Abstract
Glutamatergic inputs arising from the parabrachial nucleus to neurons in the lateral sector of the central amygdala were studied in vitro. Tetanic stimulation of these inputs led to LTP that did not require activation of NMDA receptors or a rise of postsynaptic calcium. LTP was accompanied by a reduction in the paired-pulse ratio, indicating that LTP results from an increase in transmitter release probability. Activation of adenylyl cyclase with forskolin potentiated these inputs with a similar reduction in paired-pulse facilitation and occluded LTP induction. LTP was inhibited by the protein kinase A blocker H89. Low-frequency stimulation led to LTD that required activation of postsynaptic NMDA receptors and a rise in postsynaptic calcium. There was no change in paired-pulse facilitation with LTD. LTD was blocked by protein phosphatase blockers calyculin and okadaic acid. We conclude that parabrachial inputs to the lateral sector of the central amygdala show presynaptic LTP that requires activation of a presynaptic protein kinase A via a calcium-dependent adenylyl cyclase while LTD at the same synapses is postsynaptic and requires a rise in postsynaptic calcium and activation of protein phosphatase.
Fear is an emotional response that evolved as a survival instinct and is evoked by a variety of threatening stimuli. In humans, inappropriate fear responses are seen as the main manifestation of anxiety disorders. Studies over the last 20 years have established that the amygdala, a complex temporal lobe structure, plays a central role in the processing of emotional stimuli, particularly fear. Cellular studies of the neuronal correlates of fear have largely used a simple Pavlovian conditioning protocol, fear conditioning, to address questions about the locus of changes that may underlie fear and anxiety. In fear conditioning, a relatively neutral stimulus (the conditioned stimulus, CS) is paired with an aversive stimulus (the unconditioned stimulus, UCS). Following a few pairings the previously benign CS comes to evoke behavioural, autonomic and endocrine responses typical of the fear state. A converging body of evidence has established that conditioned and unconditioned stimuli converge on single neurons within the lateral amygdala (LA) (Sah et al. 2003; Rodrigues et al. 2004). These inputs are glutamatergic, and form dual-component synapses on pyramidal neurons in the LA (Weisskopf & LeDoux, 1999; Mahanty & Sah, 1999). Coincident activation of synapses activated by the CS and UCS is thought to lead to NMDA receptor-dependent long-term potentiation (LTP) of the efficacy of inputs relaying CS information. This information is processed locally and then transmitted to the central nucleus of the amygdala (CeA) from which descending projections are responsible for initiating the behavioural components of fear conditioning (LeDoux, 2000; Davis & Whalen, 2001; Maren, 2001). In this model, the central amygdala simply acts as a relay station for initiating the physiological changes that accompany fear-related behaviours. However, a converging body of evidence over the last 10 years has shown that the central nucleus receives input from virtually all areas that project to the basolateral complex. Thus, the emerging view is that the central nucleus, rather than being a static output nucleus, is an important site of plasticity in a number of learning protocols that engage the amygdala (Pare et al. 2004; Samson & Pare, 2005).
In fear conditioning, the UCS is typically a mild footshock that activates, among other modalities, ascending nociceptive inputs. The experience of pain has a strong emotional component and it is well known that perception of pain is altered in a variety of anxiety disorders. Accumulating evidence indicates that the amygdala is a key structure in the central mechanisms of nociception (Neugebauer et al. 2004). Thus, both the basolateral complex and the central nucleus receive nociceptive inputs from cortical and subcortical structures, a network that has been called the ‘nociceptive amygdala’. In particular, the central amygdala receives abundant nociceptive inputs from the spinal cord and the trigeminal nucleus via the pontine parabrachial nucleus (pPB) (Bernard et al. 1993; Neugebauer et al. 2004). The CeA has three divisions (medial, lateral and capsular) which receive afferents from different sources (Sah et al. 2003). Of these, nociceptive inputs from the pPB innervate neurons in the lateral and capsular regions of the CeA (Bernard et al. 1993). These inputs, together with the neurons they innervate, have been shown to undergo long-term changes following persistent peripheral pain (Neugebauer & Li, 2003; Neugebauer et al. 2003). However, the cellular changes that initiate these changes are still not understood.
Persistent changes in synaptic transmisson that underlie learned behaviours are of two types: LTP or long-term depression (LTD). Both these forms of plasticity occur at glutamatergic inputs and are typically initiated by calcium influx via NMDA receptors (Bliss & Collingridge, 1993; Malenka & Bear, 2004). Inputs from the pPB to CeA neurons are glutamatergic and form typical dual-component synapses that express both AMPA and NMDA receptors (Lopez de Armentia & Sah, 2003). The NMDA subtype of ionotropic glutamate receptor is a heteromultimeric protein containing two obligatory NR1 subunits and two or more NR2 subunits (Cull-Candy et al. 2001). Four types of NR2 subunits, NR2A, NR2B, NR2C and NR2D, are known. At birth, when most forebrain synapses form, there is little or no expression of NR2A subunits and postsynaptic NMDA receptors are composed of NR1/NR2B subunits. With development, NR2A subunit expression rises and, at mature synapses, receptors may contain NR1/NR2A subunits or NR1/NR2A/NR2B subunits (Sheng et al. 1994; Tovar & Westbrook, 1999).
It has recently been proposed that calcium influx via receptors that contain NR2B subunits initiates processes that lead to LTD, whereas influx via receptors that contain NR2A subunits initiates LTP (Liu et al. 2004; Massey et al. 2004). However, these studies have been done at synapses where the exact subunit composition of synaptic NMDA receptor(s) is not known. Furthermore, in cortical neurons it has been suggested that the NR2B-containing receptors that underlie LTD induction are extrasynaptic (Massey et al. 2004). We have shown that, unlike other synapses in the forebrain, NMDA receptors at synapses in the CeA do not undergo a developmental switch in subunit composition, but remain as NR1/NR2B receptors into adulthood (Lopez de Armentia & Sah, 2003). In this study we examine the properties of synaptic plasticity at pPB inputs to the lateral sector of the central amygdala (CeL). We show that tetanic stimulation of these inputs evokes LTP that has a presynaptic locus and does not require activation of NMDA receptors. In contrast, low-frequency stimulation leads to LTD that requires calcium influx via postsynaptic NMDA receptors.
Methods
Experiments were performed on acute rat brain slices maintained in vitro. All experimental procedures were in accordance with guidelines of the University of Queensland Institutional Animal Ethics Committee. Wistar rats (21–27 days old) were anaesthetized with intraperitoneal pentobarbitone (50 mg kg−1), decapitated and coronal slices (400 μm) were prepared using standard methods (Mahanty & Sah, 1999). Following a recovery period of 30 min at 32°C, slices were maintained at room temperature in oxygenated (95% O2–5% CO2) Ringer solution containing (mm): NaCl 118, KCl 2.5, NaHCO3 25, NaH2PO4 1.2, MgCl2, 1.3, CaCl2 2.5 and glucose 10. For recordings, slices were transferred to the recording chamber and superfused with Ringer solution at 32–34°C.
Whole-cell recordings were made from neurons in the lateral division of the central amygdala (Sah et al. 2003) using either the ‘blind’ approach or infrared differential interference videomicroscopy. Electrodes were filled with an intracellular solution containing (mm): KMeSO4 135, NaCl 8, Hepes 10, Mg2ATP 2, Na3GTP 0.3 (pH 7.3, mosmoles l−1 290 mosmol kg−1). Access resistance was 7–20 MΩ and was monitored throughout the experiment. Experiments were discarded if the series resistance changed by more than 20% during the course of the experiment. Signals were recorded using an Axopatch 1-D amplifier (Axon Instruments, Foster City, CA, USA) filtered at 5 kHz and digitized at 10 kHz (Instrutech, ITC 16, Long Island, NY, USA). All cells described in this study had a membrane potential more negative than −50 mV. Electrical activity was recorded in voltage clamp and analysed using Axograph 4.9 (Axon Instruments).
Synaptic responses from the region of the internal capsule (just ventral to the central nucleus) were evoked electrically using a concentric bipolar electrode. A second stimulating electrode was also placed in the BLA and used as a control input. Stimuli (50 μs, 5–30 V) were applied by a constant-voltage isolated stimulator (Digitimer, DS2A, Hertfordshire, UK). For baseline measurements, afferent fibres were stimulated at 0.1 Hz. LTP was induced by stimulating fibres at 100 Hz for 1 s, repeated twice with an interval of 20 s. For induction of LTD, afferent fibres were stimulated at 1 Hz for 900 pulses. During the induction of LTP and LTD, neurons were depolarized at −50 mV. Picrotoxin (100 μm) was added to the physiological solution in all experiments to block inhibitory synaptic transmission.
All values are expressed as mean ± s.e.m. For amplitude comparison between responses, excitatory postsynaptic currents (EPSCs) from 5 min prior to the LTP or LTD induction protocol were averaged and compared with the averaged responses recorded in the last 5 min using a two-tailed paired t test. For comparison between populations, chi-square tests were used. d-APV and MCPG were purchased from Tocris Cookson Inc. (Bristol, UK) and picrotoxin, H-89, BAPTA-AM, okadaic acid, calyculin A, 8-bromo-cAMP and forskolin from Sigma-Aldrich.
Results
LTP of the excitatory synaptic transmission in the medial input of the CeL
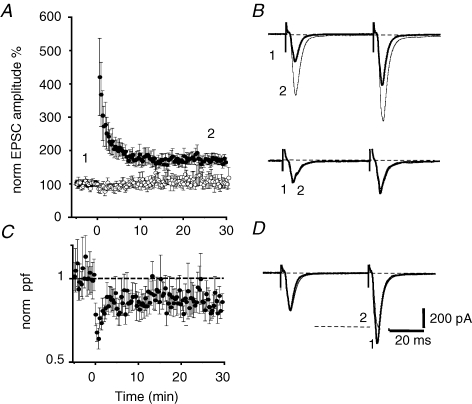
Whole-cell recordings were made from neurons in the lateral sector (CeAL) of the central amygdala. Inputs arising in the basolateral nucleus and those arising in the parabrachial nucleus (Neugebauer et al. 2003) were alternatively stimulated at 0.1 Hz. As previously described (Lopez de Armentia & Sah, 2003), in the presence of GABAA receptor blockers, stimulation of afferents to the CeA evoked a glutamatergic dual-component excitatory synaptic current (EPSC) in all CeA neurons. Tetanic stimulation (2 × 100 Hz, 1 s, separated by 20 s) of pPB inputs caused a long-lasting potentiation of the EPSC amplitude (n = 17). This increase in EPSC amplitude was maintained for the duration of the recording up to 30 min (Fig. 1A). The averaged potentiated response 30 min after the tetanus was 168 ± 10% of the baseline response (P < 0.0001, n = 12). This potentiation was input specific as EPSCs evoked by stimulation of a second input arising in the BLA (Lopez de Armentia & Sah, 2004) did not show any change (Fig. 1B) after the tetanus (111 ± 20% of the baseline response, P = 0.80, n = 10). LTP at glutamatergic inputs to CeA neurons in the medial sector (CeAM) has been shown to have a presynaptic locus as judged by changes in paired-pulse facilitation (Fu & Shinnick-Gallagher, 2005; Samson & Pare, 2005). To test if LTP was due to a change in the release probability, inputs were stimulated twice with a 50 ms interval to examine paired-pulse facilitation (PPF). Following LTP induction at pPB inputs there was a clear reduction in PPF (86 ± 4%, P < 0.02, n = 10; Fig. 1C), suggesting that, as in CeAM neurons (Samson et al. 2005), LTP at glutamategic inputs to CeAL neurons is associated with an increase in transmitter release probability.
Figure 1.
Tetanic stimulation of the parabrachial input induces LTP at the synapses onto CeAL neurons A, average normalized EPSC amplitude plotted against time for 14 neurons before and after tetanic stimulation of the medial input (•) and a control input from the BLA (^) that was not tetanized. The inputs were stimulated with paired pulses with an interpulse interval of 50 ms. Representative EPSCs from the indicated time points are shown in B for the tetanized input (upper traces) and the control input (lower traces). C, the average normalized paired-pulse ratios for the same cells shown in A are plotted against time. There is an increase in release probability with LTP as shown by the reduction in paired-pulse ratio. EPSCs from the input showing LTP have been normalized to the first EPSC and overlaid in D to illustrate the change in paired-pulse ratio during LTP.
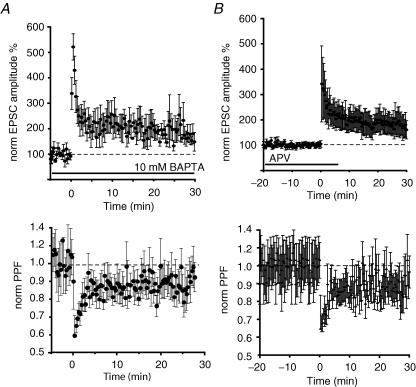
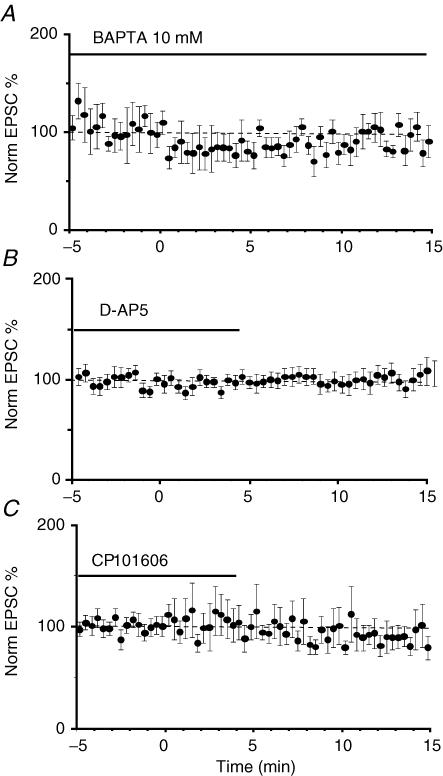
To test if induction of LTP at these synapses requires a rise in postsynaptic calcium, cells were loaded with the calcium chelator BAPTA (10 mm) for 10 min before delivery of the tetanus. BAPTA-loaded neurons showed a potentiated response 30 min after the tetanus (165 ± 45% of the baseline, n = 5, Fig. 2A) indicating that a rise of calcium in the postsynaptic neuron was not necessary for the induction of LTP. The potentiation in the synaptic responses was also associated with a decrease in the PPF ratio (n = 5, Fig. 2A). At synapses on CeAM neurons, while LTP does not require a rise in postsynaptic calcium, activation of NMDA receptors is still necessary (Samson et al. 2005). We next tested if LTP in the CeAL required activation of NMDA receptors. d-APV (30 μm) was applied for 20 min prior to the tetanus to ensure the homogeneous distribution of the drug in the slice, a concentration at which it effectively blocks NMDA receptors at these synapses but has no effect on baseline transmission (Lopez de Armentia & Sah, 2003). LTP induction was not blocked by APV, with the average potentiation being 212 ± 52%, not significantly different from that seen under control conditions (P = 0.06, n = 6, Fig. 2B). A reduction in PPF was also observed after the tetanus, again not significantly different from control conditions (86 ± 7% of the baseline, P = 0.16, n = 6). These results indicate that LTP of pPB afferents does not require the activation of NMDA receptors or a rise in postsynaptic calcium.
Figure 2.
LTP does not require NMDA receptor activation or a rise in postsynaptic calcium A, averaged normalized EPSC amplitude is plotted for four neurons loaded with the calcium chelator BAPTA (10 mm). The normalized paired-pulse ratio for these cells is plotted in the lower panel. B, average normalized EPSC is plotted for 6 cells. Following a short baseline, d-APV (30 μm) was applied and had no effect on baseline transmission. Tetanic stimulation in the presence of APV leads to LTP. Subsequent washout of APV has no effect on synaptic transmission. The normalized paired-pulse ratio for these cells is plotted in the lower panel.
It has been suggested that group I metabotropic glutamate receptors (mGluR) are involved in the induction of LTP in the nociceptive pathway to the CeL (Neugebauer et al. 2003). To test this possibility we perfused the slice with 1 mm MCPG, a group I and II mGluR antagonist, 10 min before the tetanus. Application of MCPG had no effect on either the parabrachial input or the BLA input (not shown). Furthermore, in the presence of MCPG, LTP induction was not blocked, with the average EPSC increasing to 209 ± 31% of baseline 30 min after the tetanus (n = 4, not illustrated) indicating that activation of mGluRs is not involved in the induction of LTP at pPB input to CeAL neurons.
Activation of adenylyl cyclase is necessary for LTP induction
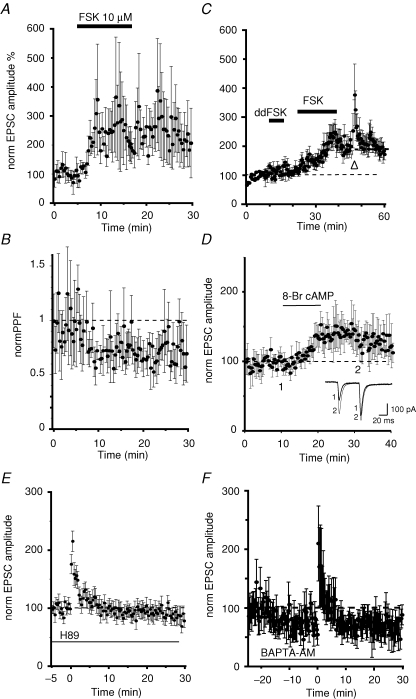
NMDA receptor-independent changes in synaptic transmission have been described at a number of different synapses (Zalutsky & Nicoll, 1990; Salin et al. 1996; Margrie et al. 1998). A common feature of LTP at these synapses is activation of presynaptic adenylyl cyclase, which leads to an increase in the probability of transmitter release. To test if a similar mechanism is functional at CeAL synapses we first applied the diterpene forskolin that directly activates adenylyl cyclase (Laurenza et al. 1989). As has been described at other synapses (Weisskopf et al. 1994; Margrie et al. 1998), application of forskolin (10 μm) enhanced the EPSC by 191 ± 34% (n = 4) with an associated reduction of PPF (79 ± 6% of control; n = 4, Fig. 3B), indicating a presynaptic action. Application of the inactive isomer of forskolin, 1,9 dideoxy forskolin, that has no direct effect on adenylyl cyclase but does have the cAMP-independent actions of forskolin (Laurenza et al. 1989), had no effect on the EPSC (n = 5; Fig. 3C). If the actions of forskolin and LTP induction share similar mechanisms we predicted that induction of LTP should be occluded in the presence of forskolin (Weisskopf et al. 1994). In agreement with this, tetanization of the input following application of forskolin did not lead to LTP (n = 5; Fig. 3C). These results suggest that tetanic stimulation of pPB inputs to CeAL neurons activates adenylyl cyclase and leads to an increase in release probability. In support of this proposal, application of the membrane-permeable cAMP analogue 8-bromo-cAMP also potentiated the EPSC with a change in PPF (n = 4; Fig. 3D). Activation of adenylyl cyclase and the generation of cAMP activates the serine threonine kinase protein kinase A. To test if LTP results from activation of protein kinase A we blocked the downstream actions of cAMP by H-89, a selective blocker of the catalytic site of cAMP-dependent protein kinase A. Slices were soaked in 1 μm H-89 for 30 min before the experiment started and the tetanus was applied in the presence of the inhibitor. Under these conditions we were unable to induce LTP in any of the 14 neurons examined (Fig. 3E). The averaged response 30 min after the tetanus was 84 ± 10% of the baseline (P = 0.19, n = 11), and no changes in PPF were observed after the tetanus (112 ± 18% of baseline, P = 0.50, n = 11). These results show that tetanic stimulation of pPB afferents to neurons in the CeAL activates adenylate cyclase and activation of protein kinase A and a subsequent increase in release probability. Adenylate cyclase has multiple isoforms some of which are activated via a G-protein-coupled receptor while other can be activated by a rise in cytosolic calcium (Sunahara et al. 1996). As tetanic stimulation will lead to a prolonged rise in presynaptic calcium we wondered if LTP at pPB inputs may be triggered by activation of a calcium-dependent adenylyl cyclase. To test for this possibility we buffered presynaptic calcium rises by bath application of the membrane-permeant calcium chelator BAPTA-AM (5 μm). Application of BAPTA-AM reduced EPSC's amplitude by 33% at pPB synapses. Moreover, LTP was completely blocked by BAPTA-AM (n = 7; Fig. 3F), indicating that a rise in presynaptic calcium is necessary for activation of adenylyl cyclase.
Figure 3.
Activation of calcium-dependent adenylyl cyclase and protein kinase A is necessary for the induction of LTP A, average normalized EPSC amplitudes are plotted against time. Application of forskolin (10 μm), an activator of adenylyl cyclase, potentiates synaptic transmission with a reduction in the paired-pulse ratio (B) showing that it is due to an increase in release probability. C, the inactive isomer of forskolin (dideoxy forskolin, ddFSK) has no effect on synaptic transmission. Subsequent application of forskolin in the same neurons potentiates transmission. Tetanic stimulation delivered in the presence of forskolin (arrowhead) does not induce LTP at these inputs. D, application of the membrane-permeant analogue of cAMP, 8-bromo cAMP, potentiates the EPSC with a reduction in paired-pulse potentiation (insets). E, slices were incubated with the protein kinase A blocker H-89 (1 μm). LTP is blocked in the presence of H-89 showing that activation of protein kinase A is required for LTP induction. F, application of the membrane-permeant calcium chelator BAPTA-AM (5 μm) led to a small reduction of the EPSC amplitude. Tetanic stimulation in the presence of BAPTA-AM completely blocked LTP showing that adenyl cyclase activation requires a rise in presynaptic calcium.
Homosynaptic LTD in CeL neurons
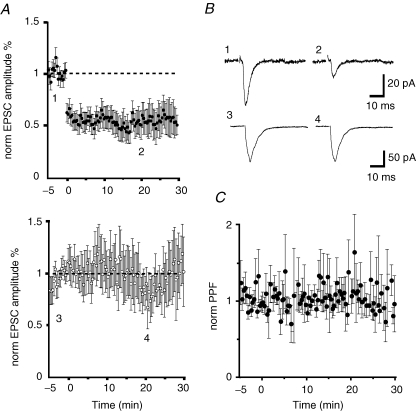
We next tested if the pPB input can also undergo LTD. Low-frequency stimulation (1 Hz, 900 pulses) of the input led to a long-lasting reduction of the EPSC amplitude. EPSC amplitude 30 min after low-frequency activation was 37 ± 11% of the baseline (P < 0.001, n = 7). LTD was homosynaptic as shown by the lack of effect on the unstimulated BLA input (102 ± 23% of the baseline, P = 0.34, n = 7; Fig. 4A and B). Unlike LTP at these inputs, following low-frequency stimulation there was no change of PPF (109 ± 17% of the baseline, P = 0.42, n = 7, Fig. 4C) indicating that the reduction of EPSC amplitude was not produced by a change in the release probability.
Figure 4.
Low-frequency stimulation of the parabrachial input to CeL neurons induces LTD A, average normalized EPSC amplitude in which the parabrachial input (•) and a control input from the BLA (^, lower panel) were alternatively stimulated at 0.1 Hz. At time 0, the parabrachial input was stimulated at 1 Hz for 900 stimuli which led to a large reduction in EPSC amplitude. Average EPSC from the time points indicated in A are shown in B for the parabrachial input (upper traces) and the BLA (lower traces). C, normalized paired-pulse ratio for the inputs shown in A. No changes were observed in the paired-pulse ratio.
The induction of LTD at many synapses requires postsynaptic calcium influx via NMDA receptors (Malenka & Bear, 2004). To test if the induction of LTD was pre- or postsynaptic we loaded postsynaptic neurons with 10 mm BAPTA and performed the low-frequency stimulation protocol after a 10 min baseline. Loading cells with BAPTA completely blocked LTD (97 ± 12% of the baseline, P = 0.67, n = 8, Fig. 5A). Thus, a rise in postsynaptic calcium is necessary for the induction of LTD. We next tested if NMDA receptors were the source of Ca2+ elevation during LTD induction. In the presence of d-APV (30 μm), LTD was absent in all cells (103 ± 24% of baseline, P = 0.78, n = 13; Fig. 5B). Synaptic NMDA receptors in CeL neurons are composed of NR1/NR2B subunits (Lopez de Armentia & Sah, 2003). Thus, as expected, the selective NR2B antagonist CP101606 (5 μm), which has no effect on baseline transmission (Lopez de Armentia & Sah, 2003), also blocked the induction of LTD (88 ± 11%, P = 0.28 n = 12, Fig. 5C). Finally, in separate experiments we hyperpolarized the cells to −80 mV during the low-frequency stimulation to block activation of NMDA receptors, and the entry of Ca2+. No changes in EPSC amplitude were observed in any of the seven neurons tested after the LTD induction protocol (data not shown). Taken together, these experiments demonstrate that induction of LTD in CeAL neurons is postsynaptic and requires a rise in postsynaptic calcium by influx via NMDA receptors.
Figure 5.
LTD of the parabrachial input requires activation of NMDA receptors and a rise in postsynaptic calcium A, averaged normalized responses from 8 neurons that were loaded with 10 mm BAPTA. At time 0, low-frequency (1 Hz) stimulation was delivered for 900 stimuli. LTD induction is blocked by postsynaptic BAPTA. B and C, slices were incubated in either the NMDA receptor antagonist d-AP5 (30 μm, B, n = 13) or the selective NR2B antagonist CP101606 (5 μm, C, n = 12). Low-frequency stimulation was delivered at time 0. LTD induction was blocked when postsynaptic NMDA receptors were antagonized.
Phosphatase activity is required for the induction of LTD
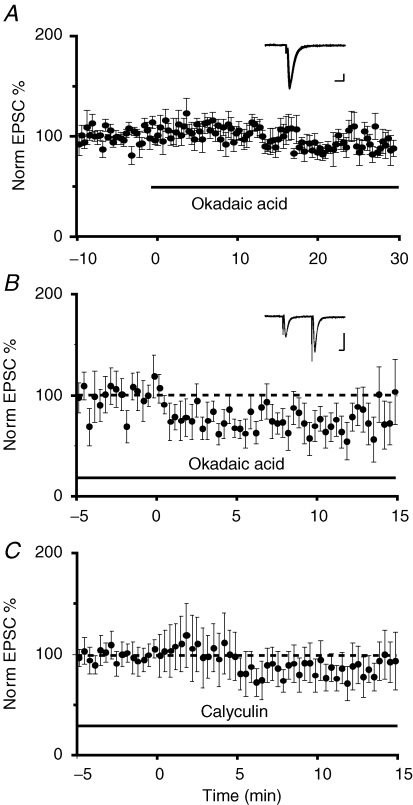
In hippocampal pyramidal neurons, activation of postsynaptic phosphatases has been implicated in the induction of LTD (Mulkey & Malenka, 1992). To test if phosphatase activity is also required for LTD at medial inputs in the CeL, we applied two inhibitors of protein phosphatases I and II, okadaic acid and calyculin A (Honkanen & Golden, 2002). For both compounds, slices were soaked in a physiological solution containing the drugs for at least 30 min before starting the experiment, and induction of LTD was performed in the presence of the drugs. These compounds are slow to act in brain slices; however, application of okadaic acid under control conditions had no detectable effect on baseline transmission over 30 min (n = 5; Fig. 6A). As shown in Fig. 6, both okadaic acid (1 μm) and calyculin A (1 μm) blocked LTD. Although a reduction of EPSC amplitude was observed after the low-frequency stimulation, the response had returned to baseline after 30 min when the slices were in the presence of okadaic acid (87 ± 11% of baseline P = 0.08, n = 5, Fig. 6B) or in the presence of calyculin A (92 ± 20% of baseline P = 0.78, n = 10 Fig. 6C).
Figure 6.
Activation of phosphatase I and II are required for LTD A, average normalized EPSC amplitude is plotted against time for 6 cells in which the phosphatase I blocker okadaic acid was applied at time zero. Okadaic acid had no effect on basal transmission. The inset shows superimposed traces from one cell before and after application of okadaic acid. Scale bars are 100 pA and 10 ms B, average normalized EPSC amplitude is plotted against time in the presence of okadaic acid (1 μm) in the perfusing Ringer solution. The parabrachial input was stimulated at 1 Hz (900 pulses) at time 0 min (n = 8). LTD was absent in the presence of the protein phosphatase blocker. The inset shows superimposed traces from one cell before and after low-frequency stimulation. Scale bars, 50 pA and 10 ms C, similarly, incubation of slices in the protein phosphatase II blocker calyculin A (1 μm) also blocked the induction of LTD (n = 13).
Discussion
We have shown that tetanic stimulation of glutamatergic, presumed parabrachial, inputs to the lateral sector of the CeA leads to input-specific LTP of these inputs. This LTP does not require activation of NMDA receptors, or a rise in postsynaptic calcium, and is accompanied by a clear change in PPF indicating that LTP at these inputs is due to a change in transmitter release probability. Low-frequency stimulation led to LTD of the same inputs. In contrast to LTP, LTD required a rise in postsynaptic calcium, and activation of NMDA receptors. LTD was not accompanied by a change in PPF, suggesting a postsynaptic locus for this plasticity. We conclude that glutamatergic synapses made by inputs from the parabrachial nucleus to CeA neurons undergo LTP and LTD by separate and independent mechanisms.
Two types of LTP have been described at mammalian central synapses. At most synapses, the induction of LTP requires activation of postsynaptic NMDA receptors with a consequent rise in postsynaptic calcium (Malenka & Bear, 2004). This calcium rise triggers a second messenger cascade that is initiated by activation of CaMKII, and ultimately potentiates synaptic transmission. While the exact locus of the final changes that mediate NMDA receptor-dependent LTP have been debated, current models suggest a postsynaptic locus for this change (Malenka & Bear, 2004). In contrast, at mossy fibre synapses to CA3 pyramidal neurons (Weisskopf et al. 1994) and parallel fibre synapses in the cerebellum (Salin, 1996), both also glutamatergic synapses, tetanic stimulation leads to LTP that does not require NMDA receptor activation. This LTP results from activation of a presynaptic type I adenylyl cyclase, with a consequent increase in release probability. Similarly, at glutamatergic synapses to CeL neurons, we have shown that LTP does not require activation of NMDA receptors or a rise in postsynaptic calcium. Consistent with these findings, the ability to induce LTP at these inputs was not prone to ‘washout’ and could be reliably induced even with prolonged whole-cell recordings (up to 30 min). Transmitter release probability at these synapses is higher following LTP, as shown by the reduction in PPF (Zucker, 1989). Activation of adenylyl cyclase with forskolin, or exogenous application of cAMP (Fig. 3), potentiates transmission by increasing the release probability. Furthermore, forskolin application occludes LTP induction and LTP is blocked by the protein kinase A (PKA) inhibitor H-89. Together, these results show that LTP at glutamatergic inputs to CeAL neurons results from presynaptic activation of adenylyl cyclase, the generation of cAMP and the activation of PKA. As LTP at these synapses was blocked by buffering presynaptic calcium by bath application of the membrane-permeant calcium chelator BAPTA-AM, these results show that the presynaptic adenylyl cyclase is activated by a sustained rise in presynaptic calcium during tetanic stimulation. The cell bodies of origin of both mossy and parallel fibres express high levels of calcium-dependent adenylyl cyclase, consistent with calcium-dependent activation of PKA at the axon terminals of these neurons (Xia et al. 1991). Glutamatergic inputs to CeAL neurons that we have activated are thought to originate in the lateral parabrachial area (Bernard et al. 1993; Neugebauer et al. 2003). However, whether type I adenylyl cyclase is present in these neurons is not known.
Low-frequency stimulation of these inputs to CeL neurons led to LTD of synaptic transmission. In contrast to LTP, the induction of LTD required NMDA receptor activation and a rise in postsynaptic calcium. Furthermore, unlike LTP, there was no change in PPF, suggesting that the reduction in EPSC amplitude is not due to a reduction in the probability of transmitter release. At central synapses, both NMDA receptor-dependent and NMDA receptor-independent forms of LTD have been described (Malenka & Bear, 2004). For example, at mossy fibre synapses onto CA3 interneurons, LTD at synapses that express calcium-impermeable AMPA receptors requires NMDA receptor activation, and its expression appears to be postsynaptic. In contrast, in the same neurons, at synapses that express rectifying, calcium-permeable AMPA receptors, LTD does not require NMDA receptors and is associated with a change in release probability, suggesting a presynaptic locus (Lei & McBain, 2004). While they are not defined as interneurons, neurons in the CeA are GABAergic neurons (Swanson & Petrovich, 1998) and express calcium-impermeable AMPA receptors at glutamatergic inputs (Lopez de Armentia & Sah, 2004). While the mechanism that underlies LTD at calcium-impermeable AMPA receptors in CA3 interneurons was not explored, LTD induction at Schaffer collateral–CA1 pyramidal cell synapses, which also express non-rectifying calcium-impermeable AMPA receptors (Hestrin et al. 1990), requires activation of phosphatase I and II (Mulkey & Malenka, 1992) and the subsequent removal of synaptic AMPA receptors (Malinow & Malenka, 2002). The fact that the induction of LTD at CeL synapses is postsynaptic, and is not accompanied by a change in release probability, suggests that this LTD is likely to be due to a postsynaptic change. We have shown that LTD at these inputs involves activation of phosphatase I and II, and it is tempting to suggest that the final mechanism of this LTD may also involve trafficking of postsynaptic AMPA receptors at these synapses.
NMDA receptors are heteromeric proteins and have an obligatory NR1 subunit, and one of the NR2 subunits (Cull-Candy et al. 2001). While newly formed glutamatergic synapses contain NR1/NR2B subunits, adult synapses are thought to contain receptors that are either a combination of NR1/NR2A and NR1/NR2B or trimeric NR1/NR2A/NR2B subunits (Sheng et al. 1994; Lopez de Armentia & Sah, 2003). That different NR2 subunits may be responsible for initiating different forms of plasticity was initially suggested by the finding that selective antagonism of different receptor types reveals different contributions to plasticity (Hrabetova et al. 2000). It has recently been suggested that the presence of different types of NR2 subunits leads to fundamentally different forms of synaptic plasticity. Thus, it has been proposed that the activation of receptors containing NR2B subunits selectively leads to LTD, whereas activation of receptors containing NR2A subunits leads to LTP (Liu et al. 2004; Massey et al. 2004; but see Bartlett et al. 2006; Morishita et al. 2007). At adult glutamatergic synapses at CA1 pyramidal neurons, functional NMDA receptors are either NR1/NR2A dimers or contain both NR2A/NR2B subunits (Sheng et al. 1994). In contrast, in cortical neurons, NR2B-containing receptors are thought to be extrasynaptic (Rumbaugh & Vicini, 1999) and, in agreement with this, NR2B-dependent LTD at cortical synapses has been shown to require activation of extrasynaptic NMDA receptors (Massey et al. 2004). NR2A and NR2B subunits have different size C terminal domains (Cull-Candy et al. 2001; Sheng, 2001), and interact with different binding partners. Thus, it has been proposed that calcium flux via these two subunits activates different transduction machinery to initiate LTP or LTD (Liu et al. 2004). However, as both LTP and LTP in hippocampal pyramidal neurons requires a rise in postsynaptic calcium (Malenka & Bear, 2004), the mechanisms that may underlie separation of LTP and LTD induction by calcium influx via these two receptors at single synapses are not clear.
Excitatory synapses on CeL neurons express NMDA receptors that do not appear to contain NR2A subunits (Lopez de Armentia & Sah, 2003). We have shown that tetanic stimulation of these synapses, which would lead to calcium influx via NMDA receptors containing NR2B subunits, evokes LTP that has a presynaptic locus. This LTP does not require NMDA receptor activity, or a rise in postsynaptic calcium. However, low-frequency stimulation, that would also lead to a rise in postsynaptic calcium, activates protein phosphatases and leads to LTD. To account for these findings, it is conceivable that the machinery required for NMDA receptor-dependent LTP is simply not expressed in CeL neurons. Another interesting possibility is that the proteins necessary for the induction of NMDA receptor-dependent LTP may be anchored to NR2A subunits, thus not present at synapses on CeL neurons. In contrast, those necessary for NMDA receptor-dependent LTD are anchored to NR2B subunits. The fact that tetanic stimulation does not lead to LTD, even when LTP has been blocked (for example, in the presence of the PKA blocker H-89) suggests that the transduction mechanisms necessary for induction of LTD require a prolonged rise in postsynaptic calcium.
A plethora of studies over the last 20 years has shown that the amygdala has a key role in the processing of emotions and in the laying down of emotion-related memories (LeDoux, 2000; Davis & Whalen, 2001; Sah et al. 2003). The relationship between emotion-loaded affective states and the perception of pain is well known (Craig, 2006). Consistent with its role in emotional processing, nociceptive information reaches the amygdala at all levels (Samson et al. 2005), and plasticity of these inputs has been suggested to underlie the emotional modifications that accompany states with persistent pain (Neugebauer et al. 2004). Our results show that nociceptive inputs into the central amygdala can undergo different types of plasticity, depending on the stimulus patterns that they receive. Understanding the mechanisms that initiate these changes suggests that targeting them in the central amygdala may be an alternative approach to the control of pain.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia. We thank Rowan Tweedale and members of the lab for comments on the manuscript.
References
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacol. 2006;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Craig KD. Emotions and psychobiology. In: McMahon Sb, Koltzenburg M., editors. Wall and Melzack's Textbook of Pain. Elsevier Press; 2006. pp. 231–239. [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Op Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Fu Y, Shinnick-Gallagher P. Two intra-amygdaloid pathways to the central amygdala exhibit different mechanisms of long-term potentiation. J Neurophysiol. 2005;93:3012–3015. doi: 10.1152/jn.00871.2004. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Nicoll RA, Perkel DJ, Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkanen RE, Golden T. Regulators of serine/threonine protein phosphatases at the dawn of a clinical era? Curr Med Chem. 2002;9:2055–2075. doi: 10.2174/0929867023368836. [DOI] [PubMed] [Google Scholar]
- Hrabetova S, Serrano P, Blace N, Tse HW, Skifter DA, Jane DE, Monaghan DT, Sacktor TC. Distinct NMDA receptor subpopulations contribute to long-term potentiation and long-term depression induction. J Neurosci. 2000;20:RC81. doi: 10.1523/JNEUROSCI.20-12-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenza A, Sutkowski EM, Seamon KB. Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action? Trends Pharmacol Sci. 1989;10:442–447. doi: 10.1016/S0165-6147(89)80008-2. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lei S, McBain CJ. Two loci of expression for long-term depression at hippocampal mossy fiber-interneuron synapses. J Neurosci. 2004;24:2112–2121. doi: 10.1523/JNEUROSCI.4645-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P. Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B synapses in the adult central amygdala. J Neurosci. 2003;23:6876–6883. doi: 10.1523/JNEUROSCI.23-17-06876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P. Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala. J Neurophysiol. 2004;92:1285–1294. doi: 10.1152/jn.00211.2004. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Excitatory synaptic inputs to pyramidal neurons of the lateral amygdala. Eur J Neurosci. 1999;11:1217–1222. doi: 10.1046/j.1460-9568.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Margrie TW, Rostas JAP, Sah P. Long term potentiation of synaptic transmission in the avian hippocampus. J Neurosci. 1998;18:1207–1216. doi: 10.1523/JNEUROSCI.18-04-01207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacol. 2007;52:71–76. doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term deression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol. 2003;89:716–727. doi: 10.1152/jn.00799.2002. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW., 4th Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci. 2003;23:52–63. doi: 10.1523/JNEUROSCI.23-01-00052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Vicini S. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J Neurosci. 1999;19:10603–10610. doi: 10.1523/JNEUROSCI.19-24-10603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- Samson RD, Duvarci S, Pare D. Synaptic plasticity in the central nucleus of the amygdala. Rev Neurosci. 2005;16:287–302. doi: 10.1515/revneuro.2005.16.4.287. [DOI] [PubMed] [Google Scholar]
- Samson RD, Pare D. Activity-dependent synaptic plasticity in the central nucleus of the amygdala. J Neurosci. 2005;25:1847–1855. doi: 10.1523/JNEUROSCI.3713-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M. The postsynaptic NMDA-receptor–PSD-95 signaling complex in excitatory synapses of the brain. J Cell Sci. 2001;114:1251. doi: 10.1242/jcs.114.7.1251. [DOI] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, LeDoux JE. Distinct populations of NMDA receptors at subcortical and cortical inputs to principal cells of the lateral amygdala. Journal of Neurophysiology. 1999;81:930–934. doi: 10.1152/jn.1999.81.2.930. [DOI] [PubMed] [Google Scholar]
- Xia ZG, Refsdal CD, Merchant KM, Dorsa DM, Storm DR. Distribution of mRNA for the calmodulin-sensitive adenylate cyclase in rat brain: expression in areas associated with learning and memory. Neuron. 1991;6:431–443. doi: 10.1016/0896-6273(91)90251-t. [DOI] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short term synaptic plasticity. Ann Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]