Abstract
The aim of the study was to examine local muscle metabolism in response to graded exercise when the involved muscle mass is too small to elicit marked hormonal changes and local blood flow restriction. Nine healthy overnight fasted male subjects performed knee extension exercise with both thighs kicking at 25% of maximal power (Wmax) for 45 min (23 ± 1% of pulmonary 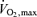 ) followed by 35 min of kicking with one thigh at 65% and the other at 85% Wmax (40 ± 1%
) followed by 35 min of kicking with one thigh at 65% and the other at 85% Wmax (40 ± 1%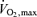 ). Primed constant infusion of [U-13C] palmitate and [2H5]glycerol was carried out. Blood was sampled from a femoral artery and both femoral veins, and thigh blood flow was determined by thermodilution. Muscle biopsies were obtained from m. vastus lateralis of both thighs. From rest through exercise at 25, 65 and 85% Wmax the thigh blood flow (0.3 ± 0.1, 2.5 ± 0.2, 3.5 ± 0.2, 4.1 ± 0.3 l min−1) and oxygen uptake (0.02 ± 0.01, 0.27 ± 0.03, 0.48 ± 0.04, 0.55 ± 0.05 l min−1) increased (P < 0.05). The plasma fatty acids oxidized in the thigh (5 ± 1, 114 ± 15, 162 ± 30, 180 ± 31 μmol min−1) increased (P < 0.05) with exercise intensity, whereas the total thigh fat oxidation (19 ± 6, 312 ± 64, 356 ± 93, 323 ± 120 μmol min−1) increased (P < 0.05) from rest, but remained unchanged through exercise. The thigh glycerol uptake (1 ± 1, 16 ± 4, 24 ± 10, 39 ± 8 μmol min−1) increased significantly from rest through exercise at 25–65 and 85% Wmax, respectively. Glucose uptake and glycogen breakdown always increased with exercise intensity. In conclusion, in the presence of a high blood flow and oxygen supply and only small hormonal changes, total fat oxidation in muscle increases from rest to light exercise, but then remains constant with exercise intensity up to heavy exercise. However, with increasing exercise intensity, oxidation of plasma free fatty acids increases and accordingly oxidation of other fat sources decreases. These findings are in contrast to whole body measurements performed during graded exercise involving a large muscle mass during which fat oxidation peaks at around 60% of
). Primed constant infusion of [U-13C] palmitate and [2H5]glycerol was carried out. Blood was sampled from a femoral artery and both femoral veins, and thigh blood flow was determined by thermodilution. Muscle biopsies were obtained from m. vastus lateralis of both thighs. From rest through exercise at 25, 65 and 85% Wmax the thigh blood flow (0.3 ± 0.1, 2.5 ± 0.2, 3.5 ± 0.2, 4.1 ± 0.3 l min−1) and oxygen uptake (0.02 ± 0.01, 0.27 ± 0.03, 0.48 ± 0.04, 0.55 ± 0.05 l min−1) increased (P < 0.05). The plasma fatty acids oxidized in the thigh (5 ± 1, 114 ± 15, 162 ± 30, 180 ± 31 μmol min−1) increased (P < 0.05) with exercise intensity, whereas the total thigh fat oxidation (19 ± 6, 312 ± 64, 356 ± 93, 323 ± 120 μmol min−1) increased (P < 0.05) from rest, but remained unchanged through exercise. The thigh glycerol uptake (1 ± 1, 16 ± 4, 24 ± 10, 39 ± 8 μmol min−1) increased significantly from rest through exercise at 25–65 and 85% Wmax, respectively. Glucose uptake and glycogen breakdown always increased with exercise intensity. In conclusion, in the presence of a high blood flow and oxygen supply and only small hormonal changes, total fat oxidation in muscle increases from rest to light exercise, but then remains constant with exercise intensity up to heavy exercise. However, with increasing exercise intensity, oxidation of plasma free fatty acids increases and accordingly oxidation of other fat sources decreases. These findings are in contrast to whole body measurements performed during graded exercise involving a large muscle mass during which fat oxidation peaks at around 60% of 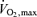 .
.
Apart from in extreme conditions, substrate utilized by skeletal muscle is derived from carbohydrate and fat originating from extramuscular and intramuscular sources. During exercise the choice of muscle substrate is primarily affected by exercise intensity and duration, substrate storage prior to exercise and state of training (Saltin & Gollnick, 1983; Galbo, 1983). During whole body exercise, utilization of carbohydrate, derived both from plasma glucose and from muscle glycogen, increases as exercise intensity increases (Wahren, 1970; Saltin & Karlsson, 1971; Romijn et al. 1993; van Loon et al. 2001). In contrast, whole body fat oxidation reaches its maximum at moderate exercise intensities ranging between 55 and 65% of maximal oxygen uptake (Galbo, 1992; Achten et al. 2002). The higher fat oxidation at moderate versus both higher and lower exercise intensities has been ascribed to a higher contribution of fat recruited from both plasma fatty acids (FA) and other fat sources including plasma derived and muscle derived triacylglycerol (Romijn et al. 1993; van Loon et al. 2001).
During whole body exercise the autonomic neuroendocrine response is markedly increased as exercise intensity is increased and this influences the muscle substrate delivery through a change in exogenous substrate mobilization and a change in blood flow distribution (Galbo, 1983). Consequently, when exercise intensity is high, the muscle delivery of plasma FA may actually decline due to adipose tissue blood flow restriction and/or increased fatty acid reesterification (Bulow & Madsen, 1981; Romijn et al. 1995), which in turn may lead to a decreased plasma FA uptake and oxidation compared to that observed at a moderate exercise intensity (van Loon et al. 2001). Thus during whole body exercise, muscle substrate utilization at higher intensities is a reflection of both systemic and local muscle factors, and it is not clear how exercise intensity without the concomitant intensity-dependent whole body autonomic neuroendocrine response will affect muscle substrate utilization. In addition, we and others have previously shown that oxidation of plasma long chain fatty acids in the legs during bicycle exercise only represents 25–45% of whole body plasma fatty acid oxidation (Burguera et al. 2000; Helge et al. 2001; Roepstorff et al. 2002; Kiens, 2006). Thus, lipid oxidation based on whole body measurements may not be an accurate reflection of lipid metabolism in working muscle. This was recently confirmed by Friedlander et al. (2007).
To address these issues we used the two-legged knee extension exercise model, which elicits a markedly lower increase in the autonomic neuroendocrine response than that observed during whole body exercise. With this model oxygen delivery for contracting muscle and muscle substrate metabolism is also not restricted by central circulatory capacity (Andersen et al. 1985), because no more than 6–6.5 kg of muscle is fully recruited during contractions (Andersen & Saltin, 1985) and muscle metabolism can be directly quantified. Thus, the present study aimed to investigate the effect of contraction intensity per se on human thigh muscle substrate utilization studied directly by the leg balance technique, stable isotopes and muscle biopsies.
Methods
Subjects
Nine untrained healthy male subjects, age 25 ± 1 year, height 182 ± 2 cm, weight 76 ± 3 kg and maximal oxygen uptake 3.9 ± 0.2 l O2 min−1 (mean ± s.e.m.) participated in the study. Subjects were fully informed of the nature and the possible risks associated with the study before they volunteered to participate. The study was approved by the Ethics Committee for Medical Research in Copenhagen and adhered to the declaration of Helsinki II.
Experimental protocol
Prior to the experiment subjects were accustomed to exercise in the knee extension ergometer, and maximal work capacity (Wmax) was determined for each leg (Andersen et al. 1985). In brief an incremental one leg exercise test was performed and pulmonary oxygen uptake, carbon dioxide excretion and heart rate were measured. When the incremental exercise could be performed using only the quadriceps muscle, additional muscle was recruited to support the quadriceps muscle, such as gluteal, hamstring and stomach muscle. The one leg Wmax was then established as the maximal work capacity that can be performed without other muscle involvement. In addition the experimental exercise protocol was rehearsed for each subject, namely 45 min kicking with both legs at 25% Wmax followed by 35 min of kicking with one leg at 65% and one leg at 85% Wmax. In particular the synchronized kicking with two legs with different workloads was practiced. Selection of the leg eligible for high (85% Wmax) versus moderate (65% Wmax) intensity was done by randomised stratification, such that the dominant and non-dominant leg were similarly represented. Also prior to the experimental day, whole body maximal oxygen uptake was determined on a Krogh bicycle ergometer using a progressive incremental exercise test.
For 2 days prior to the experiment, subjects were asked to consume a diet low in foods containing 13C such as cane sugar and maize (corn) and furthermore to refrain from vigorous physical activity. The subjects reported to the laboratory in the morning at 8 a.m. after a 10 h fast. Initially subjects were placed for 15–30 min in a supine position. After this, catheters were placed in the femoral artery and both femoral veins under local anaesthesia by an aseptic technique, and the tips were advanced to ∼2 cm above and below the inguinal ligament in the retrograde and antegrade direction, respectively. A thermistor (Edslab Probe 94-030-2.5-F, Baxter Healthcare) for measuring venous blood temperature was advanced 8 cm proximal to the catheter tip in both veins. A catheter was also inserted into an antecubital vein for the infusion of stable isotope tracer. The catheters were kept patent by intermittent flushing with sterile sodium citrate. Subjects were then placed in a semi-supine position and rested for half an hour. Then blood was sampled simultaneously from the femoral artery and veins, and thigh venous blood flow was measured by the thermodilution method by use of bolus injections of 5 ml ice-cold isotonic saline (Andersen et al. 1985); the average of three sequential determinations performed with 2–3 min intervals was used.
Prior to exercise two incisions were made in both thighs under local analgesia (2–3 ml lidocaine, 20 mg ml−1) and a needle biopsy was obtained with suction from both vastus lateralis muscles approximately 12–18 cm above the knee. At this point the bicarbonate pool was primed with a bolus of NaH13CO3 (0.1 mg kg−1, 99% enriched) and the glycerol pool with a bolus of [1,1,2,3,3-2H5]glycerol (1.5 mg kg−1, 99% enriched). After this a continuous infusion of [U-13C]palmitate using a calibrated syringe pump (Vial Medical SE 200B, Simonsen & Weel, Copenhagen, Denmark) set at a constant rate of 0.015 μmol kg−1 min−1 and a continuous infusion of [1,1,2,3,3-2H5]glycerol using a calibrated syringe pump (Harward Apparatus, Plymouth Meeting, PA, USA) set at a rate of 0.1 mg kg −1 min−1 through the 90 min of rest and at 0.2 mg kg−1 min−1 through the 80 min of exercise were initiated. After 80 and 85 min of rest, blood was sampled and blood flow measured. Prior to each blood sample and blood flow measurement a cuff placed just below each knee was inflated to suprasystolic pressure to minimize contribution from the lower leg. After 90 min, subjects started to perform knee extension exercise at 25% of Wmax with both legs kicking simultaneously in two independent knee extension ergometers at a frequency of 60 kicks min−1. During the 45 min of exercise blood samples were taken at 15, 30 and 40 min. After blood was sampled, blood flow was measured in both thighs by continuous infusions of ice-cold isotonic saline (15–20 s) according to the thermodilution principle (Andersen et al. 1985). After 45 min, exercise was stopped shortly, approximately 2–3 min, and a muscle biopsy was obtained from the vastus lateralis muscle of both thighs. In each thigh the biopsy was obtained through the same incision as the first biopsy, but in the opposite direction, distal – proximal, of the first biopsy. Subsequently exercise was continued for 35 min, one leg exercising at 65% of Wmax and the other leg at 85% of Wmax. After 15, 30 and 35 min blood was sampled, and blood flow was measured at 15 and 30 min as described above. Immediately after exercise termination a biopsy was taken from the vastus lateralis muscle of both thighs through the second incision. Heart rate was recorded continuously during exercise with a PE 3000 Sports Tester (Polar Electro, Finland). Throughout the exercise subjects had free access to water. Two subjects were not able to fully complete the second exercise period; one subject missed the last 30 min of exercise and the other subject only the very last blood sample and blood flow measurement. Therefore all data points including moderate and high intensity have n = 8 except for the last data point where n = 7.
Pulmonary oxygen uptake and carbon dioxide excretion at rest and during exercise were measured using the Douglas bag technique as previously described (Helge et al. 1996). Throughout the exercise, just prior to each blood sample, expired air was sampled into Douglas bags and subsequently small aliquots were collected into evacuated glass tubes (Vacutainer, Becton Dickinson, Meylan, Cedex, France) for the analysis of 13CO2 enrichment.
On a separate day two of the subjects volunteered to do an acetate recovery experiment. The experimental protocol was similar to that described above, but without the infusion of the fatty acid and glycerol tracers as well as muscle biopsies. After the initial preparations and placement of the arterial and venous femoral catheters a NaH13CO3 (0.085 mg kg−1, 99% enriched) prime was followed by a constant infusion of [1,2-13C]acetate (0.075 μmol kg−1min−1) for 90 min followed by 45 and 35 min of knee extension exercise performed with both legs simultaneously as described above. Blood flow measurements and blood sampling were performed as described above.
Materials
[U-13C]Palmitate, [1,1,2,3,3-2H5]glycerol, [1,2-13C] acetate and sodium[13C]bicarbonate (99% enriched) were purchased from Cambridge Isotope Laboratories, Andover, MA, USA. Preparation of the tracers for infusion has been described elsewhere (Roepstorff et al. 2002). In brief the palmitic acid tracer in solution was added to methanolic potassium hydroxide to form the potassium salt, dried under nitrogen, redissolved in sterile water, passed through a 0.22 μm sterile filter and added and thereby complexed to sterile 20% (w/v) human albumin (State Serum Institute, Copenhagen, Denmark). The [1,1,2,3,3-2H5]glycerol tracer and the [1,2-13C]acetate were dissolved in sterile isotonic saline and passed through a sterile 0.22 μm filter immediately prior to infusion.
Analytical procedures
Arterial and venous plasma palmitate concentration and enrichment were determined by gas chromatograph–mass spectrometry (GC-MS, Automass II, Finnigan France) as previously described (Roepstorff et al. 2002; van Hall et al. 2002c). The enrichment of 13CO2 in expired air and in arterial and venous blood was analysed by gas chromatograph isotope ratio–mass spectrometry; GC-IRMS (GC-IRMS, Deltaplus, Finnigan Matt, Germany) as previously described (Roepstorff et al. 2002; van Hall et al. 2002c). Plasma glycerol and acetate concentrations and enrichments were determined by gas chromatography–mass spectrometry (GC-MS, Automass II, Finnigan, France) as recently described (van Hall et al. 2002a,van Hall 2002b; Roepstorff et al. 2004).
Blood glucose and lactate were analysed on a glucose and lactate analyser (Yellow Springs Instruments, Yellow Springs, OH, USA). Plasma glycerol was analysed as described in Wieland (1974). Insulin in arterial plasma was determined using a radio immunoassay kit (Insulin RIA100, Pharmacia, Sweden) and catecholamines in arterial plasma were determined by a radioenzymatic procedure (Christensen et al. 1980). Blood oxygen saturation, haematocrit, PCO2, PO2 and pH were measured with the Astrup technique (ABL 30, Radiometer, Copenhagen, Denmark). Haemoglobin was determined spectrophotometrically on the haemoximeter by the cyan-methaemoglobin method (Drabkin & Austin, 1935).
The biopsies were frozen in liquid nitrogen within 10–15 s of sampling and were stored at −80°C until further analysis. Before biochemical analysis, muscle biopsy samples were freeze dried and dissected free of connective tissue, visible fat and blood using a stereomicroscope. Muscle glycogen concentration was determined as glucose residues after hydrolysis of the muscle sample in 1 m HCL at 100°C for 2 h (Lowry & Passonneau, 1972).
Calculations
Thigh volume was determined from measurements of the length, circumference and skinfold at three sites of the thigh and subsequently this volume was used to calculate the quadriceps femoris muscle mass as described by Kiens et al. (1993) and modified by Radegran et al. (1999). Uptake and release of substrates and metabolites over the thigh were calculated from femoral arterial and venous differences multiplied by plasma or blood flow, according to the Fick principle. The oxygen and carbon dioxide content in the blood was calculated (Siggaard-Andersen et al. 1988) and from this the respiratory quotient (RQ) across the thigh was calculated (Helge et al. 2001). Indirect calorimetry calculations were performed according to the stoichiometric equations given by Frayn (1983). Fatty acid (FA) oxidation was determined by converting the rate of fat oxidation (g kg−1 min−1) to its molar equivalent, assuming an average molecular weight of fatty acids to be 286 g mol−1 (Frayn, 1983).
The thigh fatty acid (FA) extraction was calculated using the tissue balance approach:
where Cpalm is concentration of palmitate in artery (art) and vein, and Epalm is enrichment of palmitate in artery and vein.
As palmitate kinetics are assumed to be representative for all fatty acids, the thigh FA uptake, FA release and plasma FA oxidation were calculated according to the following equations:
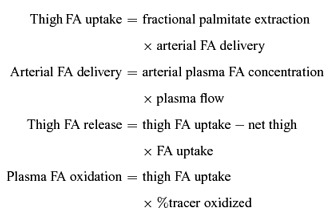 |
where C is the acetate correction factor calculated as described below. The percentage of tracer taken up by the thigh which was oxidized was calculated as:
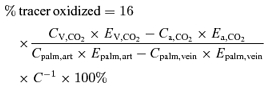 |
where CV,CO2 is concentration of CO2 in artery (art) and vein, and Epalm is enrichment of CO2 in artery (art) and vein.
The estimated oxidation of fatty acids in the thigh originating from sources other than plasma FA was calculated as:
The acetate correction factor across the leg was calculated as described by van Hall et al. (2002b). The glycerol tracer calculations were carried out as described by van Hall et al. (2002c). In both cases leg balance equations were applied with the underlying principle being similar to the calculations for the palmitate tracer outlined above.
Whole body fatty acid rate of appearance (Ra) and rate of disappearance (Road) were calculated using the steady state equations at rest (Wolfe, 1992). Non-steady state Steele equations (Steele, 1959) modified for use with stable isotope methodology were applied during exercise (Romijn et al. 1993; Roepstorff et al. 2002). Plasma fatty acid oxidation and percentage oxidized of Ra were calculated as described in Roepstorff et al. (2002). In addition the contribution of thigh plasma fatty acid oxidation to whole body plasma fatty oxidation was calculated.
Statistics
The statistical analysis was performed with SigmaStat 2.03 (SPSS Inc., Chicago, IL, USA) and data are given as means ± s.e.m., if not otherwise stated. If data were not normally distributed a log transformation was performed prior to the analysis of variance (ANOVA). One-way and two-way ANOVAs with repeated measures for the time factor were performed to test for changes from rest to exercise and subsequently between exercise intensities and/or times. In the presence of significant effects or interactions the Student–Newman–Keuls test was used as a post hoc test to discern statistical differences. In all cases, an α of 0.05 in two-tailed testing was taken as the level of significance.
Results
Knee extension exercise was performed for 45 min at 25% Wmax (13 ± 1 W) and for 35 min at 65% and 85% of Wmax (32 ± 3 and 44 ± 3 W), respectively. At rest whole body oxygen uptake was 0.3 ± 0.1 l min−1. During the initial 45 min of exercise whole body oxygen uptake increased (P < 0.05) to 0.9 ± 0.1 l min−1 and during the last 35 min a further increase (P < 0.05) was noted to 1.5 ± 0.1 l min−1. This was equivalent to a workload of 23 ± 1% and 40 ± 1%, respectively, of whole body maximal oxygen uptake. Whole body respiratory exchange ratio was similar at rest and during the initial 45 min of exercise, 0.83 ± 0.02 and 0.84 ± 0.01, respectively. During the following 35 min an increase (P < 0.05) to 0.90 ± 0.01 was noted. Prior to exercise heart rate was 62 ± 2 b.p.m. Heart rate increased from rest to 95 ± 2 b.p.m. after 10 min of exercise and no further increase was observed through the first 45 min, whereas during the following 35 min of exercise a continuous increase (P < 0.05) was observed to 141 ± 4 b.p.m.
Through the initial 45 min both thighs were exposed to a workload of 25% Wmax, and since similar data were observed (P > 0.05), the 25% Wmax data will be presented as an average of the values from the two thighs. The thigh blood flow (0.3 ± 0.1, 2.5 ± 0.2, 3.5 ± 0.2, 4.1 ± 0.3 l min−1) and also oxygen uptake (0.02 ± 0.01, 0.27 ± 0.03, 0.48 ± 0.04, 0.55 ± 0.05 l min−1) increased (P < 0.05) with exercise intensity from rest through exercise at 25, 65 and 85% Wmax, respectively. The calculated thigh RQ also increased (P < 0.05) with exercise intensity (0.82 ± 0.04, 0.83 ± 0.03, 0.88 ± 0.03, 0.91 ± 0.03) from rest through exercise at 25, 65 and 85% of Wmax, respectively.
Thigh FA kinetics
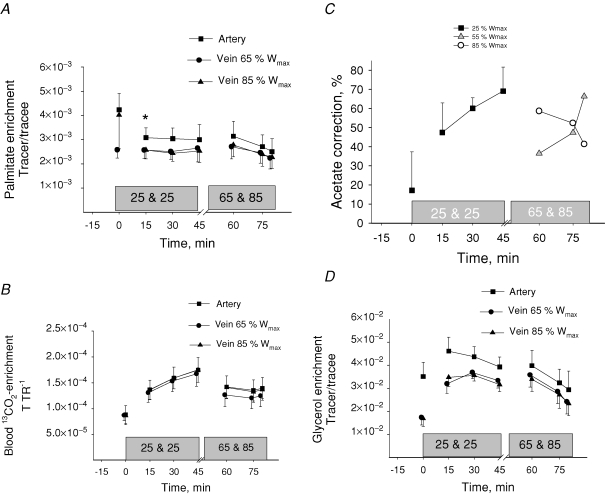
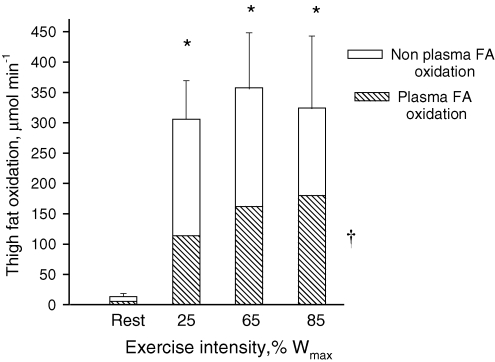
From rest through the initial 45 min of exercise arterial plasma FA concentration increased (P < 0.05) from 612 ± 51 to 723 ± 108 μmol l−1. After 15 min of the next 35 min of exercise the arterial plasma FA concentration was 610 ± 68 μmol l−1 and it increased (P < 0.05) to 804 ± 136 μmol l−1 at the end of exercise (Table 1). The FA delivery to the thighs increased (P < 0.05) with exercise intensity from rest to exercise at 85% Wmax (Table 1). The femoral venous FA concentration remained unchanged from rest through the graded exercise (Table 1). The net thigh FA uptake increased (P < 0.05) with exercise intensity (Table 1). After 15 min of exercise the plasma palmitate enrichment was lower (P < 0.05) than at rest and it remained at this level through the remainder of the exercise at 25% Wmax (Fig. 1). During the last 35 min the arterial palmitate enrichment only decreased insignificantly compared to that observed at the prior exercise level (Fig. 1A). The arterial and venous blood 13CO2 enrichment increased (P < 0.05) from rest to 15 min of exercise and no further changes were observed (Fig. 1B). The acetate correction factor (n = 2) is shown in Fig. 1C. The acetate correction factor is somewhat low during the moderate and high intensity exercise and this may be due to the 2–3 min of inactivity before continuation of exercise (Van Loon et al. 2003). The thigh FA uptake and the thigh FA release both increased (P < 0.05) with increasing exercise intensity (Table 1). The fractional extraction of FA decreased (P < 0.05) with increasing exercise intensity (Table 1), and the percentage FA uptake oxidized increased (P < 0.05) from rest to exercise (Table 1). Thigh plasma FA oxidation increased (P < 0.05) as exercise intensity was increased (Table 1). If the acetate carbon recovery factor is not applied in the calculations a non-corrected thigh plasma FA oxidation shows a similar trend as the corrected values (Table 1). Total thigh fat oxidation calculated from leg RQ was higher (P < 0.05) during exercise than at rest, but there was no difference in total thigh fat oxidation between exercise intensities (Fig. 2).
Table 1.
Thigh fatty acid (FA) kinetics during graded knee extension exercise
| Rest | 25% Wmax | 65% Wmax | 85% Wmaz | ANOVA Rest & Ex. | ANOVA Intensity | |
|---|---|---|---|---|---|---|
| Femoral arterial FA concentration (μmol l−1) | 615 ± 54 * | 687 ± 80 | 720 ± 111 | 720 ± 111 | † | |
| FA delivery (μmol min−1) | 85 ± 11 * | 902 ± 86 # | 1369 ± 155 | 1612 ± 169 | † | ‡ |
| Femoral venous FA concentration (μmol l−1) | 651 ± 38 | 638 ± 44 | 673 ± 59 | 679 ± 58 | ||
| Net thigh FA uptake (μmol min−1) | 0 ± 3 * | 59 ± 15 | 71 ± 11 | 81 ± 18 | † | |
| Fractional extraction (%) | 38 ± 2 * | 21 ± 2 # | 16 ± 2 | 14 ± 2 | † | ‡ |
| Total thigh FA uptake (μmol min−1) | 31 ± 4 * | 183 ± 16 | 206 ± 23 | 224 ± 25 | † | |
| Thigh FA release (μmol min−1) | 31 ± 2 * | 124 ± 14 | 136 ± 16 | 142 ± 19 | † | |
| % FA uptake oxidized | 17 ± 21 * | 66 ± 10 | 76 ± 16 | 80 ± 14 | † | |
| Thigh plasma FA oxidation (μmol min−1) | 5 ± 1 * | 114 ± 15 | 162 ± 30 | 180 ± 31 $ | † | ‡ |
| Non-corrected thigh plasma FA oxidation (μmol min−1) | 1 ± 5 * | 67 ± 5 | 81 ± 15 | 91 ± 15 | † |
Tracer data are averaged across the last 15 min of rest, the last 30 min during the initial 45 min of exercise and the last 20 min during the final 35 min of exercise. Other data are averaged across the measurement period. Values are means ± s.e.m.
P < 0.05 Rest versus 25, 65 & 85% Wmax;
ANOVA effect of exercise intensity including rest.
P < 0.05 ANOVA effect of exercise intensity;
P < 0.05 25% Wmaxversus 65% Wmax and 85% Wmax;
P < 0.05 25% and 65% Wmaxversus 85% Wmax.
Figure 1.
Arterial and venous plasma palmitate enrichment (A), blood enrichment of 13CO2 (B), acetate correction factor (C) and arterial and venous blood glycerol enrichment (D) during rest and knee extension exercise at 25, 65 and 85% Wmax Tracer glycerol infusion was increased from 0.1 mg kg −1 min−1 at rest to 0.2 mg kg −1 min−1 during exercise. Values are means ± s.e.m., n = 9. *P < 0.05 Rest versus 15 min.
Figure 2.
Contribution of plasma and non-plasma FA to total thigh fat oxidation during rest and knee extension exercise at 25, 65 and 85% Wmax Data were calculated from leg oxygen uptake and RQ and stable isotope determined plasma fatty acid oxidation. Values are means ± s.e.m., n = 9. *P < 0.05, Rest versus Exercise. †P < 0.05, ANOVA effect of exercise intensity on plasma palmitate oxidation. See Table 1 for further details.
Thigh glycerol kinetics
From rest through the initial 45 min of exercise arterial plasma glycerol concentration increased (P < 0.05) from 56 ± 5 to 105 ± 13 μmol l−1. Subsequently, after 35 min of exercise a further increase (P < 0.05) to 147 ± 13 μmol l−1 was noted (Table 2). The thigh glycerol delivery increased (P < 0.05) with exercise intensity from rest to 85% Wmax (Table 2). Also the femoral venous glycerol concentration was higher (P < 0.05) during exercise at 65 and 85% Wmax than at rest and during exercise at 25% Wmax (Table 2). At rest the arterial glycerol enrichment was higher (P < 0.05) than the venous glycerol enrichment. The infusion rate of labelled glycerol was doubled from rest to exercise and the arterial glycerol enrichment did not change significantly with exercise, whereas the venous glycerol enrichment increased (P < 005, Fig. 1D). The glycerol uptake was lower (P < 0.05) at rest and during exercise at 25% Wmax than during exercise at 65 and 85% Wmax (Table 2). The total glycerol release increased (P < 0.05) as exercise intensity was increased (Table 2). The net thigh glycerol release remained unchanged from rest through the graded exercise (Table 2).
Table 2.
Thigh glycerol kinetics during graded knee extension exercise
| Rest | 25% Wmax | 65% Wmax | 85% Wmax | ANOVA Rest & Ex. | ANOVA Intensity | |
|---|---|---|---|---|---|---|
| Femoral arterial glycerol concentration (μmol l−1) | 56 ± 5* | 100 ± 6# | 147 ± 13 | 147 ± 13 | † | ‡ |
| Glycerol delivery (μmol min−1) | 8 ± 1* | 132 ± 13# | 264 ± 38 | 301 ± 37 | † | ‡ |
| Femoral venous glycerol concentration (μmol l−1) | 81 ± 7* | 107 ± 7# | 155 ± 14 | 155 ± 13 | † | ‡ |
| Net thigh glycerol release (μmol min−1) | 3.8 ± 1.1 | 11.2 ± 2.0 | 9.4 ± 7.5 | 10.9 ± 6.0 | ||
| Fractional extraction (%) | 21.0 ± 9.8 | 11.6 ± 3.2 | 7.9 ± 3.6 | 10.8 ± 1.7 | ||
| Thigh glycerol uptake (μmol min−1) | 1.4 ± 1.1* | 15.1 ± 3.6 | 24.3 ± 9.7 | 39.3 ± 8.0$ | † | ‡ |
| Total glycerol release (μmol min−1) | 5.2 ± 1.0* | 26.5 ± 3.9 | 33.7 ± 7.4 | 50.1 ± 7.8$ | † | ‡ |
Tracer data are averaged across the last 15 min of rest, the last 30 min of the initial 45 min of exercise and the last 20 min during the final 35 min of exercise. Other data are averaged across the measurement period. Values are means ± s.e.m.
P < 0.05 Rest versus 25, 65 and 85% Wmax;
ANOVA effect of exercise intensity including rest.
P < 0.05 ANOVA effect of exercise intensity;
P < 0.05 25% Wmaxversus 65% Wmax and 85% Wmax;
P < 0.05 25% and 65% Wmaxversus 85% Wmax.
Thigh carbohydrate metabolism
The femoral arterial and venous glucose concentrations were higher (P < 0.05) during exercise than at rest (Table 3). The thigh glucose uptake increased (P < 0.05) with exercise intensity from rest to exercise at 85% Wmax (Table 3). The thigh glucose extraction was lower (P < 0.05) at 25% Wmax than at rest or during exercise at 65 and 85% Wmax. Arterial blood lactate concentration was identical at rest and during exercise at 25% Wmax, 0.5 ± 0.1 and 0.5 ± 0.1 mmol l−1, respectively. During the last 35 min of knee extension exercise the arterial lactate concentration was increased (P < 0.05) to1.3 ± 0.3 mmol l−1. The femoral arterial and venous lactate concentrations were higher (P < 0.05) during exercise at 65 and 85% than during rest and exercise at 25% Wmax (Table 3). The net thigh lactate release was higher (P < 0.05) during exercise at 85% Wmax than at rest and during exercise at 25 and 65% of Wmax (Table 3). The femoral venous pH was significantly decreased from rest and across the exercise intensities (Table 3).
Table 3.
Thigh carbohydrate metabolism during graded knee extension exercise
| Rest | 25% Wmax | 65% Wmax | 85% Wmax | ANOVA Rest & Ex. | ANOVA Intensity | |
|---|---|---|---|---|---|---|
| Femoral arterial glucose conc. (mmol l−1) | 4.7 ± 0.1* | 4.8 ± 0.2 | 5.0 ± 0.1 | 5.0 ± 0.1 | † | |
| Glucose delivery (mmol l−1) | 1.3 ± 0.1* | 11.8 ± 1.4# | 17.5 ± 1.7 | 20.7 ± 2.0$ | † | ‡ |
| Femoral venous glucose conc. (mmol l−1) | 4.5 ± 0.1* | 4.7 ± 0.1 | 4.7 ± 0.1 | 4.7 ± 0.1 | † | |
| Net thigh glucose uptake (mmol min−1) | 0.05 ± 0.01* | 0.30 ± 0.05# | 0.75 ± 0.15 | 0.98 ± 0.19 | † | ‡ |
| Thigh glucose extraction (%) | 4.3 ± 0.8** | 2.5 ± 0.5§ | 4.1 ± 0.5 | 5.1 ± 0.4 | † | ‡ |
| Femoral arterial lactate conc. (mmol l−1) | 0.5 ± 0.1*** | 0.5 ± 0.1# | 1.3 ± 0.1 | 1.3 ± 0.1 | † | ‡ |
| Femoral venous lactate conc. (mmol l−1) | 0.5 ± 0.1*** | 0.5 ± 0.1 | 1.3 ± 0.2 | 1.6 ± 0.2$ | † | ‡ |
| Femoral venous pH | 7.39 ± 0.01*** | 7.36 ± 0.01# | 7.31 ± 0.01 | 7.30 ± 0.01 | † | ‡ |
| Net thigh lactate release (mmol min−1) | 0.01 ± 0.01 **** | 0.16 ± 0.08 | − 0.03 ± 0.15 | 0.88 ± 0.16$ | † | ‡ |
| Muscle glycogen breakdown | — | 50 ± 13§ | 105 ± 23 | 165 ± 25 | — | ‡ |
| (mmol (kg d.w.)−1) | ||||||
| Muscle glycogen breakdown rate | — | 1.1 ± 0.3§ | 3.0 ± 0.7 | 4.7 ± 0.7 | — | ‡ |
| (mmol (kg d.w.)−1 min−1) |
Data are averaged across each exercise period. The values for Rest and 25% Wmax are averaged across both legs. Values are means ± s.e.m.
P < 0.05 Rest versus 25, 65 and 85% Wmax;
P < 0.05 Rest versus 25% Wmax;
P < 0.05 Rest versus 65 and 85% Wmax;
P < 0.05 Rest versus 85% Wmax.
ANOVA effect of exercise intensity including rest.
P < 0.05 ANOVA effect of exercise intensity;
P < 0.05 25% Wmaxversus 65% Wmax and 85% Wmax; P < 0.05 25% and 65% Wmaxversus 85% Wmax;
P < 0.05 25% Wmaxversus 85% Wmax.
Before exercise muscle glycogen concentration was similar in the two thighs 496 ± 32 and 516 ± 28 mmol (kg d.w.)−1. After 45 min of exercise at 25% Wmax, muscle glycogen concentration had decreased (P < 0.05) similarly in both thighs by 10 ± 3%. After the following 35 min, muscle glycogen concentration was reduced (P < 0.05) to 329 ± 36 and 284 ± 40 mmol (kg d.w.)−1 in the thighs that exercised at 65 and at 85% Wmax, respectively. The muscle glycogen breakdown and the breakdown rate both increased (P < 0.05) as exercise intensity was increased (Table 3).
Thigh substrate utilization
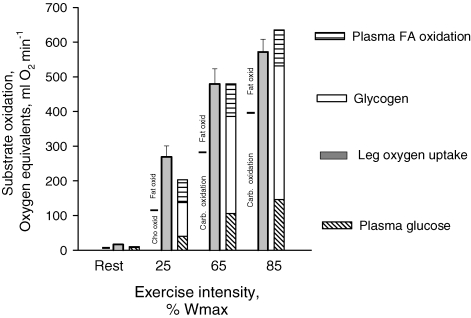
Total thigh substrate utilization was calculated as the percentage contribution in oxygen equivalents derived from the measured substrates, e.g. glycogen, glucose, lactate, glycerol and oxidized plasma FA (Fig. 3). At rest and during exercise at 25% Wmax, 34 and 20% of the substrate utilized could not be accounted for, respectively. In contrast at 65 and 85% Wmax the energy corresponding with the oxygen utilized could be fully accounted for. Both at rest and during exercise at 25% Wmax the utilization of carbohydrate determined by indirect calorimetry (leg RQ) was very similar to the metabolism of carbohydrate determined by the substrate balance approach (Fig. 3). In contrast, during exercise at 65 and 85% Wmax the carbohydrate oxidation determined by indirect calorimetry was lower than carbohydrate metabolism determined by the substrate balance approach. Accordingly, lipid oxidation was lower calculated from substrate utilization than calculated from leg RQ, and lipid oxidation at 65 and 85% Wmax was covered entirely by FA oxidation when calculated from substrate balance (Fig. 3).
Figure 3.
Contribution of endogenous and exogenous substrates to total thigh oxidation determined as the substrate balance across the thigh during rest and knee extension exercise at 25, 65 and 85% Wmax Total glycogen utilization in the thigh was determined as net breakdown (in quadriceps muscle) multiplied by the estimated active muscle (quadriceps) mass in the thigh. Values are means of determinations in 9 subjects. The partition between non-protein fat and carbohydrate oxidation calculated from thigh RQ is indicated to the left of each condition.
Hormones
The arterial adrenaline and noradrenaline concentrations remained unchanged from rest through the first 45 min of exercise at 25% Wmax being 0.73 ± 0.15 and 1.3 ± 0.3 nmol l−1, respectively. After 15 min of the last 35 min of exercise the arterial adrenaline and noradrenaline concentration had increased (P < 0.05) compared to the low intensity exercise being 1.2 ± 0.3 and 4.4 ± 0.8 nmol l−1, respectively. The arterial noradrenaline concentration was not further increased whereas an increase (P < 0.05) to 1.9 ± 0.6 nmol l−1 was observed in the arterial adrenaline concentration at the end of exercise (P < 0.05). The arterial plasma insulin concentration was 7.0 ± 0.8 μU ml−1 at rest and remained unchanged throughout the whole experiment.
Whole body FA kinetics
Whole body Ra and Road both increased (P < 0.05) with exercise intensity (Table 4). The whole body plasma fatty acid oxidation increased (P < 0.05) from rest through exercise, and similarly the percentage of Ra oxidized was increased (P < 0.05) from rest through the exercise (Table 4). The percentage contribution of plasma fatty acid oxidation in both thighs to whole body fatty acid oxidation was higher (P < 0.05) during the initial 45 min exercise, where both legs worked at low intensity, than at rest and during the last 35 min of exercise at moderate and high intensity combined (Table 4). It is noteworthy, that oxidation of plasma FA in the legs only covered 25–40% of whole body plasma FA oxidation (Table 4).
Table 4.
Whole body fatty acid (FA) kinetics during graded knee extension exercise
| Rest | 15–45 min | 60–80 min | ANOVA Rest & Ex. | ANOVA Intensity | |
|---|---|---|---|---|---|
| Ra FA (mmol min−1) | 1.22 ± 0.17* | 1.43 ± 0.09 | 1.63 ± 0.15 | † | ‡ |
| Road FA (mmol min−1) | 1.21 ± 0.17* | 1.42 ± 0.09 | 1.61 ± 0.15 | † | ‡ |
| FA oxidation (μmol min−1) | 68.2 ± 14.7 *$ | 535 ± 68# | 1285 ± 180 | † | ‡ |
| % Ra FA oxidized | 6 ± 1 *$ | 36 ± 4# | 88 ± 11 | † | ‡ |
| % FA ox. in thighs in whole body | 15 ± 2* | 40 ± 5# | 25 ± 5 | † | ‡ |
Whole body tracer data are averaged across the last 15 min of rest, the last 30 min during the initial 45 min of exercise and the last 20 min during the final 35 min of exercise. Values are means ± s.e.m.
ANOVA effect of exercise intensity including rest;
ANOVA effect of exercise intensity;
P < 0.05 Rest versus 60–80 min;
P < 0.05 Rest versus 15–45 min;
P < 0.05) 15–45 min versus 60–80 min.
Discussion
In the present study a main finding was that thigh total fat oxidation was not influenced by intensity within a wide range of intensities from 25 to 85% of maximal power output. However, as contraction intensity was increased a progressive increase in thigh plasma fatty acid oxidation was observed and, accordingly, oxidation of fat from other sources must have decreased. These findings differ from findings during whole body exercise involving a larger muscle mass, a fact which points at the importance of extramuscular factors in regulation of muscle metabolism. Alternatively, whole body measurements do not accurately reflect muscle metabolism. Finally, we also observed an increase in muscle glycerol uptake as contraction intensity and arterial glycerol concentrations increased.
In earlier studies, when progressive graded whole body exercise was performed, fat oxidation determined by indirect calorimetry increased from rest and peaked at about 60% of maximal oxygen uptake (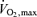 ); it then decreased at higher exercise intensities (Galbo, 1992; Achten et al. 2002). This pattern of fat utilization during exercise is consistent with the substrate utilization stipulated by some of the early Scandinavian work (Krogh & Lindhard, 1920; Christensen & Hansen, 1939) as well as the cross-over concept (Brooks & Mercier, 1994). Also when whole body exercise was studied on three separate days, Romijn et al. (1993) found that total fat oxidation was higher at moderate exercise at 65%
); it then decreased at higher exercise intensities (Galbo, 1992; Achten et al. 2002). This pattern of fat utilization during exercise is consistent with the substrate utilization stipulated by some of the early Scandinavian work (Krogh & Lindhard, 1920; Christensen & Hansen, 1939) as well as the cross-over concept (Brooks & Mercier, 1994). Also when whole body exercise was studied on three separate days, Romijn et al. (1993) found that total fat oxidation was higher at moderate exercise at 65%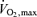 compared to exercise performed at 25 and 85%
compared to exercise performed at 25 and 85%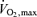 . Furthermore, in that study stable isotope tracers were used to demonstrate that whole body plasma fatty acid rate of disappearance peaked at moderate compared to the higher as well as the lower exercise intensity (Romijn et al. 1993). These findings were extended by van Loon et al. (2001) who during incremental exercise directly measured plasma FA oxidation from 13CO2 in expired air and confirmed a decline in the oxidation rate of both plasma FA and other lipid sources at the highest work intensity (72% of
. Furthermore, in that study stable isotope tracers were used to demonstrate that whole body plasma fatty acid rate of disappearance peaked at moderate compared to the higher as well as the lower exercise intensity (Romijn et al. 1993). These findings were extended by van Loon et al. (2001) who during incremental exercise directly measured plasma FA oxidation from 13CO2 in expired air and confirmed a decline in the oxidation rate of both plasma FA and other lipid sources at the highest work intensity (72% of 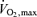 ) compared to 57% and 44 of
) compared to 57% and 44 of 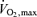 . The decline in plasma FA and total fat oxidation was found both relative to total energy expenditure as well as in absolute numbers. Finally, it was recently shown that leg lipid oxidation in fed postabsorptive subjects decreased when they performed cycle ergometer exercise at 65% of
. The decline in plasma FA and total fat oxidation was found both relative to total energy expenditure as well as in absolute numbers. Finally, it was recently shown that leg lipid oxidation in fed postabsorptive subjects decreased when they performed cycle ergometer exercise at 65% of 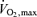 compared to when they exercised at 45% of
compared to when they exercised at 45% of 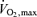 (Friedlander et al. 2007).
(Friedlander et al. 2007).
In contrast, in the present study where fat metabolism and plasma FA kinetics were measured directly across a limited muscle mass, we observed that thigh total fat oxidation calculated from RQ values (Fig. 2) was not influenced by exercise intensity within a wide range of intensities from 25 to 85% of maximal one leg work capacity (Wmax). In this study we did not investigate the fat oxidation pattern during exercise at workloads between 85 and 100% Wmax and further studies are needed to determine the contribution of fat to total oxidation at these very high intensities, at which, however, measurements are rendered difficult by the possible lack of steady state. When exercise intensity was increased in this study, a progressive increase in thigh plasma fatty acid oxidation (Table 1, Fig. 2) was observed and, accordingly, oxidation of fat from other sources must have decreased. Intense whole body exercise induces sympathoadrenal activity and plasma catecholamine concentrations that are higher than when exercise is performed with a limited muscle mass as in the present study, although a modest increase in plasma catecholamine concentrations was noted at the high exercise intensity. Therefore, our findings point to the importance of extramuscular factors in regulation of muscle lipid metabolism. One such extramuscular factor could be the plasma FA delivery to the exercising muscles. The high sympatho-adrenal response during whole body exercise involving a large muscle mass impairs blood flow in adipose tissue and, in turn, FA release (Bulow & Madsen, 1981) leading to a decrease in plasma FA concentrations and accordingly a reduction in cellular plasma fatty acid uptake and oxidation. Recent studies have, however, indicated that other factors than delivery and uptake of plasma fatty acids also are involved in regulation of cellular fatty acid oxidation. Thus, elevation of plasma FA concentration by infusion of Intralipid and heparin during exercise at 85% of 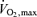 only slightly restored fat oxidation compared with the levels observed during exercise at 65% of
only slightly restored fat oxidation compared with the levels observed during exercise at 65% of 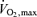 (Romijn et al. 1995). Similarly, in the study by van van Loon et al. (2001) the reduction in plasma FA oxidation rate at the highest work intensity was observed even when plasma FA concentrations did not decrease. Furthermore, Kiens et al. (1999) observed an increase in intramyocellular FA concentrations concomitantly with a decrease in plasma FA concentrations when whole body exercise intensity was increased from 65% to 90% of
(Romijn et al. 1995). Similarly, in the study by van van Loon et al. (2001) the reduction in plasma FA oxidation rate at the highest work intensity was observed even when plasma FA concentrations did not decrease. Furthermore, Kiens et al. (1999) observed an increase in intramyocellular FA concentrations concomitantly with a decrease in plasma FA concentrations when whole body exercise intensity was increased from 65% to 90% of 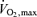 indicating that intramuscular FA availability was not limiting fat oxidation during intense whole body exercise.
indicating that intramuscular FA availability was not limiting fat oxidation during intense whole body exercise.
These findings perhaps suggest that thigh versus whole body exercise exerts a different effect on intramuscular factors, which may influence regulation of fat oxidation. During high intensity exercise, lactate is produced and accumulated in muscle and Starritt et al. (2000) proposed that a reduced muscle pH, as a consequence of muscle lactate accumulation, may decrease CPT-1 activity and thus impair transport of fatty acids across the mitochondrial membrane. Albeit we did find a minor increase in lactate release and a lower venous pH at the high compared to low and moderate intensity exercise, the femoral venous plasma lactate concentration was most likely too low to reflect anything but a small intramuscular lactate accumulation in the present study. While it cannot be excluded that such lactate accumulation contributed to the observed decrease in fractional extraction of FA with exercise intensity, it obviously did not prevent a progressive increase in plasma FA uptake in exercising muscle. Furthermore, the fact that intramuscular lactate accumulation may be higher during whole body exercise than during exercise with a smaller muscle group may reflect catecholamine mediated muscle blood flow restriction and enhancement of glycogenolysis (Saltin, 1988; Richter et al. 1982), rather than differences in purely intracellular, contraction mediated mechanisms. Higher muscle glycogeneolysis during whole body exercise than during exercise with a small muscle mass may also increase acetylcarnitine and decrease free carnitine concentration in muscle leading to inhibited lipid oxidation due to a relative lack of free carnitine as substrate for CPT-1 (Roepstorff et al. 2004).
During exercise plasma FA oxidation could not cover the entire fat oxidation implying that other fat sources contribute to fat oxidation as found in many previous studies. The other likely fat sources are the intramuscular triacylglycerols and the plasma VLDL triacylglycerols. In a prior publication using the same subjects and protocol as the present paper, we did not observe any significant changes in biochemically determined muscle triacylglycerol stores during exercise (Stallknecht et al. 2004) as also found previously during one-legged knee-extensor exercise (Kiens et al. 1993). However, measured by the microdialysis technique, glycerol release from muscle was detected during exercise at 25% Wmax, whereas this was not the case at 65 and 85% Wmax suggesting that muscle triacylglycerol was contributing to fat utilization during exercise at 25% Wmax. Nevertheless, the relative role of muscle and plasma triacylglycerol, respectively, as fuel sources in the present study remains elusive.
Another novel finding in the present study was that leg glycerol uptake increased with increasing exercise intensity. Whereas no net glycerol exchange could be detected across the thigh during exercise, the use of a glycerol tracer allowed us to measure the simultaneous uptake and release of glycerol. Leg tracer-determined glycerol uptake has previously been demonstrated during constant low intensity one-legged knee-extensions (van Hall et al. 2002c), and it was found that uptake increased with time as did the arterial glycerol concentration. Similarly, in the present study the increase in glycerol uptake paralleled the increase in glycerol delivery (Table 2). Taken together, these data indicate that the main regulator of muscle glycerol uptake during exercise is the glycerol delivery. The observation of leg glycerol release during exercise is in agreement with data published by Bergman et al. (1999) and van Hall et al. (2002c) and is probably due to release from adipose tissue draining to the femoral vein.
It may be argued that the high exercise load in the present study was not comparable to the muscle load during whole body high intensity exercise. We consider this unlikely for the following reasons. First, in the present study thigh muscle mass and thigh oxygen uptake at the high intensity averaged 2.88 ± 0.11 kg and 0.55 ± 0.05 l O2 min−1, respectively. It follows that peak oxygen utilization was approximately 0.19 l O2 min−1 per kg of active muscle. Assuming that intense whole body exercise requires activation of approximately 15 kg of muscle and the same oxygen utilization, the oxygen demand of the active muscle would be 15 kg × 0.19 l min−1 kg−1 = 2.85 l O2 min−1. Assuming an oxygen requirement of non-muscle tissue, of 0.5 l O2 min−1, the total oxygen utilization would amount to 3.35 l O2 min−1, which is 86% of 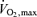 for the subjects of the present study and, accordingly, in relative terms similar to the highest thigh exercise intensity. Secondly, we found that the contribution of glucose derived from the circulation and from muscle glycogen were increased as exercise intensity increased. This is similar to the observations in the earlier studies mentioned above, and other evidence in the literature (Wahren, 1970; Saltin & Karlsson, 1971). Based on these arguments we consider the high intensity exercise applied in the present study to be in good agreement with the load skeletal muscle experiences during intense whole body exercise.
for the subjects of the present study and, accordingly, in relative terms similar to the highest thigh exercise intensity. Secondly, we found that the contribution of glucose derived from the circulation and from muscle glycogen were increased as exercise intensity increased. This is similar to the observations in the earlier studies mentioned above, and other evidence in the literature (Wahren, 1970; Saltin & Karlsson, 1971). Based on these arguments we consider the high intensity exercise applied in the present study to be in good agreement with the load skeletal muscle experiences during intense whole body exercise.
Finally, it may be worth noting that plasma FA oxidation in working skeletal muscle accounted for only 25–40% of whole body plasma FA oxidation (Table 4). This is in line with previous observations during bicycle exercise (Burguera et al. 2000; Helge et al. 2001; Roepstorff et al. 2002) and indicates that whole body plasma FA oxidation during exercise only to a limited extent reflects leg FA oxidation. Recently this conclusion was supported by (Friedlander et al. 2007). The finding underscores the importance of measurements using the leg balance technique compared to extrapolating whole body measurements to muscle during exercise.
Conclusion
In the present study an attempt was made to directly study the effect of contraction intensity on muscle substrate recruitment and oxidation under very limited influence of autonomic neurohumoral stimulation. Under these conditions muscle total fat oxidation remains unchanged from low to high exercise intensities, while uptake and oxidation of plasma free fatty acids increase progressively. These findings differ from whole body measurements during whole body exercise in which fat oxidation peaks at around 60% of 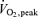 and is lower at both lower and higher exercise intensities. Whole body exercise elicits a marked neurohumoral response suggesting that autonomic neurohumoral mechanisms may modulate local contraction mediated regulation of muscle metabolism. Furthermore, whole body measurements may not accurately reflect local muscle metabolism. Finally, thigh glycerol uptake increases with increasing exercise intensity and arterial glycerol concentration, suggesting that glycerol may be oxidized in exercising muscle.
and is lower at both lower and higher exercise intensities. Whole body exercise elicits a marked neurohumoral response suggesting that autonomic neurohumoral mechanisms may modulate local contraction mediated regulation of muscle metabolism. Furthermore, whole body measurements may not accurately reflect local muscle metabolism. Finally, thigh glycerol uptake increases with increasing exercise intensity and arterial glycerol concentration, suggesting that glycerol may be oxidized in exercising muscle.
Acknowledgments
The skilled technical assistance of Irene Beck Nielsen, Betina Bolmgren, Winnie Taagerup, Heidi Storgaard, Birgitte Jessen and Nina Pluszek is acknowledged. The study was supported by grants from the Danish National Research Foundation grant no. 504-14, the Danish Sports Research Council (J.nr. 981001-19 and 980501-20), the Copenhagen Muscle Research Centre, the Danish Medical and Natural Science Research Council and an integrated project (LSHM-CT-2004-005272) funded by the European Commission.
References
- Achten J, Gleeson M, Jeukendrup AE. Determination of the exercise intensity that elicits maximal fat oxidation. Med Sci Sports Exerc. 2002;34:92–97. doi: 10.1097/00005768-200201000-00015. [DOI] [PubMed] [Google Scholar]
- Andersen P, Adams RP, Sj′gaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman BC, Butterfield GE, Wolfel EE, Casazza GA, Lopaschuk GD, Brooks GA. Evaluation of exercise and training on muscle lipid metabolism. Am J Physiol Endocrinol Metab. 1999;276:E106–E117. doi: 10.1152/ajpendo.1999.276.1.E106. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the ‘crossover’ concept. J Appl Physiol. 1994;76:2253–2261. doi: 10.1152/jappl.1994.76.6.2253. [DOI] [PubMed] [Google Scholar]
- Bulow J, Madsen J. Influence of blood flow on fatty acid mobilization from lipolytically active adipose tissue. Pflugers Arch. 1981;390:169–174. doi: 10.1007/BF00590202. [DOI] [PubMed] [Google Scholar]
- Burguera B, Proctor D, Dietz N, Guo Z, Joyner M, Jensen MD. Leg free fatty acid kinetics during exercise in men and women. Am J Physiol Endocrinol Metab. 2000;278:E113–E117. doi: 10.1152/ajpendo.2000.278.1.E113. [DOI] [PubMed] [Google Scholar]
- Christensen EH, Hansen O. Untersuchungen ûber die verbrennungsvorgänge bei langdaurnder, schwerer muskelarbeit. Skand Arch Physiol. 1939;81:153–159. [Google Scholar]
- Christensen NJ, Vestergaard P, Sorensen T, Rafaelsen OJ. Cerebrospinal fluid adrenaline and noradrenaline in depressed patients. Acta Psych Scand. 1980;61:178–182. doi: 10.1111/j.1600-0447.1980.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Drabkin DL, Austin FH. Spectrophotometric studies II. Preparations from washed blood cells, nitric oxide hemoglobin and sulfhemoglobin. J Biol Chem. 1935;122:51–65. [Google Scholar]
- Frayn K. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Friedlander AL, Jacobs KA, Fattor JA, Horning MA, Hagobian TA, Bauer TA, Wolfel EE, Brooks GA. Contributions of working muscle to whole body lipid metabolism are altered by exercise intensity and training. Am J Physiol Endocrinol Metab. 2007;292:E107–E116. doi: 10.1152/ajpendo.00148.2006. [DOI] [PubMed] [Google Scholar]
- Galbo H. Hormonal and Metabolic Adaptation to Exercise. New York: Georg Thieme; 1983. pp. 1–116. [Google Scholar]
- Galbo H. Exercise physiology: Humoral function. Sport Sci Rev. 1992;1:65–93. [Google Scholar]
- Helge JW, Richter EA, Kiens B. Interaction of training and diet on metabolism and endurance during exercise in man. J Physiol. 1996;292:293–306. doi: 10.1113/jphysiol.1996.sp021309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helge JW, Watt PW, Richter EA, Rennie MJ, Kiens B. Fat utilization during exercise; adaptation to fat rich diet increases utilization of plasma FA and VLDL-TG. J Physiol. 2001;537:1009–1020. doi: 10.1111/j.1469-7793.2001.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006;86:205–243. doi: 10.1152/physrev.00023.2004. [DOI] [PubMed] [Google Scholar]
- Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilization during submaximal exercise in man: Effect of endurance training. J Physiol. 1993;469:459–478. doi: 10.1113/jphysiol.1993.sp019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiens B, Roemen TH, Van der Wusse GJ. Muscular long-chain fatty acid content during graded exercise in humans. Am J Physiol Endocrinol Metab. 1999;276:E352–E357. doi: 10.1152/ajpendo.1999.276.2.E352. [DOI] [PubMed] [Google Scholar]
- Krogh A, Lindhard J. The relative value of fat and carbohydrate as sources of muscular energy. Biochem J. 1920;14:290–363. doi: 10.1042/bj0140290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. [Google Scholar]
- Radegran G, Blomstrand E, Saltin B. Peak muscle perfusion and oxygen uptake in humans: importance of precise estimates of muscle mass. J Appl Physiol. 1999;87:2375–2380. doi: 10.1152/jappl.1999.87.6.2375. [DOI] [PubMed] [Google Scholar]
- Richter EA, Ruderman NB, Gavras H, Belur ER, Galbo H. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. Am J Physiol Endocrinol Metab. 1982;242:E25–E32. doi: 10.1152/ajpendo.1982.242.1.E25. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Wojtaszewski JF, Richter EA, Kiens B. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab. 2004;288:E133–E142. doi: 10.1152/ajpendo.00379.2004. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Steffensen CH, Madsen M, Stallknecht B, Kanstrup IL, Richter EA, Kiens B. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am J Physiol Endocrinol Metab. 2002;282:E435–E447. doi: 10.1152/ajpendo.00266.2001. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Gastadelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol Endocrinol Metab. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Zhang XJ, Wolfe RR. Relationship between fatty acid delivery and fatty acid oxidation during strenuos exercise. J Appl Physiol. 1995;79:1939–1945. doi: 10.1152/jappl.1995.79.6.1939. [DOI] [PubMed] [Google Scholar]
- Saltin B. Capacity of blood flow delivery to exercising skeletal muscle in humans. Am J Cardiol. 1988;62:30E–35E. doi: 10.1016/s0002-9149(88)80007-9. [DOI] [PubMed] [Google Scholar]
- Saltin B, Gollnick P. Skeletal muscle adaptability: significance for metabolism and performance. In: Peachey LD, editor. Handbook of Physiology, Skeletal Muscle. Bethesda, MD, USA: American Physiological Society; 1983. pp. 555–631. section 10. [Google Scholar]
- Saltin B, Karlsson J. Muscle glycogen utilization during work at different intensities. Adv Exp Med Biol. 1971;11:289–299. [Google Scholar]
- Siggaard-Andersen O, Wimberley PD, Fogh-Andersen N. Measured and derived quantities with mdern pH and blood gas equipment: calculation algorithms with 54 equations. Scand J Clin Lab Invest. 1988;48:7–15. [Google Scholar]
- Stallknecht B, Kiens B, Helge JW, Richter EA, Galbo H. Interstitial glycerol concentrations in human skeletal muscle and adipose tissue during graded exercise. Acta Physiol Scand. 2004;180:367–377. doi: 10.1111/j.1365-201X.2004.01264.x. [DOI] [PubMed] [Google Scholar]
- Starritt EC, Howlett RA, Heigenhauser GJ, Spriet LL. Sensitivity of CPT I to malonyl-CoA in trained and untrained human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E462–E468. doi: 10.1152/ajpendo.2000.278.3.E462. [DOI] [PubMed] [Google Scholar]
- Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–432. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- van Hall G, Bulow J, Sacchetti M, AlMulla N, Lyngso D, Simonsen L. Regional fat metabolism in human splanchnic and adipose tissues; the effect of exercise. J Physiol. 2002a;543:1033–1046. doi: 10.1113/jphysiol.2002.022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, Sacchetti M, Radegran G. Whole body and leg acetate kinetics at rest, during exercise and recovery in humans. J Physiol. 2002b;542:263–272. doi: 10.1113/jphysiol.2001.014340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, Sacchetti M, Radegran G, Saltin B. Human skeletal muscle fatty acid and glycerol metabolism during rest, exercise and recovery. J Physiol. 2002c;543:1047–1058. doi: 10.1113/jphysiol.2002.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LJC, Greenhaff PL, Constantin-Teodosiu D, Saris WHM, Wagenmakers AJM. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536:295–304. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon LJ, Koopman R, Schrauwen P, Stegen J, Wagenmakers AJ. The use of the [1,2-13C]acetate recovery factor in metabolic research. Eur J Appl Physiol. 2003;89:377–383. doi: 10.1007/s00421-003-0810-x. [DOI] [PubMed] [Google Scholar]
- Wahren J. Human forearm muscle metabolism during exercise. IV. Glucose uptake at different work intensities. Scand J Clin Lab Invest. 1970;25:129–135. doi: 10.3109/00365517009049194. [DOI] [PubMed] [Google Scholar]
- Wieland O. Glycerol assay. In: Bergmeyer HV, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. pp. 1404–1406. [Google Scholar]
- Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine. New York: Wiley-Liss; 1992. [Google Scholar]