experiments to be reported, we have searched for a dose of amiloride that attenuated the exercise pressor reflex, but had no effect on the pressor responses to tendon stretch and capsaicin injection into the arterial supply of skeletal muscle. Finding such a dose of amiloride would enable us to examine the role played by ASIC in the generation of the metabolic component of the exercise pressor reflex.
Method
General
The Institutional Animal Care and Use Committee of the University of California, Davis approved all procedures. Adult cats (n = 51), weighing between 2.5 and 4.6 kg, were initially anaesthetized by inhalation of a mixture of halothane (5%) and oxygen. The trachea was cannulated, and the lungs were ventilated with the anaesthetic gas mixture (1.5-2% halothane). The right common carotid artery and external jugular vein were cannulated for monitoring blood pressure and administering fluids, respectively. The tip of the carotid arterial catheter was located in the thoracic aorta and was pointed towards the leg. Blood pressure was measured by connecting the carotid arterial cannula to a Statham P23XL transducer. Arterial PO2, PCO2 and pH were measured periodically (model ABL 700 Series, Radiometer) and were maintained within normal limits either by adjusting ventilation or by administering sodium bicarbonate (8.5%i.v.). Prior to the decerebration procedure, dexamethasone (4 mg) was injected intravenously to reduce swelling of the brain stem. Additionally, the left common carotid artery was ligated in order to reduce bleeding during the decerebration. The cat was then placed in a Kopf stereotaxic frame and spinal unit and a mid-collicular decerebration was performed. The gaseous anaesthetic was gradually discontinued, and the lungs were ventilated with room air.
The left hind limb was fixed in place at the ankle and knee by clamps, and the left triceps surae muscles, calcaneal tendon and sciatic nerve were exposed. The tendon was severed from the calcaneal bone, attached to a force transducer (model FT-10, Grass Instruments), and stretched with a rack and pinion so that it developed a resting tension of approximately 400 g. Snares were placed around the left common iliac artery and vein in the abdomen for occluding the blood flow to the hind limb. At the conclusion of the experiment the cat was humanely killed with an overdose of pentobarbital (Cardinal) followed by an injection of saturated KCl solution.
Recording activity of muscle spindles, group III and IV afferents
We recorded the impulse activity of individual spindles, group III and IV triceps surae muscle afferents from the distal cut end of the left L7 or S1 dorsal roots. In these cats, a lumbosacral laminectomy was performed to expose the L6 to S2 spinal roots. The left peroneal, sural, gluteal, femoral and obturator nerves, as well as the muscular branch of the sciatic nerve, were cut. The neural signals were passed through a high-impedance probe (Grass HIP511), amplified (Grass P511), and filtered (0.1 to 3 kHz band pass). The action potentials were displayed on a computer monitor (Spike 2) as well as on a storage oscilloscope (Hewlett-Packard). An afferent's receptive field was identified as being in the triceps surae muscles if a burst of impulses were discharged in response to stretch of the muscle in the case of a muscle spindle and by either noxious or non-noxious probing of the muscle in the case of group III and IV afferents. Noxious probing consisted of vigorously pinching the muscles with the fingers, whereas non-noxious probing consisted of either gently stroking the triceps surae with a blunt rod or gently squeezing the muscles with the fingers.
We classified afferents as spindle, group III or group IV by their conduction velocities. Afferents with conduction velocities above 30 m s−1, which were inhibited by twitch, and which were stimulated by muscle lengthening were classified as spindles. Afferents with conduction velocities between 2.5 and 30 m s−1 were classified as group III, and afferents with conduction velocities of < 2.5 m s−1 were classified as group IV. We calculated conduction velocity by measuring the conduction time and distance from a stimulating electrode placed under the tibial nerve close to its exit from the triceps surae muscles and the recording electrode placed under the dorsal root filament. The criterion for a response by an afferent to a manipulation was an increase greater than or equal to 0.2 impulses s−1.
Reflex protocols
To test the effects of amiloride on the exercise pressor reflex we recorded the blood pressure and heart rate responses before, and then 30 and 60 min after one of two doses of amiloride (5 μg kg−1 or 0.5 μg kg−1) to each of four manoeuvers. These were tendon stretch, static contraction of the triceps surae muscles, injection of lactic acid (0.2–0.5 ml; 24 mm) into the popliteal artery, and injection of capsaicin (1–2 μg, 0.25 ml) into the popliteal artery. The concentration of amiloride injected to achieve the large dose (5 μg kg−1) was 200 μm, whereas the concentration injected to achieve the small dose (0.5 μg kg−1) was 20 μm. Only one of the two doses of amiloride was given to each cat.
Tendon stretch was accomplished by stretching the calcaneal tendon with a rack and pinion for 60 s. Static contraction was accomplished by stimulation of the tibial nerve for 60 s at or below twice motor threshold (0.025 ms pulse duration; 40 Hz for reflex experiments and 15–20 Hz for afferent recording experiments). Next, each cat briefly underwent neuromuscular blockade with rocuronium bromide (0.5–0.7 mg kg−1; i.v.) and the tibial nerve stimulated again. In every instance, stimulation after paralysis no longer generated a pressor response. Moreover, no tension was generated by the triceps surae muscles. Injections of lactic acid, capsaicin, as well as a neutral pH saline solution were accomplished by gently inserting a 30-gauge needle into the popliteal artery and then injecting the compounds over approximately 10 s into the vasculature of the triceps surae muscles. The manoeuvers were randomized to eliminate any order effect. Before injecting either capsaicin or lactic acid into the popliteal artery, we paralysed the cat by injecting intravenously rocuronium bromide (0.5–0.7 mg kg−1). Once we identified a muscle spindle, group III or IV afferent with a receptive field in the triceps surae muscles and established its resting level of activity, we recorded the response of the afferent to a variety of manoeuvers, depending on its classification.
Electrophysiological protocols
When a muscle spindle was identified, the cat then underwent neuromuscular blockade. We recorded its responses to two manoeuvers. The first was injection of 1–2 mg of succinylcholine into the catheter placed into the carotid artery, and the second was stretch of the calcaneal tendon. Both manoeuvers were performed before and 30 and 60 min after one of two doses of amiloride (5 μg kg−1 or 0.5 μg kg−1) was injected into the popliteal artery and trapped there for 10 min by tightening the arterial and venous snares. Succinylcholine was used because this agent has been shown to stimulate muscle spindles (Granit et al. 1953; Waldrop et al. 1984). Moreover, succinylcholine has no effect on the discharge of group III and IV muscle afferents (Waldrop et al. 1984).
When a group III or group IV muscle afferent was identified, we recorded its responses to at least one of the four manoeuvers described above (see Reflex protocol). All manoeuvers were performed before, and then 30 and 60 min after one of two doses of amiloride (5 μg kg−1 or 0.5 μg kg−1) was injected into the popliteal artery. The amiloride injectate was trapped in the artery for 10 min. Injections of capsaicin and lactic acid were performed subsequent to neuromuscular blockade of the cat with rocuronium bromide (0.5–0.7 mg kg−1).
Data analysis
Blood pressure, heart rate, muscle tension and impulse activity were all recorded with Spike 2 data acquisition system (CED, Cambridge) and stored on a computer hard drive (Dell). Mean arterial pressure is expressed in mmHg, heart rate in beats per minute, and afferent activity is expressed in impulses per second. The tension–time index was calculated by integrating the area between the tension trace and the baseline level (Spike 2) and is expressed in kgs. Peak developed tension was calculated by subtracting the resting tension from the peak tension and is expressed in kilograms. All values are expressed as the mean ± standard error of the mean (s.e.m.). Two-way repeated measures ANOVA followed by Tukey post hoc tests were used to determine statistical significance, except in the case of tension–time indices and peak developed tension in which case one-way repeated measure ANOVAS were used to determine significance. The criterion for statistical significance was set at P < 0.05.
Results
Reflex experiments
The reflex pressor responses to arterial injections of lactic acid and capsaicin as well as to tendon stretch and static contraction were measured before, and then 30 and 60 min after popliteal arterial injection of two doses of amiloride (5 μg kg−1 and 0.5 μg kg−1). The effects of the large dose of amiloride (5 μg kg−1) are reported first and are then followed by the small dose (0.5 μg kg−1). The cardioaccelerator responses to the four manoeuvers were, for the most part, modest and are given in Table 1.
Table 1.
Effects of two doses of amiloride injected into the popliteal artery on the cardioaccelerator responses to lactic acid, static contraction, capsaicin and tendon stretch
| Large dose (5.0 μg kg−1) | Small dose (0.5 μg kg−1) | |||
|---|---|---|---|---|
| Change | Baseline HR | Change | Baseline HR | |
| Lactic acid | ||||
| Before | 5 ± 2* | 217 ± 6 | 7 ± 3** | 196 ± 6 |
| 30 min | 2 ± 1† | 228 ± 6 | 1 ± 1† | 210 ± 7 |
| 60 min | 2 ± 1† | 242 ± 8 | 7 ± 2** | 240 ± 7 |
| Static contraction | ||||
| Before | 9 ± 3*** | 213 ± 7 | 17 ± 4*** | 183 ± 6 |
| 30 min | 4 ± 2**† | 222 ± 7 | 7 ± 2† | 201 ± 6 |
| 60 min | 2 ± 1† | 243 ± 7 | 15 ± 2*** | 224 ± 9 |
| Capsaicin | ||||
| Before | 16 ± 4* | 208 ± 6 | 13 ± 2*** | 193 ± 7 |
| 30 min | 10 ± 3* | 220 ± 7 | 14 ± 3*** | 205 ± 7 |
| 60 min | 3 ± 1† | 234 ± 9 | 15 ± 3*** | 240 ± 10 |
| Tendon stretch | ||||
| Before | 8 ± 3** | 212 ± 6 | 7 ± 2** | 188 ± 6 |
| 30 min | 3 ± 1† | 228 ± 6 | 8 ± 2* | 200 ± 6 |
| 60 min | 0 ± 0† | 242 ± 7 | 9 ± 3** | 228 ± 7 |
Asterisks denote significance from baseline value (
P < 0.05;
P < 0.01;
P < 0.001). Daggers denote significance from value before amiloride injection (
P < 0.05).
Effects of amiloride (5 μg kg−1) on responses to lactic acid and capsaicin
The large dose of amiloride (5 μg kg−1) abolished the pressor response to popliteal arterial injection of lactic acid as well as attenuated the response to capsaicin (Fig. 1). Before amiloride, the pressor response to lactic acid averaged 28 ± 5 mmHg (P < 0.001; n = 9), whereas 30 and 60 min afterwards it averaged 2 ± 1 mmHg (P = 0.35; n = 9) and 0 ± 1 mmHg (P = 0.88; n = 9), respectively. The differences between the pressor response to lactic acid before amiloride and those 30 and 60 min afterwards were significant (P < 0.001). Before amiloride, the pressor response to capsaicin averaged 71 ± 15 mmHg (P = 0.001; n = 8), whereas 30 and 60 min afterwards it averaged 42 ± 9 mmHg (P = 0.001; n = 8) and 36 ± 6 mmHg (P = 0.004; n = 8), respectively. The differences between the pressor response to capsaicin before amiloride and those 30 min (P = 0.009) and 60 min (P = 0.03) afterwards were significant.
Figure 1.
Effects of two doses of amiloride on the pressor responses to lactic acid (0.2–0.5 ml; 24 mm) and capsaicin (1–2 μg, 0.25 ml) injected into the popliteal artery Note that lactic acid and capsaicin were injected before giving amiloride, 30 min after giving amiloride, and 60 min after giving amiloride. Filled bars represent baseline values and open bars represent peak values (vertical brackets represent s.e.m.). Asterisks (*) signify significant difference (P < 0.05) between baseline and its corresponding peak effect. Horizontal brackets signify significant difference (P < 0.05) between pressor response before injection and that either 30 or 60 min after giving amiloride.
Effects of amiloride (5 μg kg−1) on responses to static contraction
The large dose of amiloride (5 μg kg−1) significantly attenuated the pressor response to static contraction of the triceps surae muscles (Fig. 2). Before amiloride, the pressor response to static contraction averaged 48 ± 5 mmHg (P = 0.001; n = 9), whereas 30 and 60 min afterwards it averaged 18 ± 4 mmHg (P = 0.007; n = 9) and 15 ± 4 mmHg (P = 0.02; n = 9), respectively. The differences between the pressor response to contraction before amiloride and those 30 min (P = 0.001) and 60 min (P = 0.002) afterwards were significant. The tension–time indices before, 30 and 60 min after amiloride injection were not significantly different (P = 0.45; n = 9, Table 2).
Figure 2.
Effects of two doses of amiloride on the pressor responses to static contraction and tendon stretch Note that both contraction and stretch were done before giving amiloride, 30 min after giving amiloride, and 60 min after giving amiloride. Filled bars represent baseline values and open bars represent peak values (vertical brackets represent s.e.m.). Asterisks (*) signify significant difference (P < 0.05) between baseline and its corresponding peak effect. Horizontal brackets signify significant difference (P < 0.05) between pressor response before injection and that either 30 or 60 min after giving amiloride.
Table 2.
The tension–time indices for static contraction and tendon stretch during reflex experiments
| 0.5 μg kg−1 | 5.0 μg kg−1 | |||
|---|---|---|---|---|
| Tendon stretch | Static contraction | Tendon stretch | Static contraction | |
| Before amiloride | 153 ± 10 | 146 ± 13 | 161 ± 9 | 161 ± 12 |
| 30 min | 153 ± 10 | 144 ± 11 | 170 ± 11 | 169 ± 15 |
| 60 min | 154 ± 9 | 143 ± 12 | 166 ± 11 | 159 ± 10 |
There were no significant differences (P > 0.05) between corresponding means before and 30 or 60 min after amiloride injections (0.5 μg kg−1 or 5.0 μg kg−1; i.a.). All values are expressed in kg s.
Effects of amiloride (5 μg kg−1) on responses to tendon stretch
The large dose of amiloride (5 μg kg−1) significantly attenuated the pressor response to tendon stretch (Fig. 2). Before amiloride, the pressor response averaged 34 ± 6 mmHg (P = 0.001; n = 11), whereas 30 and 60 min afterwards it averaged 16 ± 4 mmHg (P = 0.001; n = 11) and 10 ± 3 mmHg (P = 0.02; n = 11), respectively. The differences between the pressor response to stretch before amiloride and those 30 min (P = 0.01) and 60 min (P = 0.03) afterwards were significant. The tension–time indices before, 30 and 60 min after amiloride were not significantly different (P = 0.31; n = 11; Table 2).
Effects of amiloride (0.5 μg kg−1) on responses to lactic acid and capsaicin
The small dose of amiloride significantly attenuated (P < 0.05) the pressor response to lactic acid injection, but had no effect on the pressor response to capsaicin injection (P > 0.05; Fig. 1). Before amiloride, the pressor response to popliteal arterial injections of lactic acid averaged 36 ± 5 mmHg (P = 0.001; n = 7), whereas 30 and 60 min afterwards it averaged 12 ± 4 mmHg (P = 0.09; n = 7) and 23 ± 6 mmHg (P = 0.005; n = 7). The difference between the pressor response to lactic acid before amiloride and that after 30 min was significant (P = 0.02). Before amiloride the pressor response to popliteal arterial injections of capsaicin averaged 55 ± 11 mmHg, and remained unchanged 30 and 60 min afterwards, averaging 53 ± 10 and 56 ± 10 mmHg, respectively (P = 0.46; n = 7).
Effects of amiloride (0.5 μg kg−1) on responses to static contraction
The small dose of amiloride significantly attenuated (P < 0.05) the pressor response to static contraction (Fig. 2). Before amiloride, the pressor response to static contraction averaged 42 ± 7 mmHg (P = 0.003; n = 7), whereas 30 min afterwards it averaged 15 ± 5 mmHg (P = 0.19; n = 7) and 60 min afterwards it recovered to 31 ± 7 mmHg (P = 0.01; n = 7). Importantly, the difference between the pressor response before amiloride and the pressor response 30 min afterwards was significant (P = 0.009; n = 7). Likewise, the difference between the pressor response 30 min after and 60 min after was also significant (P = 0.01; n = 7). The tension–time indices before, 30 min after and 60 min after amiloride were not significantly different (P = 0.58; n = 7, Table 2).
Effects of amiloride (0.5 μg kg−1) on the responses to tendon stretch
The small dose of amiloride had no effect on the pressor response to tendon stretch (P > 0.05; Fig. 2). Before amiloride the pressor response to stretch of the calcaneal tendon averaged 34 ± 6 mmHg and remained unchanged 30 and 60 min afterwards, averaging 30 ± 6 and 31 ± 5 mmHg (P = 0.59; n = 7), respectively. The tension–time indices before, 30 and 60 min after amiloride were not significantly different (P = 0.79; n = 7, Table 2).
Electrophysiology experiments
We recorded the responses of groups I–IV triceps surae muscle afferents to their effective stimuli before and after the large (5.0 μg kg−1) or small dose (0.5 μg kg−1) of amiloride injected into the popliteal artery.
Spindles
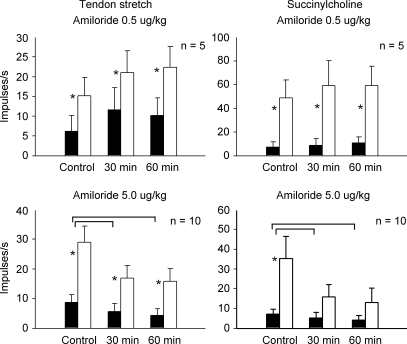
We measured the responses of 10 muscle spindles (conduction velocity: 99 ± 10 m s−1) to tendon stretch and to injection of succinylcholine, both of which evoked repeatable responses. The large dose of amiloride (5.0 μg kg−1) significantly attenuated the responses of each muscle spindle afferent to tendon stretch and to succinylcholine (P < 0.001; n = 10; Fig. 3). The tension–time indices developed during stretch (Table 3, P = 0.56; n = 10) and the baseline tensions (Table 3, P = 0.43, n = 10) were the same before and after the large dose of amiloride.
Figure 3.
Effects of two doses of amiloride on the responses of muscle spindles to tendon stretch and succinylcholine injection (1–2 mg) Note that both stretch and succinylcholine injection were done before giving amiloride, 30 min after giving amiloride, and 60 min after giving amiloride. Filled bars represent baseline values and open bars represent peak values (vertical brackets represent s.e.m.). Asterisks (*) signify significant difference (P < 0.05) between baseline and its corresponding peak effect. Horizontal brackets signify significant difference (P < 0.05) between the spindles responses before giving amiloride and that 30 or 60 min after giving amiloride.
Table 3.
Baseline tension and tension–time indices for stretch of the calcaneal tendon during experiments recorded from muscle spindles before and 30 and 60 min after amiloride injections (0.5 μg kg−1 or 5.0 μg kg−1; i.a.)
| Control | Amiloride 30 min | Amiloride 60 min | |
|---|---|---|---|
| Amiloride large dose (5 μg kg−1) | |||
| Muscle spindle | N = 10 | N = 10 | N = 10 |
| Baseline tension (kg) | 0.40 ± 0.09 | 0.38 ± 0.08 | 0.38 ± 0.08 |
| Tension–time index (kg s) | 84 ± 6 | 88 ± 7 | 83 ± 7 |
| Amiloride small dose (0.5 μg kg−1) | |||
| Muscle spindle | N = 5 | N = 5 | N = 5 |
| Baseline tension (kg) | 0.26 ± 0.06 | 0.23 ± 0.07 | 0.24 ± 0.07 |
| Tension–time index (kg s) | 57 ± 4 | 59 ± 3 | 59 ± 4 |
There were no significant differences (P > 0.05) between corresponding means.
We also examined the effects of the low dose of amiloride (0.5 μg kg−1) on the responses of five other spindle afferents (conduction velocity: 105 ± 16 m s−1) to tendon stretch and to succinylcholine. The low dose had no effect on the responses of any of the five spindle afferents to tendon stretch or to succinylcholine (P = 0.15; Fig. 3). The tension–time indices developed during stretch (Table 3, P = 0.66; n = 5) and the baseline tensions (Table 3, P = 0.36, n = 5) were the same before and after amiloride. Similarly, the low dose of amiloride had no effect on the responses of any of the five muscle spindle afferents to succinylcholine (P = 0.34; Fig. 3).
Group III afferents
We recorded the activity of 11 group III afferents whose receptive fields were in the left triceps surae muscles (conduction velocity: 14.5 ± 3.2 m s−1; range: 5.4–24.1 m s−1). Each of the 11 responded to non-noxious probing of the triceps surae muscles; 10 of the 11 responded to stretch of the calcaneal tendon. Five of the 11 group III afferents were tested with the large dose of amiloride (5 μg kg−1) and six were tested with the low dose of amiloride (0.5 μg kg−1).
Of the five group III afferents (mean conduction velocity: 12.6 ± 2.1 m s−1; range: 5.4–23.8 m s−1) tested with the large dose of amiloride, four responded to tendon stretch, three responded to popliteal artery injection of lactic acid, and five responded to static contraction. The large dose attenuated the responses of the four group III afferents to tendon stretch (Fig. 4). The tension–time indices developed during stretch were the same before and after amiloride (P = 0.77, n = 4).
Figure 4.
Histograms showing the effects of amiloride (5.0 μg kg−1) on the responses of a group III afferent (conduction velocity, 14.4 m s−1) to lactic acid injection (0.5 ml; 24 mm) to tendon stretch and to static contraction Before indicates the control response before injection of amiloride; 30 and 60 min indicate the responses of the group III afferent to the three stimuli 30 and 60 min after amiloride was injected. Arrows signify the injection of lactic acid. Horizontal filled bar represents the 60 s period of time that either the calcaneal tendon was stretched or the triceps surae muscles were contracted. Intervals between vertical tick marks on the horizontal axes represent 10 s. A sample of the action potential counted is shown as an inset in the far left panels.
The large dose of amiloride blocked the responses of the three group III afferents to lactic acid (Fig. 4). In addition, the large dose significantly attenuated the responses of the five group III afferents to static contraction (Fig. 4). The tension–time indices developed during contraction were the same before and after amiloride (P = 0.54, n = 5).
Of the six group III afferents (conduction velocity: 17.9 ± 3.1 m s−1; range: 9.3–24.1 m s−1) tested with the low dose of amiloride (0.5 μg kg−1), each responded to tendon stretch. Four of the six responded to lactic acid, and four responded to static contraction. The low dose of amiloride did not attenuate the responses of group III afferents to tendon stretch. The tension–time indices developed during stretch were the same before and after amiloride (P = 0.87, n = 6).
The small dose of amiloride attenuated the responses of each of the four group III afferents to lactic acid. Likewise, the low dose attenuated the responses of each of the four group III afferents to static contraction. The tension–time indices developed during contraction were the same before and after amiloride (P = 0.44, n = 4). Only 1 of the 11 group III afferents responded to popliteal arterial injection of capsaicin. The low dose of amiloride appeared to have no effect on its response to capsaicin.
Group IV afferents
We recorded the impulse activity of seven group IV afferents whose receptive fields were in the left triceps surae muscles (conduction velocity: 1.1 ± 1.2 m s−1; range: 0.7–2.3 m s−1). Six of the seven responded to noxious probing of the triceps surae muscles. None responded to tendon stretch or to non-noxious probing of the muscles. Four of the seven group IV afferents were tested with the large dose of amiloride (5 μg kg−1) and three were tested with the small dose (0.5 μg kg−1). Three of the four tested with the large dose responded to popliteal arterial injection of lactic acid and capsaicin. Each of the four group IV afferents challenged with the large dose of amiloride responded to static contraction.
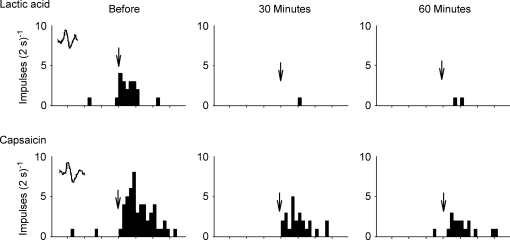
The large dose of amiloride prevented the responses of the three group IV afferents to arterial injection of lactic acid (Fig. 5). Similarly, the large dose attenuated each of the responses of the three group IV afferents to arterial injection of capsaicin (Fig. 5). In addition, the large dose of amiloride attenuated the responses of the four group IV afferents to static contraction. The tension–time indices during contraction were the same before and after amiloride (P = 0.34, n = 4).
Figure 5.
Histograms showing the effects of amiloride (5.0 μg kg−1) on the responses of a group IV afferent (conduction velocity, 1.6 m s−1) to lactic acid (0.5 ml; 24 mm) and capsaicin (1 μg) injection into the popliteal artery Thirty and 60 min indicate the responses of the group III afferent to the three stimuli 30 and 60 min after amiloride was injected into the popliteal artery. Arrows signify the injection of either lactic acid or capsaicin. Intervals between vertical tick marks on the horizontal axes represent 10 s. A sample of the action potential counted is shown as an inset in the far left panels.
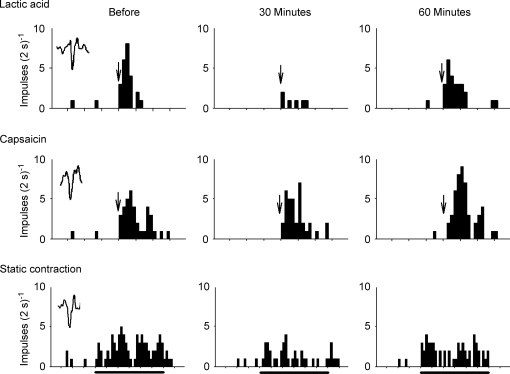
The small dose of amiloride (0.5 μg kg−1) attenuated the responses of all group IV afferents to lactic acid injection and to static contraction (Fig. 6). The tension–time indices developed during contraction were the same before and after amiloride (P = 0.66, n = 3). The small dose of amiloride had no effect on the responses of three group IV afferents to arterial injection of capsaicin (Fig. 6).
Figure 6.
Histograms showing the effects of amiloride (0.5 μg kg−1) on the responses of a group IV afferent (conduction velocity, 0.9 m s−1) to lactic acid (0.5 ml; 24 mm) and capsaicin (1 μg) injection into the popliteal artery as well as to static contraction Thirty and 60 min indicate the responses of the group IV afferent to the three stimuli 30 and 60 min after amiloride was injected into the popliteal artery. Arrows signify the injection of either lactic acid or capsaicin. Horizontal filled bar represents the 60 s period of time that the triceps surae muscles were contracted. Intervals between vertical tick marks on the horizontal axes represent 10 s. A sample of the action potential counted is shown as an inset in the far left panels.
Discussion
The purpose of our experiments was to find a dose of amiloride, injected into the arterial supply of the triceps surae muscles, that attenuated or abolished the pressor response to popliteal arterial injection of lactic acid, an ASIC agonist (Immke & McCleskey, 2001), but had no effect on the pressor responses to both tendon stretch, which stimulates mechanogated channels (Hayes & Kaufman, 2001), and popliteal arterial injections of capsaicin, a TRPV 1 receptor agonist (Caterina et al. 1997). If a dose of amiloride could be found that fulfilled these requirements, we could then use it to determine if ASICs played a role in the exercise pressor reflex (Mitchell et al. 1983). We found a dose of amiloride (0.5 μg kg−1) that greatly attenuated the pressor response to popliteal arterial injections of lactic acid, but did not attenuate the pressor responses to injections of capsaicin or to tendon stretch. These findings are consistent with the hypothesis that this dose of amiloride selectively blocked ASICs on the endings of group III and IV afferents innervating the triceps surae muscles. We then showed that this dose of amiloride, injected into the popliteal artery, attenuated the exercise pressor reflex, which was induced by statically contracting the triceps surae muscles.
We also found that a second dose of amiloride (5 μg kg−1), which was an order of magnitude higher than the first dose, not only abolished the pressor response to lactic acid, but also attenuated markedly the pressor responses to capsaicin, tendon stretch and static contraction of the triceps surae muscles. Moreover, this higher dose of amiloride attenuated markedly the responses of muscle spindles to both succinylcholine and to tendon stretch, two stimuli which presumably do not activate ASICs (Drew et al. 2004). These findings are consistent with the hypothesis that in our experiments the high dose of amiloride not only blocked ASICs but also blocked voltage-gated sodium channels, thereby impairing impulse conduction in the afferent fibres.
Previous studies in rats have used amiloride to block the pressor responses to injection of acidic solutions into the arterial supply of hind limb muscles. Specifically, these studies have shown that femoral arterial injections of amiloride at a dose of 6 μg kg−1 attenuated by half the reflex pressor responses to diprotonated phosphate (Gao et al. 2006) and to lactic acid (Li et al. 2004). Our findings in cats suggest that the dose of amiloride used in these previous studies (Li et al. 2004; Gao et al. 2006) blocked voltage-gated sodium channels as well as ASICs. If sodium channels were blocked by amiloride, this effect could have impaired impulse conduction in thin fibre afferents stimulated by the acidic solutions.
Amiloride has also been shown to prevent the responses of afferents innervating the lungs, kidneys and carotid sinus (Kopp et al. 1998; Drummond et al. 1998; Carr et al. 2001) to a variety of mechanical stimuli. For example, amiloride attenuated the increases in cytosolic calcium concentrations evoked by a pressure stimulus in cultured baroreceptor cell bodies (Kopp et al. 1998). Likewise, amiloride attenuated the responses to punctate stimulation of vagal myelinated mechanoreceptors innervating the trachea and bronchus (Carr et al. 2001). Patch-clamp studies revealed that the attenuation induced by amiloride was caused by blockade of voltage-gated sodium channels (Carr et al. 2001). This finding led the authors to conclude that amiloride did not selectively block mechanotransduction, but instead reduced excitability in mechanosensitive afferents by blocking voltage-gated sodium channels (Carr et al. 2001).
In our experiments, both doses of amiloride markedly attenuated the exercise pressor reflex. We do not think that a reduction in afferent excitability caused by blockade of voltage-gated sodium channels was the cause of the attenuation of the exercise pressor reflex induced by the low dose of amiloride. If this dose caused a reduction in afferent excitability, then one might expect that low dose would also attenuate the reflex pressor responses to capsaicin injection and to tendon stretch. Moreover, the low dose of amiloride would be expected to attenuate the responses of spindle afferents to succinylcholine and stretch. None of these effects were observed in our experiments, thereby leading us to speculate that the low dose of amiloride blocked ASICs. This speculation is supported by our finding that this low dose of amiloride significantly attenuated the reflex pressor response to lactic acid, a potent stimulant of these channels (Immke & McCleskey, 2001). On the other hand, the attenuation of the pressor reflexes by the high dose of amiloride seems best explained by a reduction in afferent excitability caused by blockade of voltage-gated sodium channels as well as blockade of ASICs.
Three genes and two splice variants encode the four ASICs found in sensory nerves. ASIC3, which has also been termed DRASIC, is unique because it is found, for the most part, only in dorsal root ganglia (Waldmann & Lazdunski, 1998). In addition, in vitro evidence suggests that ASIC3 opens when the pH in the interstitium of muscle decreases from 7.4 to 7.0 (Immke & McCleskey, 2001), a concentration which occurs during exercise. Moreover, ASIC3 is more sensitive to lactic acid than it is to other acids (Immke & McCleskey, 2001). In ASIC3 knockout mice, multiple injections of acid into skeletal muscle failed to cause a long lasting hypersensitivity to mechanical stimuli that was found in their wild type counterparts (Sluka et al. 2003). These findings, considered together, lead us to speculate that the ASIC3 was the channel blocked by the low dose of amiloride in our experiments.
Dorsal root ganglion cells innervating rodent skeletal muscle have been reported to bind antibodies to both ASIC3 and calcitonin gene-related peptide (CGRP), but not to the P2X3 receptor (Molliver et al. 2005). These findings led Molliver et al. (2005) to speculate that during exercise, lactic acid accumulation in the muscle interstitium stimulated thin fibre afferents, thereby evoking the metabolic component of the exercise pressor reflex, while simultaneously releasing CGRP, a vasodilatory peptide, from their sensory endings. There may be, however, more than one substance evoking the metabolic component of the exercise pressor reflex. Likewise, there may be more than one substance causing vasodilatation in exercising muscle. For example, ATP is known to evoke the exercise pressor reflex (Hanna & Kaufman, 2003), and its metabolite, adenosine, is known to cause vasodilatation (MacLean et al. 1998). The possibility that purinergic substances evoke both effects is attractive because ASIC3 and P2X3 receptors have been reported to be found on different thin fibre muscle afferents (Molliver et al. 2005), thereby dilating vessels supplied by thin fibre afferents possessing either ASIC3 or P2X3 receptors.
In humans, the role played by lactic acid in the generation of the metabolic component of the exercise pressor reflex has been controversial (Thomas & Victor, 2003). Most of this controversy has stemmed from studies examining the pressor and muscle sympathetic nerve responses to static exercise in individuals with McArdle's disease. These patients have a myophosphoralase deficiency and as a consequence produce little lactic acid when they exercise. Two studies have shown that the pressor and muscle sympathetic nerve responses to static handgrip are almost non-existent in these patients (Pryor et al. 1990; Fadel et al. 2003), whereas two others have shown that these responses are preserved (Vissing et al. 1998, Vissing 2001). Most other studies in humans have found support for a role played by lactic acid in the generation of the exercise pressor reflex. For example, humans display a strong inverse relationship between intracellular pH in statically exercising muscle and either muscle sympathetic nerve activity or calf vascular resistance (Victor et al. 1988; Sinoway et al. 1989). Likewise, blunting lactic acid production by either depleting glycogen in muscle or giving dichloroacetate has been shown to decrease the pressor and muscle sympathetic nerve responses to exercise (Ettinger et al. 1991; Sinoway et al. 1992).
Our report is the first to show that amiloride attenuated the exercise pressor reflex. Although we attribute this attenuation by the low dose to the blockade of ASIC3 on the endings of thin fibre muscle afferents, this blockade was not complete because we found that it did not abolish the reflex pressor response to lactic acid injections. We note with interest that blockade of P2X receptors (Hanna & Kaufman, 2003), bradykinin 2 receptors (Pan et al. 1993) and prostaglandin synthesis (Stebbins et al. 1988) in skeletal muscle have been each shown to attenuate the exercise pressor reflex by at least half. These findings suggest that the ligands to each of these receptors has a role to play in the elicitation of the reflex, the full expression of which must be the result of a complex integration of afferent input in the dorsal horn of the spinal cord or the ventrolateral medulla.
Acknowledgments
This work was supported by NIH grant HL 30710 and an AHA Beginning Grant-in-Aid 0565051Y. We thank Yao Dong for her surgical assistance.
References
- Alvarez de la Rosa D, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral neruons system. PNAS. 2002;99:2326–2331. doi: 10.1073/pnas.042688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MJ, Gover TD, Weinreich D, Undem BJ. Inhibition of mechanical activation of guinea-pig airway afferent neurons by amiloride analogues. Br J Pharmacol. 2001;133:1255–1262. doi: 10.1038/sj.bjp.0704197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol. 2004;556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond HA, Price MP, Welsh MJ, Abboud FM. A molecular component of the arterial baroreceptor mechanotransducer. Neuron. 1998;21:1435–1441. doi: 10.1016/s0896-6273(00)80661-3. [DOI] [PubMed] [Google Scholar]
- Ettinger S, Gray K, Whisler S, Sinoway L. Dichloroacetate reduces sympathetic nerve responses to static exercise. Am J Physiol. 1991;261:H1653–H1658. doi: 10.1152/ajpheart.1991.261.5.H1653. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Wang Z, Tuncel M, Watanabe H, Abbas A, Arbique D, Vongpatanasin W, Haley RW, Victor RG, Thomas GD. Reflex sympathetic activation during static exercise is severely impaired in patients with myophosphorylase deficiency. J Physiol. 2003;548:983–993. doi: 10.1113/jphysiol.2003.039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Henig O, Kehoe V, Sinoway LI, Li J. Vanilloid type 1 receptor and the acid-sensing ion channel mediate acid phosphate activation of muscle afferent nerves in rats. J Appl Physiol. 2006b;100:421–426. doi: 10.1152/japplphysiol.00659.2005. [DOI] [PubMed] [Google Scholar]
- Granit R, Skoglund S, Thesleff S. Activation of muscle spindles by succinylcholine and decamethonium. Acta Physiol Scand. 1953;28:134–151. doi: 10.1111/j.1748-1716.1953.tb00964.x. [DOI] [PubMed] [Google Scholar]
- Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol. 2003;94:1437–1445. doi: 10.1152/japplphysiol.01011.2002. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol. 2001;280:H2153–H2161. doi: 10.1152/ajpheart.2001.280.5.H2153. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol. 2006;290:H2239–H2246. doi: 10.1152/ajpheart.01274.2005. [DOI] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001b;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- Kindig AE, Heller TB, Kaufman MP. VR-1 receptor blockade attenuates the pressor response to capsaicin but has no effect on the pressor response to contraction in cats. Am J Physiol Heart Circ Physiol. 2005;288:H1867–H1873. doi: 10.1152/ajpheart.00735.2004. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Matsushita K, Sigmund RD, Smith LA, Watanabe S, Stokes JB. Amiloride-sensitive Na+ channels in pelvic uroepithelium involved in renal sensory receptor activation. Am J Physiol. 1998;275:R1780–R1792. doi: 10.1152/ajpregu.1998.275.6.R1780. [DOI] [PubMed] [Google Scholar]
- Li J, Maile MD, Sinoway AN, Sinoway LI. Muscle pressor reflex: potential role of vanilloid type 1 receptor and acid-sensing ion channel. J Appl Physiol. 2004;97:1709–1714. doi: 10.1152/japplphysiol.00389.2004. [DOI] [PubMed] [Google Scholar]
- MacLean DA, La Noue KF, Gray KS, Sinoway LI. Effects of hindlimb contraction on pressor and muscle interstitital metabolite responses in the cat. J Appl Physiol. 1998;85:1583–1592. doi: 10.1152/jappl.1998.85.4.1583. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J. Cyclooxygenase products sensitize muscle mechanoreceptors in healthy humans. Am J Physiol Heart Circ Physiol. 2004;287:H1944–H1949. doi: 10.1152/ajpheart.00329.2004. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Ann Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H-L, Stebbins CL, Longhurst JC. Bradykinin contributes to the exercise pressor reflex: mechanism of action. J Appl Physiol. 1993;75:2061–2068. doi: 10.1152/jappl.1993.75.5.2061. [DOI] [PubMed] [Google Scholar]
- Pryor SL, Lewis SF, Haller RG, Bertocci LA, Victor RG. Impairment of sympathetic activation during static exercise in patients with muscle phosphorylase deficiency (McArdle's Disease) J Clin Invest. 1990;85:1444–1449. doi: 10.1172/JCI114589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotto DM, Kaufman MP. Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. J Appl Physiol. 1988;64:2306–2313. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by products of arachidonic acid metabolism. J Appl Physiol. 1990;68:861–867. doi: 10.1152/jappl.1990.68.3.861. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Stebbins CL, Kaufman MP. Reflex cardiovascular and ventilatory responses to increasing H+ activity in cat hindlimb muscle. J Appl Physiol. 1989;67:256–263. doi: 10.1152/jappl.1989.67.1.256. [DOI] [PubMed] [Google Scholar]
- Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol. 1989;66:429–436. doi: 10.1152/jappl.1989.66.1.429. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol. 1993;69:1053–1059. doi: 10.1152/jn.1993.69.4.1053. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Wroblewski KJ, Prophet SA, Ettinger SM, Gray KS, Whisler SK, Miller G, Moore RL. Glycogen depletion-induced lactate reductions attenuate reflex responses in exercising humans. Am J Physiol. 1992;263:H1499–H1505. doi: 10.1152/ajpheart.1992.263.5.H1499. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Maruoka Y, Longhurst JC. Prostaglandins contribute to cardiovascular reflexes evoked by static muscular contraction. Circ Res. 1988;59:645–654. doi: 10.1161/01.res.59.6.645. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Victor RG. In favor of a role of lactic acid on the generation of the exercise pressor reflex. Clin Auton Res. 2003;13:5–6. doi: 10.1007/s10286-003-0080-z. [DOI] [PubMed] [Google Scholar]
- Victor RG, Bertocci LA, Pryor SL, Nunnally RL. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest. 1988;82:1301–1305. doi: 10.1172/JCI113730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing J, MacLean D, Vissing SF, Sander M, Saltin B, Haller RG. The exercise metaboreflex is maintained in the absence of muscle acidosis: insights from muscle microdialysis in humans with McArdle's disease. J Physiol. 2001;537:641–649. doi: 10.1111/j.1469-7793.2001.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing J, Vissing SF, MacLean DA, Saltin B, Quistorff B, Haller RG. Sympathetic activation in exercise is not dependent on muscle acidosis. J Clin Invest. 1998;101:1654–1660. doi: 10.1172/JCI555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- Waldrop TG, Rybicki KJ, Kaufman MP. Chemical activation of group I and II muscle afferents has no cardiorespiratory effects. J Appl Physiol. 1984;56:1223–1228. doi: 10.1152/jappl.1984.56.5.1223. [DOI] [PubMed] [Google Scholar]