Abstract
Peripheral chemoreflex activation with potassium cyanide (KCN) in awake rats or in the working heart–brainstem preparation (WHBP) produces: (a) a sympathoexcitatory/pressor response; (b) bradycardia; and (c) an increase in the frequency of breathing. Our main aim was to evaluate neurotransmitters involved in mediating the sympathoexcitatory component of the chemoreflex within the nucleus tractus solitarii (NTS). In previous studies in conscious rats, the reflex bradycardia, but not the pressor response, was reduced by antagonism of either ionotropic glutamate or purinergic P2 receptors within the NTS. In the present study we evaluated a possible dual role of both P2 and NMDA receptors in the NTS for processing the sympathoexcitatory component (pressor response) of the chemoreflex in awake rats as well as in the WHBP. Simultaneous blockade of ionotropic glutamate receptors and P2 receptors by sequential microinjections of kynurenic acid (KYN, 2 nmol (50 nl)−1) and pyridoxalphosphate-6-azophenyl-2′,4′-disulphonate (PPADS, 0.25 nmol (50 nl)−1) into the commissural NTS in awake rats produced a significant reduction in both the pressor (+38 ± 3 versus +8 ± 3 mmHg) and bradycardic responses (−172 ± 18 versus −16 ± 13 beats min−1; n = 13), but no significant changes in the tachypnoea measured using plethysmography (270 ± 30 versus 240 ± 21 cycles min−1, n = 7) following chemoreflex activation in awake rats. Control microinjections of saline produced no significant changes in these reflex responses. In WHBP, microinjection of KYN (2 nmol (20 nl)−1) and PPADS (1.6 nmol (20 nl)−1) into the commissural NTS attenuated significantly both the increase in thoracic sympathetic activity (+52 ± 2% versus +17 ± 1%) and the bradycardic response (−151 ± 17 versus −21 ± 3 beats min−1) but produced no significant changes in the increase of the frequency of phrenic nerve discharge (+0.24 ± 0.02 versus +0.20 ± 0.02 Hz). The data indicate that combined microinjections of PPADS and KYN into the commissural NTS in both awake rats and the WHBP are required to produce a significant reduction in the sympathoexcitatory response (pressor response) to peripheral chemoreflex activation. We conclude that glutamatergic and purinergic mechanisms are part of the complex neurotransmission system of the sympathoexcitatory component of the chemoreflex at the level of the commissural NTS.
There is both anatomical and functional evidence that the peripheral chemoreceptor afferents make their first synapses in the nucleus tractus solitarii (NTS) terminating mainly in the commissural NTS (Donoghue et al. 1984; Finley & Katz, 1992; Mifflin, 1992; Ciriello et al. 1994; Chitravanshi et al. 1994; Chitravanshi & Sapru, 1995; Paton et al. 2001). Activation of the peripheral chemoreceptors by intravenous injection of aqueous cyanide in either conscious rats or the working heart–brainstem preparation (WHBP) of rat produces reflex bradycardia, an increase in arterial pressure (due to sympathetic activation) and tachypnoea in rats (Franchini & Krieger, 1992, Franchini 1993; Haibara et al. 1995; Boscan & Paton, 2001; Paton et al. 2002; Machado, 2004; Antunes et al. 2005b; Braga & Machado, 2006). Our interest has been in the neurotransmitters involved in mediating this pattern of response at the level of the NTS.
Previous studies by Haibara et al. (1995) in awake rats showed that selective NMDA receptor blockade in the commissural NTS attenuated the chemoreflex-evoked bradycardia but did not affect the reflex pressor response. Subsequently, we demonstrated that the antagonism of ionotropic glutamate receptors in the commissural NTS produced only a partial reduction of the pressor response to chemoreflex activation in awake rats (Haibara et al. 1999; Machado & Bonagamba, 2005). Moreover, antagonism of both ionotropic and metabotropic glutamate receptors in the commissural NTS also failed to reduce the reflex sympathoexcitation elicited by peripheral chemoreceptor activation in the WHBP (Braga & Machado, 2006). This has led us to hypothesize that neurotransmission of the sympathoexcitatory component of the chemoreflex involves neurotransmitters other than l-glutamate in NTS. Here, we propose a purinergic mechanism.
A potential role for ATP as a neurotransmitter/neuromodulator was suggested originally by Burnstock (1972). Immunohistochemical studies have demonstrated the presence of all six P2X receptor subtypes in the NTS (Yao et al. 2000) with a predominance of P2X2 and P2X3 receptors (Vulchanova et al. 1997; Llewellyn-Smith & Burnstock, 1998). At the level of the NTS, ATP is involved in cardio-respiratory regulation (Ergene et al. 1994; Barraco et al. 1996; Phillis et al. 1997; Scislo et al. 1997, Scislo 1998; Scislo & O'Leary, 1998; de Paula et al. 2004; Antunes et al. 2005a,Antunes 2005b). We reported that the bradycardic response to chemoreflex activation was attenuated by microinjection of a non-selective P2 receptors antagonist (suramin) into the commissural NTS of the WHBP (Paton et al. 2002). However, the chemoreflex-evoked sympathoexcitation and tachypnoeic responses were not evaluated in that study. Interestingly, in awake rats we showed that microinjection of ATP into the commissural NTS produced a cardiovascular and respiratory pattern of response similar to that produced by chemoreflex activation (i.e. pressor and tachypnoeic responses; see de Paula et al. 2004; Antunes et al. 2005a). Therefore, in the present study we have evaluated the effect of antagonism of P2 receptors alone and in combination with ionotropic glutamate receptors in the commissural NTS on the pressor and respiratory responses to chemoreflex. To enhance the robustness of our findings as well as to avoid the compounding effects of anaesthesia, we performed our studies in both awake rats and the WHBP.
Methods
Experiments were performed in accordance with the guidelines set by the Brazilian Committee for Animal Experimentation (COBEA) and approved by the Institutional Ethics Committee on Animal Experimentation of the School of Medicine of Ribeirão Preto, University of São Paulo. Studies were performed in both awake rats and the WHBP, and are described separately below.
I. Awake rats
Experiments were performed on adult male Wistar rats weighing 290–310 g. Four days before experiments, rats under tribromoethanol anaesthesia (250 mg kg−1, i.p., Aldrich Chemical, Milwaukee, WI, USA) were placed in a stereotaxic apparatus (David Kopf, Tujunga, CA, USA). Frequent toe pinching was used to assess the depth of anaesthesia. As soon as a flexor reflex was evident, anaesthesia was supplemented (tribromoethanol, 250 mg kg−1, i.p.). Using the approach described originally by Michelini & Bonagamba (1988), guide cannulae were implanted targeting the commissural NTS. All guide cannulae were made from 22 gauge stainless steel tube (15 mm long) and implanted in accordance with coordinates of Paxinos & Watson (1998). In all cases the tip of each guide cannula was positioned in the cerebellum approximately 1 mm above the dorsal surface of the medulla. The guide cannulae were fixed to the skull with methacrylate and watch screws and then closed with a mandrill until injections were made.
Six groups of awake rats were used. Group 1 (n = 8): two guide cannulae were implanted and aimed towards the commissural NTS (0.5 mm lateral to midline and 0.5 mm caudal to the calamus scriptorius (CS)). This group of rats was used to test the efficacy of the dose of the antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulphonate (PPADS; 0.25 nmol (50 nl)−1) in blocking the cardiovascular responses evoked following a microinjection of the agonist α,β-methylene ATP (0.0625 nmol (50 nl)−1). This dose of α,β-methylene ATP corresponded approximately to the effective dose (ED50) obtained in a group of rats (n = 17) in which the doses varied in the range from 0.00625 to 1.25 nmol (50 nl)−1 (data not shown). Despite the fact that rats in this group were fitted with two guide cannulae, microinjections were performed unilaterally. In each rat the side chosen corresponded to the side that responded to a microinjection of the agonist. Group 2 (n = 6): two guide cannulae were implanted and aimed towards the commissural NTS (0.5 mm lateral to midline and 0.5 mm caudal to the CS). In this group we evaluated the effect of bilateral antagonism of P2 receptors by PPADS (0.25 nmol (50 nl)−1) on the chemoreflex-evoked cardiovascular responses. Group 3 (n = 13): three guide cannulae were employed to target a greater region of the commissural NTS. Two of these cannulae were implanted 0.5 mm lateral to midline and 0.5 mm rostral to the CS whereas the third targeted the most caudal aspect of the commissural NTS (e.g. midline and 0.5 mm caudal to CS). In this group we made sequential microinjections of antagonists of the excitatory amino acid NMDA and of P2 receptors (kynurenic acid (KYN) 2.0 nmol (50 nl)−1 and PPADS 0.25 nmol (50 nl)−1, respectively). The order of drugs was KYN followed by PPADS (n = 6), or the reverse (n = 7). Group 4 (n = 4): three guide cannulae were employed to target a greater region of the commissural NTS similar to group 3 and in this control group we made microinjections of the vehicle (saline). Group 5 (n = 7): rats were fitted with two guide cannulae to target the most caudal aspect of the commissural NTS (e.g. 0.5 mm lateral to midline and 0.5 mm caudal to the CS). In this group both the cardiovascular and respiratory responses to chemoreflex activation were evaluated before and after bilateral microinjections of glutamatergic and purinergic receptor antagonists. This experimental protocol was performed with rats placed inside a plethysmographic chamber (6 l volume) in accordance with the method described by Malan (1973). Group 6 (n = 6): rats were fitted with two guide cannulae to target the caudal most aspect of the commissural NTS as in group 5 and in this control group both the cardiovascular and respiratory responses to chemoreflex activation were made after bilateral microinjection of the vehicle (saline).
Arterial and venous catheters
In all groups rats were anaesthetized with tribromoethanol and the femoral artery and vein were cannulated (PE-10 connected to PE-50; Clay Adams, Parsippany, NJ, USA) through a small incision (0.5 cm long) 1 day before the experiments. The arterial catheter was pushed towards the abdominal aorta for measurement of pulsatile arterial pressure (PAP) and mean arterial pressure (MAP) via a pressure transducer (model CDX III, Cobe Laboratories, Lakewood, CO, USA) connected to a physiological recorder (Narcotrace 80, Narco Bio-Systems, Austin, TX, USA). Heart rate (HR) was derived from the interval between arterial pressure pulses with a biotachometer coupler (Narco Bio-Systems, model 7302). The venous catheter was used for administration of potassium cyanide (KCN; see below). All catheters were tunnelled subcutaneously and exteriorized through the back of the neck. MAP and HR were recorded 24 h after implantation of the catheters when the rats were completely recovered from the surgical and anaesthetic procedures. All cannulae were suspended enabling animals to move freely. In group 3, a third catheter was inserted into the contralateral femoral vein for infusions of sodium nitroprusside (SNP; 3 μg (50 μl)−1 min−1) to normalize MAP after drug injections into the NTS.
Microinjections into the NTS in awake rats
All microinjections into the commissural NTS were performed without any handling or restraint of the rats and were in accordance with previous studies from our laboratory (Machado & Bonagamba, 1992, Machado 2005; Colombari et al. 1994, Colombari 1997; Haibara et al. 1995, Haibara 1999). The needle (33-gauge, Small Parts, Miami Lakes, FL, USA) used for microinjection into the NTS was 1.5 mm longer than the guide cannula and was connected by PE-10 tubing to a 1 μl syringe (Hamilton, Reno, NV, USA). After removal of the occluder, the needle was carefully inserted into the guide cannula and manual injection was initiated 30 s later. The time interval between multiple microinjections, given via the different guide cannulae, was ∼1.5 min; the volume injected was 50 nl given over a 10 s period.
Chemoreflex activation
The chemoreflex was activated by 100 μl intravenous injection of KCN (40 μg per rat; Merck, Darmstadt, Germany) in accordance with the procedures described by Franchini & Krieger (1993) and previously validated for our experimental conditions (Haibara et al. 1995, Haibara 1999; Barros et al. 2002; Machado & Bonagamba, 2005). The changes in MAP and HR were quantified using peak response values. The cardiovascular responses to chemoreflex activation were evaluated before and 3, 10, 30 and 60 min after sequential microinjection of PPADS (0.25 nmol (50 nl)−1) followed by KYN (2.0 nmol (50 nl)−1), or the drugs in the reverse sequence, into the commissural NTS.
Plethysmographic chamber
In groups 5 and 6, rats were initially conditioned to the plethysmographic chamber before any experiments were performed. After animals had adapted to this environment, control chemoreflex data were acquired. Microinjections into the NTS were performed by opening the chamber and using identical procedures to those described above. After the second microinjection, the plethysmographic chamber was closed for recording of baseline respiratory frequency (RF) and reflex changes in RF in response to chemoreflex activation. The chemoreflex was activated before (control) and 2, 5, 10, 15 and 30 min after bilateral microinjections of KYN and PPADS or saline (vehicle) into the commissural NTS. The RF was quantified 10 s before (baseline) and 10 s after chemoreflex activation. The femoral artery and vein catheters were exteriorized throughout a little hole in the top of the plethysmography chamber, which was sealed with silicone grease during recording. This allowed chemoreflex activation with i.v. injections of KCN while recording arterial blood pressure and RF.
Drug solutions
α,β-Methylene ATP (0.0625 nmol (50 nl)−1), PPADS (pyrinoxalphosphate-6-azophenyl-2′,4′-disulphonic acid tetrasodium salt (0.25 nmol (50 nl)−1)) and KYN (2.0 nmol (50 nl)−1) were obtained from Sigma Chemical (St Louis, MO, USA). KCN (40 μg (0.1 ml)−1 per rat) was obtained from Merck (Darmstadt, Germany). The drug solutions were freshly dissolved in saline (154 mm NaCl), and sodium bicarbonate was added to adjust the pH to 7.4. The pH was determined using a pH indicator (Spezialindikator, pH 6.4–8.0, Merck KGaA, 64271, Darmstadt, Germany). The dose of PPADS was determined in the group 1 of awake rats (n = 8), in which the MAP and HR responses to microinjection of α,β-methylene ATP (0.0625 nmol (50 nl)−1) into the NTS (−33 ± 6 versus −4 ± 3 mmHg and −108 ± 27 versus −20 ± 9 beats min−1, respectively) were blocked by previous microinjection of this dose into the same NTS site. The dose of KYN was determined in a previous study from our laboratory, which showed that it was effective in antagonizing the cardiovascular responses to microinjection of l-glutamate into the NTS of awake rats (Colombari et al. 1994).
II. WHBP
A series of experiments was performed in an in situ unanaesthetized decerebrated WHBP as previously described by Paton (1996). The WHBP was selected for several reasons: (a) it permitted us to directly analyse the sympathetic nerve activity component of the chemoreflex after applying antagonists into the NTS; this would be technically challenging in conscious freely moving rats; (b) it did not produce a confounding change in baseline arterial pressure during chemoreflex activation and hence eliminated any baroreflex involvement; and (c) it is also free of the undesirable effects of anaesthetics.
Rats were anaesthetized deeply with halothane (AstraZeneca do Brazil Ltda., Cotia, SP, Brazil) in a small chamber and the level of anaesthesia was assessed by the absence of response to a noxious pinch of either the paw or the tail. Following subdiaphragmatic transection, the rostral half of the animal was submerged in cooled carbogen-gassed (95% O2–5% CO2) artificial cerebrospinal fluid (ACSF), decerebrated at the precollicular level and skinned. It is important to note that the decerebration condition rendered the animals insentient. The descending aorta was isolated, the heart exposed by removal of the left ribs and the lungs. The dorsal surface of the brainstem was exposed by removal of the occipital bone and cerebellum. The WHBP was mounted in a recording chamber, the descending aorta was cannulated and perfused retrogradely with ACSF containing (mm): NaCl 125, NaHCO3 24, KCl 5, CaCl2 2.5, MgSO4 1.25, KH2PO4 1.25 and dextrose 10, and 1.25% oncotic agent Ficoll 70 (Sigma) using a roller pump (Watson-Marlow 502s, Falmouth, Cornwall, UK) via double-lumen cannula. A neuromuscular blocker (vecuronium bromide, 0.04 mg ml−1, Norcuron Organon Teknika, São Paulo, Brazil) was used. Perfusion pressure was maintained between 50 and 70 mmHg by adjusting flow rate of the perfusion pump. The perfusate was gassed with carbogen continuously, warmed to 32°C and filtered using a nylon mesh (pore size, 25 μm; Millipore, Billirica, MA, USA).
Recordings of electrocardiogram and nerve activities
Left phrenic nerve activity (PNA) was recorded from its central end using a glass suction electrode held in a micromanipulator (Narishige, Tokyo, Japan). Rhythmic ramping phrenic nerve discharge gave a continuous physiological index of preparation viability. The electrocardiogram was visible on the phrenic nerve recording which allowed us to evaluate HR. Using a window discriminator, the R-wave was captured and the inter-R wave interval displayed as instantaneous HR. Sympathetic nerve activity (tSNA) was recorded from the thoracic sympathetic chain at the level of T6–T12 using a second glass suction bipolar electrode. Signals were AC amplified, band-pass filtered (8 Hz to 3 kHz) and displayed on a computer using Spike 2 software (Cambridge Electronic Design, Cambridge, UK).
Microinjections into the commissural NTS
The CS was used as a landmark for microinjections into the commissural NTS. Drugs were applied bilaterally via a three-barrelled micropipette (tip diameter, 20–30 μm). The tip of the micropipette was driven into the medulla to a depth of 0.3–0.4 mm ventral to the dorsal surface, 0.3–0.5 mm caudal relative to CS and between 0.2 and 0.4 mm from midline. The injected volume for all drugs (∼20 nl) was determined by previous calibration of the picopump system (Picospritzer II, Parker Instruments, OH, USA). Bilateral microinjections were performed such that KYN followed by PPADS were given on one side and PPADS followed by KYN on the contralateral side. The time for all microinjections was no longer than 1.5 min.
Experimental groups and protocols
In group 1 (n = 7) we verified that the dose of PPADS (0.16 nmol (20 nl)−1) was effective in antagonizing the autonomic and respiratory responses to microinjection of α,β-methylene ATP into the commissural NTS. Thus, α,β-methylene ATP (0.4 nmol (20 nl)−1) was microinjected unilaterally into the commissural NTS before and 2, 10, 30 and 45 min after the microinjection of PPADS (0.16 nmol (20 nl)−1) into the same site.
Group 2 (n = 8) was used to evaluate the possible role of P2 receptors in the commissural NTS on the neurotransmission of the sympathoexcitatory component of the chemoreflex and for this reason PPADS was microinjected into the commissural NTS.
Group 3 (n = 9) was used to evaluate the combined role of ionotropic glutamate and P2 receptors in the commissural NTS for mediating the chemoreflex. We sequentially microinjected KYN (2.0 nmol (20 nl)−1) and PPADS (0.16 nmol (20 nl)−1) in random order. The dose of KYN used was determined in a previous study from our laboratory demonstrating that it antagonized the autonomic responses evoked by a microinjection of l-glutamate into the NTS of the WHBP (Braga et al. 2006). In groups 2 and 3, the chemoreflex was activated by KCN injections (0.05 ml of a solution 0.05%, in the perfusion system as used previously in this preparation (see Paton et al. 1999, Paton 2002; Antunes et al. 2005b; Braga & Machado, 2006) before and 2, 10, 30, 45 and 60 min after microinjections.
Histology
Awake rats
At the end of each experiment, 50 nl Evans Blue (2%) was microinjected to mark the sites of microinjection. Rats were killed with an overdose of thiopental sodium (100 mg kg−1, i.v.) and perfused intracardiacally with saline (154 mm NaCl) followed by 10% buffered formalin. Brains were removed and stored in buffered formalin for 2 days, and then serial transverse sections (15 μm thickness) were cut and stained with cresyl violet using the Nissl method. Only the rats in which the sites of microinjection were located in the commissural NTS were considered for data analysis.
WHBP
At the end of each experiment the brain was removed and fixed by immersion for 7 days in 10% buffered formalin. Histological procedures were performed in order to verify the micropipette track and the centre of microinjections in the commissural NTS. Serial transverse sections (15 μm thickness) were cut and stained with cresyl violet using the Nissl method. Only the microinjections located in commissural NTS were analysed.
Data analysis
In awake rats, data are expressed as mean ± s.e.m. The results were analysed by one-way ANOVA, and differences between individual means were compared by Student's unpaired t test. In all statistical analyses, the level of significance was P < 0.05.
In the WHBP, data were analysed off-line using Spike 2 software with custom-written scripts. Baseline and peak reflex responses in HR were measured. The rectified and integrated signals of the tSNA (100 ms time constant) were measured for a period covering 20 s before and 20 s after chemoreflex activation. Data of tSNA were normalized as percentage of control and changes in the tSNA during chemoreflex stimulation were calculated as the difference between the peak of the response and the baseline measured before each stimulus, as previously described (Braga & Machado, 2006; Braga et al. 2006). Frequency of bursts of the PNA was measured in a time-dependent way (counted every 10 s starting 40 s before the microinjection and ending 50 s after microinjection). Data are expressed as the peak increase in the frequency of the PNA response after chemoreflex activation. The statistical significance of chemoreflex-evoked changes in tSNA and HR was assessed by one-way ANOVA followed by Tukey's post hoc test (P < 0.05). All values are expressed as the mean ± s.e.m and n is the number of preparations.
Results
Awake rats
PPADS antagonism of the cardiovascular responses to microinjection of α,β-methylene ATP into the commissural NTS (Group 1)
Unilateral microinjection of α,β-methylene ATP (0.0625 nmol (50 nl)−1) into the commissural NTS (n = 8) produced hypotension (−33 ± 6 mmHg) and bradycardic (−108 ± 27 beats min−1) responses which were significantly reduced at 2 (−4 ± 3 mmHg and −20 ± 9 beats min−1, respectively; P < 0.05) and 10 min (−2 ± 2 mmHg and −2 ± 2 beats min−1, respectively; P < 0.05) after microinjection of PPADS (0.25 nmol (50 nl)−1) into the same NTS region. The antagonism produced by this dose of PPADS was reversible, as the magnitude of the cardiovascular responses to α,β-methylene ATP returned to control values 60 min later (−37 ± 9 mmHg and −136 ± 25 beats min−1, respectively).
Chemoreflex activation before and after microinjection of PPADS into the commissural NTS in awake rats (group 2)
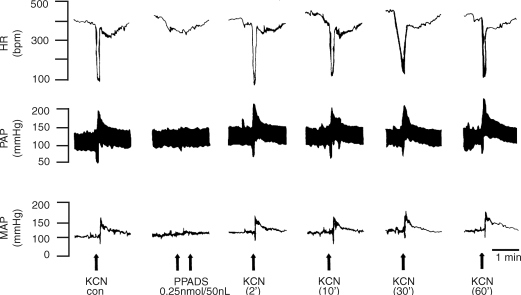
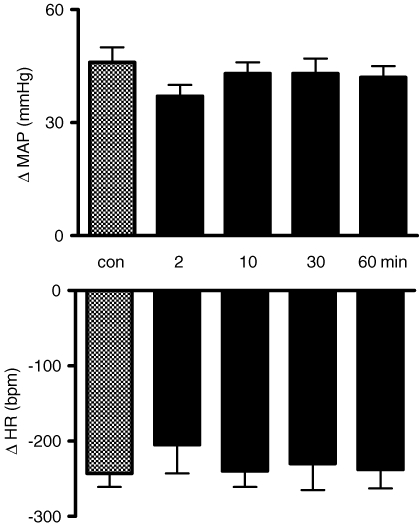
Figure 1 shows tracings from one rat, representative of the group, in which the chemoreflex activation was performed before and 2, 10, 30 and 60 min after bilateral microinjection of PPADS (0.25 nmol (50 nl)−1) into the commissural NTS depicting that both the pressor and bradycardic responses were not altered. Figure 2 summarizes changes in MAP and HR in response to chemoreflex activation before and after microinjection of PPADS into the NTS. The data show that the antagonism of P2X receptors in the commissural NTS produced no changes in the cardiovascular responses to chemoreflex activation.
Figure 1.
Representative tracings of the cardiovascular responses to chemoreflex activation before and after microinjection of PPADS into the commissural NTS Typical raw data recordings from one rat, representative of the group, showing the changes in heart rate (HR), pulsatile arterial pressure (PAP) and mean arterial pressure (MAP) in response to intravenous injection of KCN (40 μg 0.1 ml−1 per rat i.v.) before (control) and 2, 10, 30 and 60 min after microinjection of PPADS (0.25 nmol (50 nl)−1) into the commissural NTS indicated by the arrows.
Figure 2.
Changes in the pressor and bradycardic responses to chemoreflex activation before and after microinjection of PPADS into the commissural NTS Changes in the arterial pressure (ΔMAP, upper panel) and heart rate (ΔHR, bottom panel) in response to peripheral chemoreflex activation with KCN (40 μg 0.1 ml−1 per rat) before and 2, 10, 30 and 60 min after the microinjection PPADS (0.25 nmol (50 nl)−1) into the commissural NTS (n = 6).
Chemoreflex activation before and after microinjection of both PPADS and KYN into the commissural NTS in awake rats: cardiovascular responses (group 3)
Figure 3 shows tracings from one rat, representative of the group, in which chemoreflex activation was performed before and 3, 10, 30 and 60 min after sequential microinjection of KYN (2 nmol (50 nl)−1) and PPADS (0.25 nmol (50 nl)−1) and KYN again into three sites in the commissural NTS (see Methods). After these sequential microinjections there was a significant increase in basal MAP but no significant change in baseline HR. After normalizing MAP with SNP infusion, the pressor and bradycardic responses to chemoreflex activation 3 and 10 min after combined KYN and PPADS microinjections were significantly attenuated. Figure 4 summarizes the changes in MAP and HR in a group of 13 rats in response to chemoreflex activation before and after sequential microinjection of PPADS followed by KYN (n = 7) and KYN followed by PPADS (n = 6). The sequence of drugs microinjected made no difference, hence data were pooled. The upper panel of Fig. 4 illustrates that the pressor response to chemoreflex activation was statistically different at 3 (+38 ± 3 versus +8 ± 3 mmHg) and 10 min (+38 ± 3 versus +10 ± 4 mmHg) after the microinjections in relation to control (P < 0.05, n = 13). The bottom panel of the Fig. 4 illustrates that the bradycardic response to chemoreflex activation was also statistically different at 3 (−172 ± 18 versus −16 ± 13 beats min−1) and 10 min (−172 ± 18 versus −30 ± 20 beats min−1) after the microinjections of antagonists in relation to control (P < 0.05, n = 13). Thirty minutes after the sequential blockade the pressor and bradycardic responses had returned control levels.
Figure 3.
Representative tracings of the cardiovascular responses to chemoreflex activation before and after microinjections of kynurenic acid and PPADS into the commissural NTS Representative raw data recordings from one rat showing the changes in heart rate (HR), pulsatile arterial pressure (PAP) and mean arterial pressure (MAP) in response to intravenous injection of KCN (40 μg 0.1 ml−1 per rat i.v.) before (control) and 3, 10, 30 and 60 min after sequential microinjection of kynurenic acid (KYN, 2 nmol (50 nl)−1) and PPADS (0.25 nmol (50 nl)−1) into the commissural NTS indicated by the arrows. At 3, 10 and 30 min after microinjection of KYN and PPADS, peripheral chemoreflex activation was performed during the sodium nitroprusside (SNP) infusion (3 μg (50 μl)−1 min−1) to normalize the MAP.
Figure 4.
Changes in the pressor and bradycardiac responses to chemoreflex activation before and after microinjections of kynurenic acid and PPADS into the commissural NTS Changes in the arterial pressure (ΔMAP, upper panel) and heart rate (ΔHR, bottom panel) in response to peripheral chemoreflex activation with KCN (40 μg 0.1 ml−1 per rat) before and 3, 10, 30 and 60 min after sequential microinjection of PPADS (0.25 nmol (50 nl)−1) and KYN (2.0 nmol (50 nl)−1) into the commissural NTS combined with intravenous infusion of sodium nitroprusside (n = 13). *Significantly different in relation to the control response (P < 0.05).
Chemoreflex activation before and after microinjection of vehicle (saline, 50 nl) into the commissural NTS in awake rats: cardiovascular responses (group 4)
The changes in MAP in response to chemoreflex activation before and after microinjection of the vehicle (saline) into the three sites of the commissural NTS at 3 (+42 ± 1 versus +34 ± 8 mmHg), 10 (+42 ± 1 versus +44 ± 12 mmHg), 30 (+42 ± 1 versus +37 ± 6 mmHg) and 60 min (+42 ± 1 versus +37 ± 8 mmHg) were not statistically different in relation to control values (P > 0.05, n = 4). In the same way, changes in HR in response to chemoreflex activation before and after sequential microinjection of saline into the three sites of the NTS at 3 (−135 ± 54 versus −132 ± 47 beats min−1), 10 (−135 ± 54 versus −145 ± 46 beats min−1), 30 (−135 ± 54 versus −152 ± 23 beats min−1) and 60 min (−135 ± 54 versus −150 ± 15 beats min−1) were not statistically different from control values (P > 0.05, n = 4).
Baseline MAP and HR before and after NTS microinjections and during the infusion (i.v.) of sodium nitroprusside to normalize MAP in awake rats (groups 3 and 4)
Table 1 shows that the sequential microinjections of glutamatergic and purinergic receptor antagonists into the commissural NTS (n = 13) that received combined drug microinjections (PPADS followed by KYN (n = 7) and KYN followed by PPADS (n = 6)) produced a significant increase in baseline MAP, which was normalized by sodium nitroprusside infusion. Sequential microinjection of the vehicle (saline followed by saline) into the commissural NTS produced a negligible effect on baseline MAP. No significant change in baseline HR was observed after sequential microinjections of the antagonists or saline into the NTS.
Table 1.
Baseline mean arterial pressure (MAP) and heart rate (HR) before and after sequential microinjections of PPADS and KYN or saline and saline (vehicle) into the intermediate and caudal commissural NTS of awake rats
| Baseline before | Baseline after | Baseline after + SNP | ||||
|---|---|---|---|---|---|---|
| Groups | MAP | HR | MAP | HR | MAP | HR |
| KYN + PPADS n = 13 | 108 ± 3 | 355 ± 11 | 142 ± 5* | 352 ± 9 | 107 ± 2 | 362 ± 10 |
| Saline + saline n = 4 | 105 ± 5 | 335 ± 13 | 105 ± 5 | 335 ± 12 | — | — |
After sequential microinjections of antagonists of glutamate ionotropic receptors and P2 receptors into the NTS the intravenous infusion of sodium nitroprusside (SNP) was performed to normalize baseline mean arterial pressure (MAP).
Significantly different relative to the baseline before. HR, heart rate; KYN, kynurenic acid; PPADS, pyridoxalphosphate-6-azophenyl-2′,4′-disulphonate.
Chemoreflex activation before and after microinjection of both PPADS and KYN into the commissural NTS of awake rats inside the plethysmographic chamber: respiratory and cardiovascular responses (group 5)
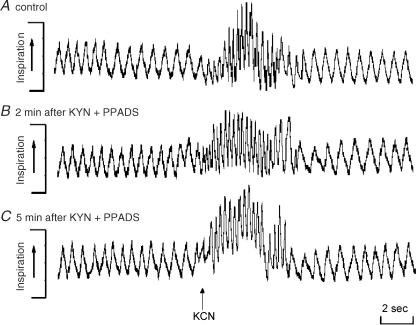
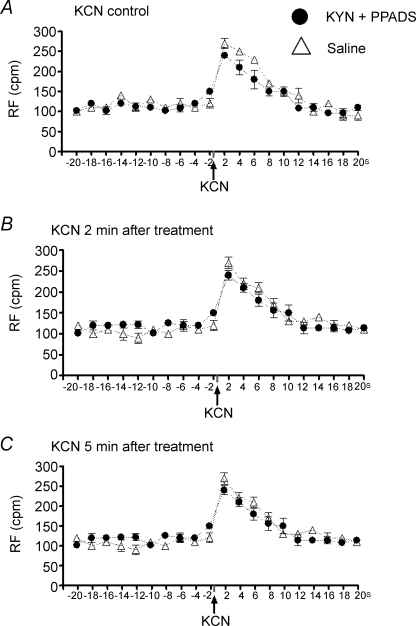
Figure 5 shows tracings of the respiratory frequency (control, Fig. 5A; 2 min, Fig. 5B; 5 min, Fig. 5C) from one rat, representative of the group, in which chemoreflex activation was performed before (control) and 2, 5, 10, 15 and 30 min after combined microinjection of PPADS (0.25 nmol (50 nl)−1) and KYN (2 nmol (50 nl)−1) into the commissural NTS. After sequential microinjections of both antagonists no significant changes in the tachypnoeic response to chemoreflex activation was observed. The data obtained in the control (Fig. 5A) and 2 min (Fig. 5B) and 5 min (Fig. 5C) after the double blockade are summarized in Fig. 6. This shows no changes in the baseline RF at 2 and 5 min after the drugs were given or in the tachypnoeic response to chemoreflex activation with KCN at 2 and 5 min after the double antagonism. The respiratory data obtained at 10, 15 and 30 min were similar to those observed at 2 and 5 min and are not shown. In this group (n = 7) the cardiovascular parameters were also simultaneously recorded while the rats were inside the plethysmographic chamber. Consistent with the experiments described above combined microinjection of PPADS and KYN reduced the magnitude of the chemoreflex-evoked pressor (+40 ± 3 versus +15 ± 2 mmHg) and bradycardic (−227 ± 10 versus −74 ± 10 beats min−1) responses 2 min after the double antagonism. In addition, baseline MAP was elevated as described above (107 ± 1 versus 123 ± 2 mmHg).
Figure 5.
Representative tracings of the respiratory responses to chemoreflex activation before and after microinjections of kynurenic acid and PPADS into the commissural NTS A representative raw data recording from one rat showing the changes in respiratory frequency (RF) in response to intravenous injection of KCN (40 μg 0.1 ml−1 per rat i.v., indicated by arrow) before (control, A) and 2 (B) and 5 min (C) after sequential microinjection of PPADS (0.25 nmol (50 nl)−1) and kynurenic acid (KYN, 2 nmol (50 nl)−1) into the commissural NTS bilaterally.
Figure 6.
Baseline respiratory frequency and tachypnoeic responses to chemoreflex activation before and after microinjections of kynurenic acid and PPADS or saline (vehicle) into the commissural NTS Changes in the respiratory frequency (RF, cycles min−1 (cpm)) in response to chemoreflex activation with KCN (40 μg 0.1 ml−1 per rat i.v., indicated by arrows) before (control, A) and 2 (B) and 5 min (C) after sequential microinjection of PPADS (0.25 nmol (50 nl)−1) and KYN (2.0 nmol (50 nl)−1, •) into commissural NTS bilaterally (n = 7) or after bilateral microinjection of the vehicle (saline, ▵, n = 6). The time scale on the x-axis corresponds to the 20 s before and 20 s after chemoreflex activation with KCN and the values represent the average of respiratory frequency every 2 s.
Chemoreflex activation before and after microinjection of the vehicle (saline) into the commissural NTS of awake rats inside the plethysmographic chamber: respiratory and cardiovascular responses (group 6)
Bilateral microinjections of saline into the commissural NTS produced no significant changes in the cardiovascular responses (data not shown), in the baseline respiratory frequency or in the tachypnoeic responses to chemoreflex activation with KCN (Fig. 6).
Histology
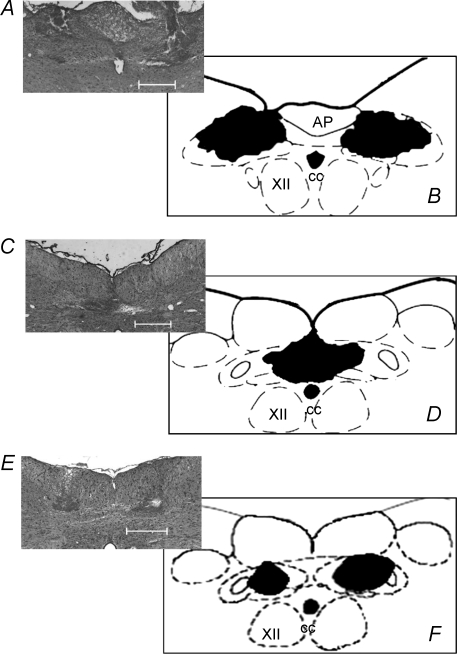
Figure 7 summarizes the microinjection sites from the awake rat experiments. Fig. 7A and C are representative photomicrographs of coronal sections of the medulla of the same rat from group 3 which contained three guide cannulae. Sites were located bilaterally in the rostral (at the level of area postrema) and in the medial aspect of the commissural NTS (caudal to CS). Figure 7B and D are schematic coronal sections of the medulla of the rat, summarizing the overlapping sites of microinjections. Figure 7E is a representative photomicrograph of a coronal section from one rat from group 5 used in the plethysmography experiments. Again, bilateral microinjections were located in the commissural NTS (caudal to CS). Figure 7F is a schematic coronal section of the medulla of the rat summarizing the overlapping sites of microinjections.
Figure 7.
Photomicrographs and schematic drawings of the sites of microinjections into the commissural NTS of awake rats A and C, are representative photomicrographs of coronal sections of the brainstem of one rat from group 3 that was fitted with three guide cannulae for targeting the intermediate regions of the commissural NTS (A) and a caudal commissural area (C). These commissural NTS regions received microinjections of KYN and PPADS (n = 13) or vehicle (saline, n = 4) while measuring the cardiovascular responses to chemoreflex activation. E, is a representative photomicrograph of a coronal section in which bilateral microinjections were made into the commissural NTS while respiratory responses were measured following chemoreflex activation before and after microinjections of KYN and PPADS (n = 7). B, D and F, are the corresponding schematic drawings summarizing the overlapping areas of all microinjections in the commissural NTS (n = 17). AP, area postrema; CC, central canal; XII, hypoglossal nuclei. The scale bars correspond to 500 μm. The schematic drawings were adapted from the atlas of Paxinos & Watson (1998).
WHBP
PPADS antagonism of the cardiovascular and respiratory responses to microinjection of α,β-methylene ATP into the commissural NTS in the WHBP
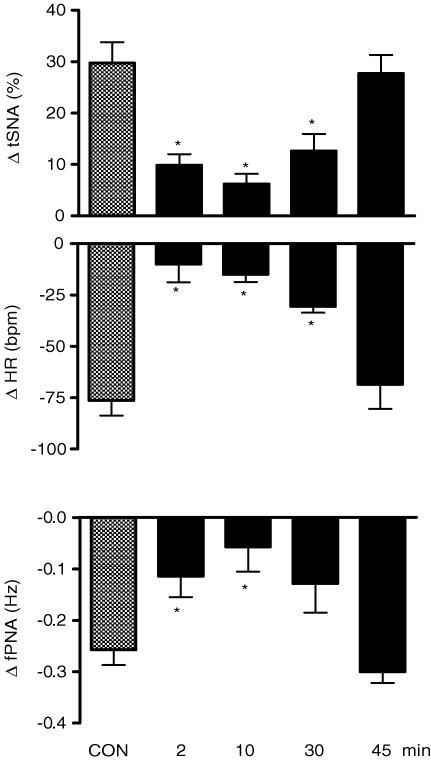
Microinjection of α,β-methylene ATP produced a significant increase in tSNA (+30 ± 4%) and bradycardia (−76 ± 29 beats min−1, P < 0.05, n = 7). Figure 8 shows that microinjection of α,β-methylene ATP (0.4 nmol (20 nl)−1) into the caudal aspect of the commissural NTS produced increases in tSNA and bradycardia, which were reduced significantly at 2 (+10 ± 2% and −10 ± 9 beats min−1, respectively), 10 (+6 ± 2% and −15 ± 4 beats min−1, respectively) and 30 min (+13 ± 3% and −31 ± 3 beats min−1, respectively) after microinjection of PPADS (0.16 nmol (20 nl)−1) into the same NTS site. In addition, microinjection of α,β-methylene ATP into the commissural NTS reduced the frequency of phrenic nerve discharge (−0.23 ± 0.03 Hz). This response was attenuated at 2 (−0.11 ± 0.04 Hz), 10 (−0.06 ± 0.05 Hz) and 30 (−0.13 ± 0.05 Hz, P < 0.05, n = 7) min after microinjection of PPADS. The antagonism produced by PPADS was reversible such that the magnitude of the autonomic and respiratory responses to α,β-methylene ATP were back to control levels 45 min later.
Figure 8.
Changes in the autonomic and respiratory responses to microinjection of α, β-methylene ATP into the commissural NTS before and after PPADS Changes in the thoracic sympathetic nerve activity (ΔtSNA), heart rate (ΔHR) and integrated frequency of phrenic nerve activity (Δ∫PNA) in response to microinjection of α,β-methylene ATP (0.4 nmol (20 nl)−1) before (control) and 2, 10, 30 and 45 min after microinjection of PPADS (0.16 nmol (20 nl)−1) into the caudal commissural NTS of the WHBP (n = 7). *Significantly different in relation to the control response (P < 0.05).
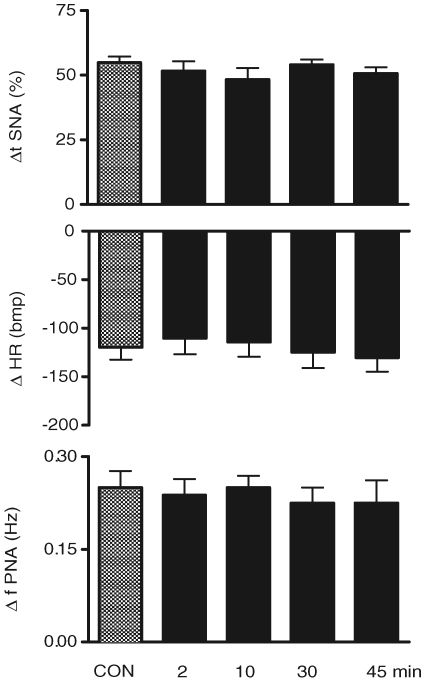
Chemoreflex activation before and after microinjection of PPADS into the commissural NTS in the WHBP
Figure 9 shows a representative tracing from one WHBP in which chemoreflex activation was performed before and 2, 10, 30 and 45 min after bilateral microinjection of PPADS (0.16 mmol (20 nl)−1) into the commissural NTS. The reflex-evoked increases in the tSNA, PNA and bradycardia were not altered after microinjections of this antagonist. Figure 10 summarizes the absence of any change in tSNA, HR and PNA in response to chemoreflex activation before (control) and 2, 10, 30 and 45 min after microinjection of PPADS into the commissural NTS (P > 0.05, n = 8).
Figure 9.
Representative tracings of the autonomic and respiratory responses to chemoreflex activation before and after microinjection of PPADS into the commissural NTS A representative tracing showing the changes in the heart rate (HR), perfusion pressure (PP), integrated thoracic sympathetic nerve activity (∫tSNA), raw thoracic sympathetic nerve activity (tSNA) and integrated phrenic nerve activity (∫PNA) in response to injection of KCN (0.05% (0.05 ml)−1 in the perfusion system) before (control) and 2, 10, 30 and 45 min after bilateral microinjection of PPADS (0.16 nmol (20 nl)−1; indicated by the arrows) into the caudal commissural NTS.
Figure 10.
Changes in the autonomic and respiratory responses to chemoreflex activation before and after microinjection of PPADS into the commissural NTS Changes in the thoracic sympathetic nerve activity (ΔtSNA), heart rate (ΔHR) and frequency of phrenic nerve activity (Δ∫PNA) in response to peripheral chemoreflex activation with KCN (0.05% (0.05 ml)−1 in the perfusion system) before and 2, 10, 30 and 45 min after the microinjection of PPADS (0.16 nmol (20 nl)−1) into the caudal commissural NTS (n = 8).
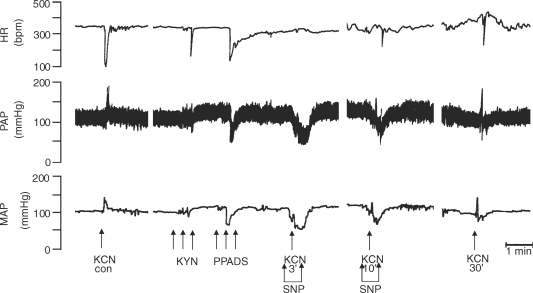
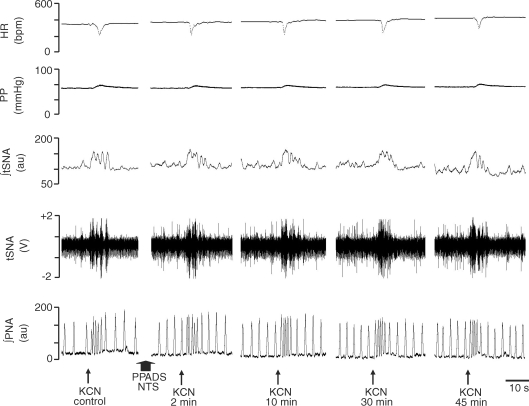
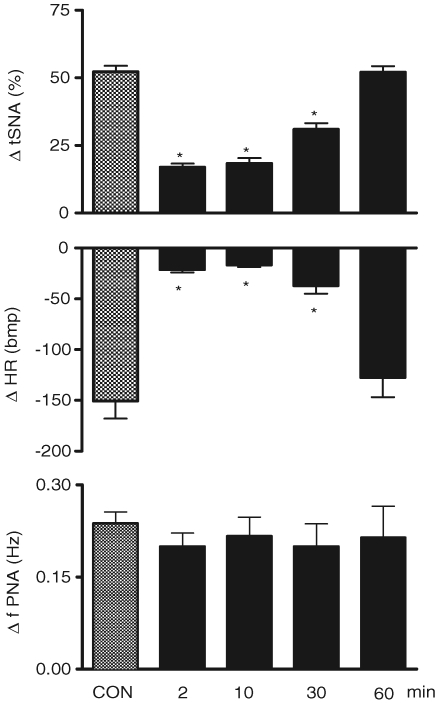
Chemoreflex activation before and after microinjection of both KYN and PPADS into the commissural NTS in the WHBP
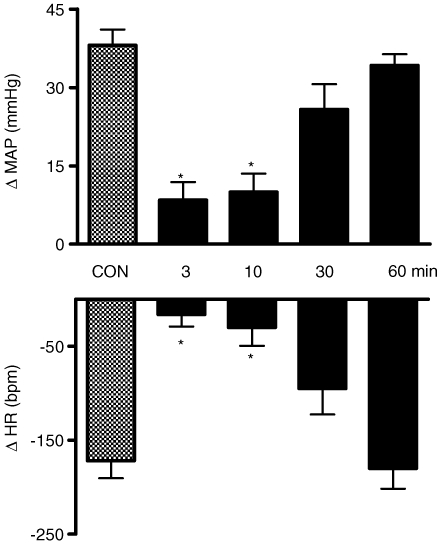
Figure 11 shows the tracings from a representative WHBP in which chemoreflex activation was performed before (control) and 2, 10, 30 and 60 min after bilateral microinjections of PPADS (0.16 nmol (20 nl)−1) followed by KYN (2 nmol (20 nl)−1) into the commissural NTS. After sequential antagonism there was a significant reduction in the sympathoexcitatory and bradycardic response to chemoreflex activation at 2, 10 and 30 min after injection of antagonists. Figure 12 summarizes these changes. Relative to the control chemoreflex-evoked increase in tSNA (+52 ± 2%), microinjection of both antagonists produced a reduction in this response at 2 (+17 ± 1%), 10 (+18 ± 2%) and 30 min (+31 ± 2%) after microinjection (P < 0.05, n = 9). Similarly, the reflex evoked bradycardia was reduced at 2 (−21 ± 3 beats min−1), 10 (−17 ± 2 beats min−1) and 30 min (−37 ± 8 beats min−1) after microinjections into the NTS relative to control (−151 ± 17 beats min−1, P < 0.05, n = 9). Sixty minutes after making the microinjections, the chemoreflex-evoked increase in the tSNA and bradycardia were back to control levels. After the microinjections of both antagonists, we observed a significant increase in the baseline frequency of the PNA at 2 min in relation to the control (0.49 ± 0.05 versus 0.32 ± 0.02 Hz), which returned to the control level 10 min later (0.40 ± 0.07 Hz). In spite of the increase in the baseline frequency of PNA at 2 min, the magnitude of the chemoreflex-evoked increase in the frequency of the PNA in response to chemoreflex activation (0.49 ± 0.05 versus 0.69 ± 0.07 Hz) was not different in relation to the control response (0.32 ± 0.02 versus 0.56 ± 0.04Hz). However, there was a clear qualitative reduction in the amplitude of baseline PNA after PPADS and KYN, as shown in the tracings of Fig. 11. This was not quantified because of uncertainty of ensuring a comparable quality of recording throughout an experiment (see Braga & Machado, 2006). It is of importance that perfusion pressure did not change after NTS microinjection of both KYN and PPADS.
Figure 11.
Representative tracings of the autonomic and respiratory responses to chemoreflex activation before and after microinjection of kynurenic acid and PPADS into the commissural NTS Typical tracings from a WHBP showing changes in the heart rate (HR), perfusion pressure (PP), integrated thoracic sympathetic nerve activity (∫tSNA), raw thoracic sympathetic nerve activity (tSNA) and integrated phrenic nerve activity (∫PNA) in response to injection of KCN (0.05% (0.05 ml)−1 in the perfusion system) before (control) and 2, 10, 30 and 60 min after sequential bilateral microinjection of KYN (2.0 nmol (20 nl)−1) and PPADS (0.16 nmol (20 nl)−1) into the commissural NTS. Arrows indicate time of microinjections.
Figure 12.
Changes in the autonomic and respiratory responses to chemoreflex activation before and after microinjection of kynurenic acid and PPADS into the commissural NTS Changes in the thoracic sympathetic nerve activity (ΔtSNA), heart rate (ΔHR) and frequency of phrenic nerve activity (Δ∫PNA) in response to peripheral chemoreflex activation with KCN (0.05% (0.05 ml)−1 in the perfusion system) before and 2, 10, 30 and 60 min after the sequential bilateral microinjection of KYN (2.0 nmol (20 nl)−1) and PPADS (0.16 nmol (20 nl)−1) into the caudal commissural NTS (n = 9). *Significantly different in relation to the control response (P < 0.05).
Histology
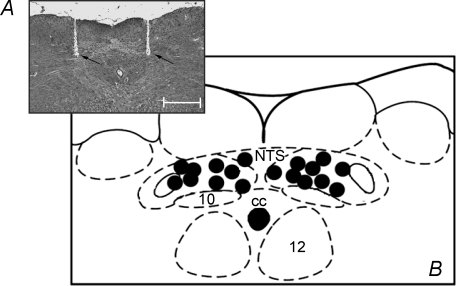
Figure 13 summarizes the microinjection sites from all WHBP experiments. Figure 13A shows a representative photomicrograph indicating the location of a bilateral microinjection in the commissural NTS. Figure 13B summarizes the centres of all bilateral microinjections (PPADS and KYN; n = 9).
Figure 13.
Photomicrograph and schematic drawing of the sites of microinjections into the commissural NTS in the working heart brainstem preparation A, is a representative photomicrograph of a coronal section of the brainstem of one rat used in the WHBP protocols showing the microinjection sites located in the caudal commissural NTS. B, is a schematic diagram depicting all microinjection sites and each black dot corresponds to the location of the centre of microinjection in one experiment. These were all located in the caudal commissural NTS and received sequential microinjections of KYN and PPADS bilaterally (n = 9). CC, central canal; 10, dorsal motor nucleus of the vagus; 12, hypoglossal nucleus. The scale bars corresponds to 500 μm. The schematic drawings were adapted from the atlas of Paxinos & Watson (1998).
Discussion
Our studies provide the first evidence that the peripheral chemoreflex-evoked pressor response and sympathoexcitation are mediated by ionotropic glutamate and P2 receptors within the commissural NTS. In addition, our data suggest that the tachypnoeic response of the chemoreflex is not mediated by these receptors within this part of the NTS. These results were replicated in two distinct preparations emphasizing their robustness.
Methodological considerations
In the present study we used two non-anaesthetized experimental models, which allowed us to obtain a body of evidence regarding the neurotransmitter substance involved in mediating the chemoreflex at the level of the NTS without the depressant effects of anaesthesia. The awake rat model has permitted microinjections into the commissural NTS and recording of both cardiovascular and respiratory variables, during chemoreflex activation. While this model has provided great insight and gives definitive measures of cardiorespiratory variables there are limitations. One of these was the change in baseline arterial pressure following antagonism of excitatory amino acid receptors in NTS. As this may have stimulated the baroreflex, and compounded interpretation of the data, we used i.v. infusions of sodium nitroprusside to restore pressure levels. Another limitation in awake animals is the technical difficulty of making sympathetic nerve recording while microinjecting. Finally, it is not a trivial task to correctly centre microinjections into the commissural NTS thus a large number of animals is required in order to obtain sufficient statistical power. The employment of the decerebrated WHBP has assisted in overcoming most of these problems. As with the awake rats, it is free of anaesthetic although this is at the expense of losing forebrain structures. It is relatively easy to access the thoracic sympathetic chain as well as the phrenic nerve thereby avoiding technical problems of plethysmography (i.e. movement-related artifacts). Further, the success of correctly targeting the commissural NTS with microinjections is vastly improved. Additionally, the perfusion pressure was not affected significantly by our NTS microinjections thereby avoiding co-activation of baroreceptors (see Figs 9 and 11).
The site of microinjections into the NTS has an important bearing on the specific subregions of the NTS involved in processing the cardiovascular and respiratory responses to chemoreflex activation. In the groups of awake rats fitted with three guide cannulae, the rationale was to cover a large rostro-caudal aspect of the commissural NTS in an attempt to ensure that most of the neural circuitry involved in processing the sympathoexcitatory component of the reflex was reached by the drugs. We found that the placement of cannulae in different aspects of the commissural NTS did not make a difference with respect to blocking the chemoreflex-evoked sympathoexcitatory response. However, because microinjections of KYN and PPADS were only made into the caudal commissural NTS in the experimental protocols in which the ventilatory response was evaluated [awake rats (groups 5 and 6) and in the WHBP], we cannot rule out the possibility that this response component depends on neuronal circuitry in the rostral and/or rostral and caudal aspects of the commissural NTS. We used a 50 nl volume and based on previous observations believe that this is not enough to spread with effective antagonism into the rostral aspect of the commissural NTS. Therefore, further studies involving combined microinjections of KYN and PPADS into different subregions of the NTS are required for a more complete evaluation of the role of these two neurotransmitters systems in the processing of the respiratory component of the chemoreflex at the commissural NTS level.
Transmission in NTS for chemoreflex-mediated responses
Our microinjections were aimed into regions of the NTS known to contain the first synapse of peripheral chemoreceptor afferents. These were shown previously to reside within the commissural NTS (Donoghue et al. 1984; Finley & Katz, 1992; Mifflin, 1992; Ciriello et al. 1994; Chitravanshi et al. 1994; Chitravanshi & Sapru, 1995; Paton et al. 2001). Microinjections were made into its rostro-caudal extent spanning approximately 1 mm from the rostral aspect of the area postrema to approximately 0.5 mm caudal to CS. Using three guide cannulae (group 3 of awake rats) we were able to target this region. In awake rats (groups 2 and 5) and the WHBP, bilateral microinjections were placed into the rostral or caudal aspect of the commissural NTS, respectively. These regions coincide with afferent termination (see above for references) as well as c-FOS expression in response to continuous activation of the chemoreflex (Cruz et al. 2004)
Our previous NTS microinjection studies in both awake rats as well as in the in situ WHBP have all failed to block the pressor/sympathoexcitatory response to chemoreflex activation. These have included using microinjections of excitatory amino acid antagonists (Haibara et al. 1995, Haibara 1999; Machado & Bonagamba, 2005; Braga & Machado, 2006), GABA agonists (Callera et al. 1997, Callera 1999), glycine (Pimentel et al. 2003), an antagonist of adenosine receptors (de Paula & Machado, 2001) and a substance P receptor antagonist (Zhang et al. 2000). Bilateral microinjection of KYN into the NTS produced only a mild reduction in the magnitude of the pressor response to chemoreflex activation, which is likely to be due to the large increase in baseline MAP caused by the blockade of the sympathoinhibitory pathways of the baroreflex (Haibara et al. 1999). In a recent study, we verified that following an infusion of SNP (i.v.) in order to normalize the increase in MAP caused by KYN, the magnitude of the pressor response to chemoreflex activation was similar to the control response (Machado & Bonagamba, 2005). In agreement with these studies, we demonstrated (Braga & Machado, 2006) that sequential antagonism of both ionotropic and metabotropic glutamate receptors in the commissural NTS did not affect the sympathoexcitation following peripheral chemoreceptor activation in the WHBP. In summary, we proposed that an additional neurotransmitter in the NTS may take part in mediating the sympathoexcitatory component of the chemoreflex. Considering that: (a) the pattern of the pressor response to microinjection of ATP into the NTS is similar to the chemoreflex response following activation with KCN (de Paula et al. 2004), and (b) both ATP and l-glutamate can interact in the central nervous system (Gu & Macdermott, 1997; de Paula et al. 2004; Shigetomi & Kato, 2004; Jin et al. 2004), the present study was based on the notion that both P2 and ionotropic glutamate receptors in the NTS participate in the expression of the sympathoexcitatory component of the chemoreflex. Considering that the respiratory response is an important component of the chemoreflex, we also assessed its dependence on ionotropic glutamate and P2 receptors. Our findings that (a) the pressor response to chemoreflex activation was almost abolished by combined microinjection of PPADS and KYN into the commissural NTS in awake rats and (b) that the sympathoexcitatory response to chemoreflex activation was abolished in the WHBP by this double antagonism in NTS strongly supports the hypothesis that these two neurotransmitters play a pivotal role in processing the sympathoexcitatory component of this reflex. However, these receptors appear not to be critical for processing the reflex respiratory response, at least in the commissural NTS.
With respect to the parasympathetic component of the peripheral chemoreflex it seems to be mediated by NMDA receptors within the NTS as it can be blocked in a dose-dependent manner by microinjection of 2-amino-5-phosphonovaleric acid (AP-5), a selective NMDA receptor antagonist, as well as by KYN, a non-selective excitatory amino acid receptor antagonist (Haibara et al. 1995, Haibara 1999; Machado & Bonagamba, 2005). We also have evidence that P2 receptors participate in mediating the parasympathetic component of the chemoreflex because NTS microinjection of either PPADS or suramin into the rostral and caudal aspects of the commissural NTS of the WHBP attenuated the reflex bradycardic response (Paton et al. 2002). In the present experiments performed in the WHBP, microinjections of PPADS, which were histologically proven to be restricted to the commissural NTS, had no effect on the chemoreflex bradycardia indicating that P2 receptors need to be blocked in both the rostral and caudal aspects of the commissural NTS subregions.
Putative mechanisms of action of purinergic cotransmission in NTS
While there is evidence concerning the neuronal mechanisms by which ATP acts on P2 receptors in the NTS, its specific involvement in mediating the autonomic and respiratory components of the chemoreflex is not fully understood. In an intracellular study performed on unidentified NTS neurons in vitro, Kato & Shigetomi (2001) showed that ATP increased the spontaneous postsynaptic currents, which were blocked by PPADS indicating that this excitatory effect was mediated by P2X receptors. More recently, Jin et al. (2004) showed that P2 receptor activation with ATP or α,β-methylene ATP in the NTS facilitated presynaptic glutamate release. This is consistent with studies by Shigetomi & Kato (2004) who showed that activation of presynaptic P2X receptors with α,β-methylene ATP triggered Ca2+-dependent glutamate release in NTS. Thus, one possibility is that ATP acting presynaptically, induces glutamate release in the NTS to mediate the chemoreflex sympathoexcitatory/pressor response. As ATP can be co-released with other neurotransmitters (Burnstock, 1986), it is possible that ATP and l-glutamate are both involved in processing both the parasympathetic and sympathetic components of the chemoreflex. This suggestion is based on findings from the present study whereby PPADS and KYN were both required to abolished the pressor/sympathoexcitation response whereas either KYN alone (Machado & Bonagamba, 2005; Braga & Machado, 2006) or PPADS alone (present study) did not affect the pressor/sympathoexcitatory response to chemoreflex activation.
With respect to the specificity of P2 receptors in NTS for mediating visceral reflexes, we showed that a P2 receptor antagonist selectively blocked the peripheral chemoreceptor-evoked excitatory synaptic input to a subpopulation of NTS neurons but not a convergent excitatory synaptic input evoked by stimulation of pharyngo-oesophageal receptors (Paton et al. 1999, Paton 2002). On the other hand, studies by Scislo et al. (1998) showed that microinjection of suramin into the NTS abolished the baroreflex regulation of HR. Thus, it is possible that P2 receptors in the NTS are not restricted to transmission of the chemoreflex but that they may modulate the parasympathetic component of the baroreflex as well, suggesting a degree of organization within the NTS that independently modulates the autonomic components of these reflexes. It is also important to emphasize that it is unlikely that the chemoreflex afferents are the only source of fibres in NTS that can release ATP as studies by Dale et al. (2002) showed that ATP is released within the NTS during the defence response evoked by hypothalamic stimulation.
Our data showing the involvement of both P2 and ionotropic glutamate receptors in the neurotransmission of the chemoreflex within the commissural NTS is consistent with the concept presented in several recent studies suggesting that ATP appears linked to chemoreception in general. This is based on the findings that ATP plays a transduction role in both central chemoreception in the rostral ventrolateral medulla as well as in peripheral chemoreception at the level of the carotid body glomus cells (Rong et al. 2003; Gourine et al. 2003, Gourine 2005; Spyer et al. 2004; Zhang & Nurse, 2004).
NTS transmitters and the respiratory responses to chemoreflex activation
The data of the present study obtained in both awake rats and the WHBP showed that combined antagonism of ionotropic glutamate and P2 receptors in the most caudal aspect of the commissural NTS produced no significant changes in the tachypnoeic response to chemoreflex activation. We suggest the following two possibilities: (a) l-glutamate and ATP may not be the neurotransmitters involved in the commissural NTS for processing the reflex ventilatory response; and (b) in spite of its involvement in transmitting the sympathoexcitatory component of the chemoreflex, the caudal commissural NTS may not be the subregion of this nucleus to mediate the ventilatory response. Therefore, further studies are required to evaluate the involvement of other transmitters, possible combinations and/or NTS subregions, including the most rostral aspect of the commissural NTS, for processing the chemoreflex-evoked tachypnoea.
Final remarks
Based on the data presented, our hypothesis that neurotransmission of the sympathoexcitatory component of the chemoreflex involves neurotransmitters other than l-glutamate alone within the NTS seems to hold. Moreover, both ionotropic glutamate and P2 receptors in the commissural NTS play a key role in the complex neurochemical mechanisms expressing the pattern of cardiovascular responses to chemoreflex activation. This might suggest redundancy within the NTS as, based on our findings, if one of these systems (glutamatergic or purinergic) fails, this reflex remains functional. We propose that this emphasizes the vital importance of this protective reflex and its essential importance for life. Further, our findings may now provide novel clues for designing pharmacological therapy for reducing sympathoexcitation during conditions of heightened peripheral chemoreflex afferent drive such as obstructive sleep apnoea and heart failure.
Acknowledgments
The authors thank Rubens F. Melo for histological technical assistance. In Brazil, these studies were funded by Fundação de Amparo á Pesquisa do Estado de São Paulo (FAPESP) (2001/11190-8, 2004/03285-7) and Conselho Nacional de Desenvolvimento Científico e tecnológico (CNPQ) (522150-95-0). In the UK, J.F.R.P. was in receipt of a Royal Society Wolfson Research Merit Award and supported by the British Heart Foundation.
References
- Antunes VR, Bonagamba LGH, Machado BH. Hemodynamic and respiratory responses to microinjection of ATP into the intermediate and caudal commissural NTS of awake rats. Brain Res. 2005a;1032:85–93. doi: 10.1016/j.brainres.2004.10.048. [DOI] [PubMed] [Google Scholar]
- Antunes VR, Braga VA, Machado BH. Autonomic and respiratory responses to microinjection of ATP into the intermediate or caudal nucleus tractus solitarius in the working heart–brainstem preparation of the rat. Clin Exp Pharmacol Physiol. 2005b;32:467–472. doi: 10.1111/j.1440-1681.2005.04213.x. [DOI] [PubMed] [Google Scholar]
- Barraco RA, O'Leary DS, Ergene E, Scislo TJ. Activation of purinergic receptor subtypes in the nucleus tractus solitarius elicits specific regional vascular response patterns. J Auton Nerv Syst. 1996;59:113–124. doi: 10.1016/0165-1838(96)00014-8. [DOI] [PubMed] [Google Scholar]
- Barros RA, Bonagamba LGH, Okamoto-Canesin R, de Oliveira M, Branco LGS, Machado BH. Cardiovascular responses to chemoreflex activation with potassium cyanide or hypoxic hypoxia in awake rats. Auton Neursci. 2002;97:110–115. doi: 10.1016/s1566-0702(02)00050-4. [DOI] [PubMed] [Google Scholar]
- Boscan P, Paton JFR. Nociceptive afferents selectively modulate the cardic component of the peripheral chemoreceptor reflex via actions within the solitary tract nucleus. Neuroscience. 2001;110:319–328. doi: 10.1016/s0306-4522(01)00585-1. [DOI] [PubMed] [Google Scholar]
- Braga VA, Antunes VR, Machado BH. Autonomic and respiratory responses to microinjection of L-glutamate into the commissural subnucleus of the NTS in the working heart-brainstem preparation of the rat. Brain Res. 2006;1093:150–160. doi: 10.1016/j.brainres.2006.03.105. [DOI] [PubMed] [Google Scholar]
- Braga VA, Machado BH. Chemoreflex sympathoexcitation is not altered by the antagonism of glutamate receptors in the commissural NTS in the working heart-brainstem preparation of rat. Exp Physiol. 2006;91:551–559. doi: 10.1113/expphysiol.2005.033100. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Phamacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- Burnstock G. Purines and cotransmitters in adrenergic and cholinergic neurons. Prog Brain Res. 1986;68:193–203. doi: 10.1016/s0079-6123(08)60239-3. [DOI] [PubMed] [Google Scholar]
- Callera JC, Bonagamba LGH, Nosjean A, Laguzzi R, Machado BH. Acitivation of GABAA but not GABAB receptors in the NTS blocked bradycardia of chemoreflex in awake rats. Am J Physiol Heart Circ Physiol. 1999;276:H1902–H1910. doi: 10.1152/ajpheart.1999.276.6.H1902. [DOI] [PubMed] [Google Scholar]
- Callera JC, Sévoz C, Laguzzi R, Machado BH. Microinjection of serotonin3 receptor agonist into the NTS of unanesthetized rats inhibits the cardiovagal component of the baro- and chemoreflex. J Auton Nerv Syst. 1997;63:127–136. doi: 10.1016/s0165-1838(96)00140-3. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Kachroo A, Sapru HN. A midline area in the nucleus commissuralis of NTS mediates the phrenic nerve responses to carotid chemoreceptor stimulation. Brain Res. 1994;662:127–133. doi: 10.1016/0006-8993(94)90804-4. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. Chemoreceptor-sensitive neurons in commissural subnucleus of nucleus tractus solitarius of the rat. Am J Physiol Regul Integr Comp Physiol. 1995;268:R851–R858. doi: 10.1152/ajpregu.1995.268.4.R851. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Hochstenbach SL, Roder S. Central projections of baroreceptor and chemoreceptor afferent fibers in the rat. In: Robin I, Barraco A, editors. Nucleus of the Solitary Tract. Boca Raton, FL: CRC Press; 1994. pp. 35–50. [Google Scholar]
- Colombari E, Bonagamba LGH, Machado BH. Mechanisms of pressor and bradycardic responses to L-glutamate microinjected into the NTS of conscious rats. Am J Physiol Regul Integr Comp Physiol. 1994;266:R730–R738. doi: 10.1152/ajpregu.1994.266.3.R730. [DOI] [PubMed] [Google Scholar]
- Colombari E, Bonagamba LGH, Machado BH. NMDA receptor antagonist blocks the bradycardic but not the pressor response to L-glutamate microinjected into the nucleus tractus solitarius (NTS) of unanesthetized rats. Brain Res. 1997;749:209–213. doi: 10.1016/S0006-8993(96)01169-9. [DOI] [PubMed] [Google Scholar]
- Cruz JC, Bonagamba LGH, Stern JE, Machado BH. FOS-. expression in NTS and PVN neurons in response to chemoreflex activation in awake rats. FASEB J. 2004;18:A667. doi: 10.1016/j.autneu.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Dale N, Gourine AV, Llaudet E, Bulmer D, Thomas T, Spyer KM. Rapid adenosine release in the nucleus tractus solitarii during defence response in rats: real-time measurement in vivo. J Physiol. 2002;544:149–160. doi: 10.1113/jphysiol.2002.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula PM, Antunes VR, Bonagamba LGH, Machado BH. Cardiovascular responses to microinjection of ATP into the nucleus tractus solitarii of awake rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1164–R1171. doi: 10.1152/ajpregu.00722.2003. [DOI] [PubMed] [Google Scholar]
- de Paula PM, Machado BH. Antagonism of adenosine A1 receptors in the nucleus tractus solitarii do not affect the chemoreflex in awake rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R2072–R2078. doi: 10.1152/ajpregu.2001.281.6.R2072. [DOI] [PubMed] [Google Scholar]
- Donoghue S, Felder R, Jordan D, Spyer KM. The central projection of carotid baroreceptor and chemoreceptor in the cat: a neurophysiological study. J Physiol. 1984;340:397–410. doi: 10.1113/jphysiol.1984.sp015072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergene E, Dunbar JC, O'Leary DS, Barraco RA. Activation of P2-purinoceptors in the nucleus tractus solitarius mediates depressor responses. Neurosci Lett. 1994;174:188–192. doi: 10.1016/0304-3940(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent neurons of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Franchini KG, Krieger EM. Carotid chemoreceptors influence arterial pressure in intact and aortic denervated rat. Am J Physiol Regul Integr Comp Physiol. 1992;262:R677–R683. doi: 10.1152/ajpregu.1992.262.4.R677. [DOI] [PubMed] [Google Scholar]
- Franchini KG, Krieger EM. Cardiovascular responses of conscious rats to carotid body chemoreceptor stimulation by intravenous KCN. J Auton Nerv Syst. 1993;42:63–70. doi: 10.1016/0165-1838(93)90342-r. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Atkinson L, Deuchars J, Spyer KM. Purinergic signaling in the medullary mechanisms of respiratory control in the rat: respiratory neurons express the P2X2 receptor subunit. J Physiol. 2003;552:197–211. doi: 10.1113/jphysiol.2003.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapse. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- Haibara AS, Bonagamba LGH, Machado BH. Neurotransmission of the sympatho-excitatory component of chemoreflex in the nucleus tractus solitarii of unanesthetized rats. Am J Physiol Regul Integr Comp Physiol. 1999;276:R69–R80. [Google Scholar]
- Haibara AS, Colombari E, Chianca DA, Jr, Bonagamba LGH, Machado BH. NMDA receptors in NTS are involved in bradycardic but not in pressor response of chemoreflex. Am J Physiol Heart Circ Physiol. 1995;269:H1421–H1427. doi: 10.1152/ajpheart.1995.269.4.H1421. [DOI] [PubMed] [Google Scholar]
- Jin Y-H, Bailey TW, Li B-Y, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci. 2004;24:4709–4717. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato F, Shigetomi E. Distinct modulation of evoked and spontaneous EPSCs by purinoceptors in the nucleus tractus solitarii of the rat. J Physiol. 2001;530:469–486. doi: 10.1111/j.1469-7793.2001.0469k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Burnstock G. Ultrastructural localization of P2X3 receptors in rat sensory neurons. Neuroreport. 1998;9:2545–2550. doi: 10.1097/00001756-199808030-00022. [DOI] [PubMed] [Google Scholar]
- Machado BH. Chemoreflex and sympathoexcitation. In: Dun NJ, Machado BH, Pilowsky PM, editors. Neural Mechanisms of Cardiovascular Regulation. Norwell, MA: Kluwer Academic Publishers; 2004. [Google Scholar]
- Machado BH, Bonagamba LGH. Microinjection of L-glutamate into the nucleus tractus solitarii increases arterial pressure in conscious rats. Brain Res. 1992;576:131–138. doi: 10.1016/0006-8993(92)90618-j. [DOI] [PubMed] [Google Scholar]
- Machado BH, Bonagamba LGH. Antagonism of glutamate receptors in the intermediate and caudal commissural NTS of awake rats produced no changes in the hypertensive response to chemoreflex activation. Auton Neurosci. 2005;117:25–32. doi: 10.1016/j.autneu.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Malan A. Ventilation measured by body pletysmography in hibernating mammals and poikilotherms. Respir Physiol. 1973;17:32–44. doi: 10.1016/0034-5687(73)90108-4. [DOI] [PubMed] [Google Scholar]
- Michelini LC, Bonagamba LGH. Baroreceptor reflex modulation by vasopressin microinjected into the nucleus tractus solitarii of conscious rats. Hypertension. 1988;11:75–79. doi: 10.1161/01.hyp.11.2_pt_2.i75. [DOI] [PubMed] [Google Scholar]
- Mifflin SW. Arterial chemoreceptor input to nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 1992;263:R368–R375. doi: 10.1152/ajpregu.1992.263.2.R368. [DOI] [PubMed] [Google Scholar]
- Paton JFR. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Paton JFR, de Paula PM, Spyer KM, Machado BH, Boscan P. Sensory afferent selective role of P2 receptors in the nucleus tractus solitarii for mediating the cardiac component of the peripheral chemoreceptor reflex. J Physiol. 2002;543:995–1005. doi: 10.1113/jphysiol.2002.021923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JFR, Deuchars J, Li Y-W, Kasparov S. Properties of solitary tract neurones responding to peripheral arterial chemoreceptor. Neuroscience. 2001;105:231–248. doi: 10.1016/s0306-4522(01)00106-3. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Li YW, Kasparov S. Reflex response and convergence of pharyngoesophageal and peripheral chemoreceptors in the nucleus of the solitary tract. Neuroscience. 1999;93:143–154. doi: 10.1016/s0306-4522(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Phillis JW, Scislo TJ, O'Leary DS. Purines and the nucleus tractus solitarius: effects on cardiovascular and respiratory function. Clin Exp Pharmacol Physiol. 1997;24:738–742. doi: 10.1111/j.1440-1681.1997.tb02124.x. [DOI] [PubMed] [Google Scholar]
- Pimentel FF, Bonagamba LGH, Machado BH. Pressor response to chemoreflex activation before and after microinjection of glycine into the NTS of awake rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1000–R1009. doi: 10.1152/ajpregu.00310.2002. [DOI] [PubMed] [Google Scholar]
- Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM, Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of ATP-gated ion chanel mediating ventilatory responses to hypoxia. J Neurosci. 2003;23:11315–11321. doi: 10.1523/JNEUROSCI.23-36-11315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scislo TJ, Augustyniak RA, Barraco RA, Woodbury DJ, O'Leary DS. Activation of P2X-purinoceptors in the nucleus tractus solitarii elicits differential inhibition of lumbar and renal sympathetic nerve activity. J Auton Nerv Syst. 1997;62:103–110. doi: 10.1016/s0165-1838(96)00116-6. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, Ergene E, O'Leary DS. Impaired arterial baroreflex regulation of heart rate after blockade of P2-purinoceptors in nucleus tractus solitarius. Brain Res Bull. 1998;47:63–67. doi: 10.1016/s0361-9230(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, O'Leary DS. Differential control of renal vs. adrenal sympathetic nerve activity by NTS A2a and P2x purinoceptors. Am J Physiol Heart Circ Physiol. 1998;275:H2130–H2139. doi: 10.1152/ajpheart.1998.275.6.H2130. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Kato F. Action potential-independent release of glutamate by Ca2+ entry through presynaptic P2X receptors elicits postsynaptic firing in the brainstem autonomic network. J Neurosci. 2004;24:3125–3135. doi: 10.1523/JNEUROSCI.0090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyer KM, Dale N, Gourine AV. ATP is a key mediator of central and peripheral chemosensory transduction. Exp Physiol. 2004;89:53–59. doi: 10.1113/expphysiol.2003.002659. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedi MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- Yao ST, Barden JA, Finkelstein DI, Bennett MR, Lawrence AJ. Comparative study on the distribution patterns of P2X1-P2X6 receptor immunoreactivity in the brainstem of the rat and the common marmoset (Callithrix jacchus): association with catecolamine cell groups. J Comp Neurol. 2000;427:485–507. [PubMed] [Google Scholar]
- Zhang CH, Bonagamba LGH, Machado BH. Blockade of NK-1 receptors in the lateral commissural nucleus tractus solitarii of awake rats had no effect on the cardiovascular responses to chemoreflex activation. Braz J Med Biol Res. 2000;33:1379–1385. doi: 10.1590/s0100-879x2000001100018. [DOI] [PubMed] [Google Scholar]
- Zhang M, Nurse CA. CO2/pH chemosensory signaling in co-cultures of rat carotid body and petrosal neurons: role of ATP and Ach. J Neurophysiol. 2004;92:3433–3445. doi: 10.1152/jn.01099.2003. [DOI] [PubMed] [Google Scholar]