Abstract
Embryonic stem cell-derived cardiomyocytes (ESdCs) have been proposed as a source for cardiac cell-replacement therapy. The aim of this study was to determine the Ca2+-handling mechanisms that determine the frequency and duration of spontaneous Ca2+ transients in single ESdCs. With laser scanning confocal microscopy using the Ca2+-sensitive dye Fluo-4/AM, we determined that spontaneous Ca2+ transients in ESdCs at the onset of beating (day 9) depend on Ca2+ entry across the plasma membrane (50%) whereas Ca2+-induced Ca2+ release is the major contributor to Ca2+ transients in ESdCs after 16 days (72%). Likewise, Ca2+ extrusion in 9-day-old ESdCs depends on Na+–Ca2+ exchange (50.0 ± 8%) whereas Ca2+ reuptake by the sarco(endo)plasmic Ca2+ ATPase (72 ± 5%) dominates in further differentiated cells. Spontaneous Ca2+ transients were suppressed by the inositol-1,4,5-trisphosphate (IP3) receptor (IP3R) blocker 2-aminoethoxydiphenyl borate (2-APB) and the phospholipase C blocker U73122 but continued in the presence of caffeine. Stimulation of IP3 production by phenylephrine or endothelin-1 had a positive chronotropic effect that could be reversed by U73122 and 2-APB. The presence of Ca2+-free solution and block of L-type Ca2+ channels by nifedipine also resulted in a cessation of spontaneous activity. Overall, IP3R-mediated Ca2+ release in ESdCs is translated into a depolarization of the plasma membrane and a whole-cell Ca2+ transient is subsequently induced by voltage-dependent Ca2+ influx. Although ryanodine receptor-mediated Ca2+ release amplifies the IP3R-induced trigger for the Ca2+ transients and modulates its frequencies, it is not a prerequisite for spontaneous activity. The results of this study offer important insight into the role of IP3R-mediated Ca2+ release for pacemaker activity in differentiating cardiomyocytes.
In early embryonic heart as well as in the adult sinoatrial (SA) node, spontaneous pacemaker activity strongly depends on the presence of Ca2+ signalling mechanisms (Sakai et al. 1983; Komuro et al. 1985; Bogdanov et al. 2001; Lipsius et al. 2001). In the early embryonic heart, spontaneous activity was shown to be initiated by Ca2+ influx, whereas in the rabbit or cat SA node, the rhythmic increase in spontaneous ryanodine receptor (RyR)-mediated Ca2+ release events and subsequent activation of the Na+/Ca2+ exchanger (NCX) seem to be the driving force for the diastolic depolarization (Huser et al. 2000; Bogdanov et al. 2001). One reason for the differences in the pacemaker mechanism could be the developmental changes in the Ca2+ handling mechanisms. While voltage-dependent L-type Ca2+ channels are expressed from day 9.5 days post coitum (dpc) (Takemura et al. 2005), T-type channels have been observed around embryonic day 14 (Cribbs et al. 2001). Influx through L-type channels is thought to be the major source of Ca2+ for excitation–contraction coupling (ECC) in early embryonic cardiomyocytes, whereas the main source of Ca2+ in adult cells during ECC is RyR-mediated Ca2+-induced Ca2+ release (CICR) (Escobar et al. 2004; Perez et al. 2005). The cause for this seems to be the rudimentary development of the sarcoplasmic reticulum (SR) and the low expression of RyRs (Gorza et al. 1997; Rosemblit et al. 1999; Escobar et al. 2004). The role of inositol-1,4,5-trisphosphate (IP3) receptor (IP3R)-mediated Ca2+ release in cardiac muscle is still debated. Recent studies have suggested a role in the generation of arrhythmic activity in atrial and ventricular myocytes or in the defined activation of cardiac transcription factors (Zima & Blatter, 2004; Proven et al. 2006; Wu et al. 2006). However, it seems that IP3-mediated Ca2+ release through IP3R channels may play a more prominent role in embryonic cardiomyocytes. IP3 production has been linked to a specific perinuclear Ca2+ release and shown to regulate mitochondrial Ca2+ uptake in neonatal rat cardiomyocytes (Jaconi et al. 2000).
Embryonic stem cells differentiate in vitro into spontaneously active multicellular cardiomyocyte preparations while recapitulating developmental stages of the early embryonic cardiomyogenesis (Boheler et al. 2002; Hescheler et al. 2002; Banach et al. 2003). They express cardiac-specific marker proteins (e.g. αMHC and MLC-2v) and transcription factors (e.g. Nkx-2.5, MEF2C and GATA-4) (Narita et al. 1997; Boheler et al. 2002; Wobus & Boheler, 2005). Shortly after the onset of spontaneous synchronized beating, the proliferation of the embryonic stem cell-derived cardiomyocytes (ESdCs) ceases and the multicellular aggregates expand by cell growth (Meyer et al. 2000; Banach et al. 2003; He et al. 2003; Puceat et al. 2003). Intracellular recordings from individual ESdCs dissociated from aggregates of beating cells show that different cellular phenotypes such as sinus node-like, atrial-like and ventricular-like cells develop (Maltsev et al. 1999; Abi-Gerges et al. 2000; Fijnvandraat et al. 2003; He et al. 2003). With the in vitro differentiation of ESdCs, expression of the Ca2+-handling proteins such as RyR (cardiac type-2 isoform), NCX1, sarcoplasmic reticulum Ca2+ ATPase (SERCA)2 and phospholamban has been described (Boheler et al. 2002; Fijnvandraat et al. 2003). Furthermore, the following voltage-dependent currents have been shown in ESdCs: If (Gryshchenko et al. 1999; Abi-Gerges et al. 2000), Ito and Isus (Gryshchenko et al. 2000), and INa, ICa,T and IL,Ca (Abi-Gerges et al. 2000; Zhang et al. 2003). Pacemaker activity in ESdC aggregates, connexin expression and conduction velocity also follow closely the development of the embryonic heart (Banach et al. 2003) and pacemaker frequency is responsive to the neurohumoral stimuli isoprenaline and acetylcholine (Ji et al. 1999; Abi-Gerges et al. 2000; Banach et al. 2003).
The spontaneous activity in early embryonic cardiomyocytes is thought to be significantly regulated by If (Satoh & Sperelakis, 1993; Robinson et al. 1997); however, it also depends critically on the presence of Ca2+ in the extracellular solution (Fujii et al. 1981a,Fujii 1981b). It has been proposed that intracellular Ca2+ oscillations in ESdCs are independent of voltage-activated Ca2+ influx (Viatchenko-Karpinski et al. 1999; Mery et al. 2005). RyR-mediated Ca2+ release has been demonstrated by the presence of Ca2+ sparks and caffeine transients in ESdCs (Sauer et al. 2001) and RyR-mediated Ca2+ release was shown to contribute to their pacemaker activity (Fu et al. 2006). However, spontaneous whole-cell Ca2+ transients continue in the presence of caffeine and in ESdCs deficient for Ry R2 expression (Sauer et al. 2001; Yang et al. 2002). By contrast, specific blocking or down-regulation of IP3R type 1 suppresses Ca2+ signalling and automaticity of ESdCs and embryonic heart tubes (Mery et al. 2005). Until now, neither the basis for IP3R activation nor the mechanism by which IP3R-mediated Ca2+ release results in the spontaneous whole-cell Ca2+ transients of the ESdCs has been determined.
In the present study, we tested the hypothesis that the developmental changes in the Ca2+ handling mechanisms of ESdCs modulate the frequency of their spontaneous activity, and that IP3-mediated Ca2+ release needs to be translated into a depolarization of the membrane potential (Vm) and subsequent activation of voltage-dependent Ca2+ channels to generate a whole-cell Ca2+ transient.
Methods
Culture and differentiation of mouse embryonic stem cells
For the experiments we used Mouse embryonic stem cells (mES) of the cell line CMV (Specialty Media; Phillipsburg, NJ, USA) transfected with a neomycin resistance gene expressed under the promoter of αMHC (Klug et al. 1996; Strom et al. 2002). The cells were propagated in culture in the presence of leukaemia inhibiting factor (ESGRO; 1000 U ml−1; Chemicon Int., Temecula, CA, USA) and differentiated as embryoid bodies (EBs) (Nagy et al. 1993; Hescheler et al. 2002; Banach et al. 2003). EBs were plated on day 7 and spontaneously beating aggregates of cardiomyocytes were observed around day 8 (Banach et al. 2003). Neomycin (300 μg ml−1) was added to the differentiating cells at day 9 of culture to select for cardiomyocytes. In early preparations (day 8–10) in which non-cardiomyocytes were not yet entirely removed by neomycin treatment, beating aggregates were microdissected under optical control. For measurements of [Ca2+]i, cardiomyocyte aggregates were collected and dissociated as previously described (Maltsev et al. 1993). Briefly, the aggregates were washed in a Ca2+- and Mg2+-free phosphate-buffered saline solution (Sigma-Aldrich, St Louis, MO, USA). After 60 min incubation in collagenase (1 mg ml−1 at 37°C; Sigma-Aldrich), the cells were incubated in Kraftbrühe (KB) solution [Klockner et al. 1999; 60 min, room temperature (20°C)]. Dissociated cells were plated onto gelatine-coated coverslips in Iscove medium supplemented with 20% fetal bovine serum (Invitrogen, Frederick, MD, USA). Experiments were carried out on the first day after dissociation. Older ESdC aggregates contain cells with different cardiac phenotypes; however, it was demonstrated that the promoter αMHC is mainly activated in sinus nodal-like and atrial-like ESdCs (Kolossov et al. 2005). By using αMHC–neomycin selected cells we can assume that in our cultures sinus nodal-like cells and atrial-like cells dominate and that the experiments were performed on spontaneously active sinus nodal-like ESdCs.
Intracellular Ca2+ measurements
To visualize changes in the intracellular Ca2+ concentration, ESdCs were incubated (15 min) at room temperature with fluo-4 acetoxymethyl ester (5 μm; fluo-4/AM; Invitrogen, Eugene, OR, USA). After washout, 15 min were allowed for de-esterification of the dye. Ca2+ measurements were performed as previously described (Sheehan & Blatter, 2003). Whole-cell Ca2+ transients were obtained from confocal linescan images through single ESdCs by averaging the signal of an individual cell. Ca2+ transients are presented as background-subtracted normalized fluorescence (F/F0).
Solutions
During the experiments cells were superfused with a Tyrode solution containing (mm): NaCl 140, KCl 5.4, CaCl2 1.5, MgCl2 1.5, glucose 10 and Hepes 5; pH adjusted to 7.38 with NaOH. To induce a depolarization of the cell Membrane potential (Vm) we used a ‘high-K+’ solution containing (mm): KCl 140, NaCl 5.4, MgCl2 1.5, glucose 10 and Hepes 5; pH adjusted to 7.38 with KOH. To determine the role of the NCX for spontaneous whole-cell Ca2+ transients, we superfused the ESdCs with a ‘low-Na+’ solution containing (mm): LiCl 128, NaCl 12, KCl 5.4, MgCl2 1.5, glucose 10 and Hepes 5; pH adjusted to 7.38 with LiOH. In both cases Ca2+ was omitted from the solutions.
Endothelin-1, phenylephrine, tetracaine, nifedipine, NiCl, BAPTA-AM, U73122 and caffeine were purchased from Sigma. Fluo-4/AM and thapsigargin were purchased from Invitrogen. Data sets were statistically evaluated using an unpaired t test. All averaged data are presented as means ± s.e.m.
Results
ESdCs exhibit spontaneous whole-cell Ca2+ transients
Embryonic stem cells develop into multicellular beating aggregates of cardiomyocytes. After dissociation, single cells maintain their spontaneous activity and rhythmic changes of [Ca2+]i can be recorded (Viatchenko-Karpinski et al. 1999). To determine the characteristics of this spontaneous activity, we monitored Ca2+ transients in ESdCs on concomitant days of differentiation. Changes in [Ca2+]i were recorded in Fluo-4/AM-loaded ESdCs with the linescan mode of the confocal microscope (4.37 ms line−1). The scan line was positioned through the centre of the cell and the fluorescence was integrated over the entire width of the cell for quantification of the transients. Rhythmic spontaneous increases in [Ca2+]i were detected in all ESdCs examined and representative examples of linescans recorded from an ESdCs at 11 days and a 17 days, respectively, are shown in Fig. 1A and B. To distinguish between AP-induced Ca2+ transients and spontaneous Ca2+ waves we considered a change in Ca2+ to be a whole-cell Ca2+ transient when Ca2+ increased simultaneously throughout the cell and its rise time did not exceed 67 ms. The frequency of spontaneous whole-cell Ca2+ transients increased with the differentiation of the ESdCs (day 8, 0.25 ± 0.02 Hz, n = 5; day 17, 1.05 ± 0.09 Hz; n = 19; Fig. 1C) whereas the transient duration at 50% amplitude (TD50) decreased (day 8, 600 ± 77 ms, n = 5; day 17, 212 ± 29 ms, n = 19; Fig. 1D). No significant change in transient amplitude (ΔF/F0: day 8, 2.39 ± 0.37, n = 7; day 17, 2.67 ± 0.3, n = 19; Fig. 1E) was observed. Whole-cell Ca2+ transients exhibited a slow diastolic rise of Ca2+ (day 9, 1.2 ± 0.17 d(ΔF/F0) s−1, n = 11; day 17, 5.8 ± 0.66 d(ΔF/F0) s−1, n = 11; Fig. 2A and B). The diastolic rise was followed by a rapid upstroke of [Ca2+]i (day 9, 26.1 ± 5 d(ΔF/F0) s−1, n = 11; day 17, 52.3 ± 4.6 d(ΔF/F0)*s−1, n = 11; Fig. 2A and B). The velocity of diastolic rise and upstroke significantly increased with differentiation of the ESdCs. The data demonstrate a change of the spontaneous Ca2+ transient in differentiating ESdCs.
Figure 1.
Developmental changes in spontaneous whole-cell Ca2+ transients in ESdCs Confocal linescan images and spatially averaged Ca2+ transients recorded from ESdCs isolated on day 11 (A) or day 17 (B) of in vitro differentiation. A slow diastolic increase of [Ca2+]i preceded the rapid upstroke of the Ca2+ transient. While the frequency of the spontaneous Ca2+ transients increased with differentiation (C), the transient duration (TD) at 50% amplitude decreased (D). However, no significant change in transient amplitude (TA) could be detected (E).
Figure 2.
The velocity of diastolic increase and upstroke of whole-cell Ca2+ transients in control conditions and in the presence of caffeine A, representative Ca2+ transients recorded from ESdCs at day 9 (black trace) and day 17 (grey trace) of differentiation. The phases of the diastolic rise and the upstroke are marked underneath the transients. B, comparison of the diastolic rise and upstroke of Ca2+ in ESdCs dissociated on day 9 (n = 6) and day 17 (n = 4) of differentiation. Both parameters exhibit a significant developmental increase (**P < 0.001). C, caffeine (10 mm) slows the diastolic rise of [Ca2+]i in ESdCs on day 9 (n = 5) and day 17 (n = 3). In both cases the decrease is significant (*P < 0.05; **P < 0.001). However, no significant difference in the diastolic rise can be determined in ESdCs between day 9 and day 17 in the presence of caffeine.
Early ESdCs already have intracellular Ca2+ stores
One significant change in the Ca2+ signalling mechanisms is the development of RyR-operated intracellular Ca2+ stores and their contribution to CICR (Gorza et al. 1997; Rosemblit et al. 1999; Escobar et al. 2004). We determined the presence of RyR-operated intracellular stores by superfusing spontaneously active ESdCs with the RyR agonist caffeine (10 mm). Already at the onset of beating (day 8), caffeine induced a rapid rise in [Ca2+]i underlining the presence of RyR-operated intracellular stores. Figure 3A and B shows representative examples of caffeine-induced Ca2+ transients in ESdCs at day 10 and day 17. The amplitude of the caffeine-induced transients (TAcaff) increased with differentiation of the ESdCs (Fig. 3C). The spontaneous activity of ESdCs continued in caffeine in 53 out of 59 experiments (ESdCs day 8–17). However, the amplitude of spontaneous whole-cell Ca2+ transients in caffeine (TAT,caff) was decreased in comparison to control conditions (TAT,ctrl). In ESdCs at day 9, the transients continued at 50% of their control amplitude, whereas the amplitude of twitch transients was reduced by 70% at day 17 (Fig. 3D). The data suggest that although RyR-operated stores are present from the onset of spontaneous activity, their contribution to the whole-cell Ca2+ transient and ECC increases during differentiation.
Figure 3.
RyR-operated Ca2+ stores gain relevance during developmental differentiation of ESdCs Confocal linescan images and spatially averaged Ca2+ transients recorded from ESdCs isolated on day 10 (A) and day 17 (B) of in vitro differentiation. Caffeine (10 mm)-induced Ca2+ transients document the presence of RyR-operated Ca2+ stores. The presence of caffeine does not suppress the occurrence of spontaneous whole-cell Ca2+ transients. C, the amplitude of caffeine-induced transients (TAcaff) increases during in vitro differentiation. D, the ratio of transient amplitude in caffeine (TAT,caff)/transient amplitude control (TAT,ctrl) decreases. The data support an increasing contribution of RyR-mediated Ca2+ release to whole-cell Ca2+ transients during differentiation. E, Ca2+ transient frequency in older ESdCs is significantly decreased in the presence of caffeine.
Ca2+ transients in the presence of caffeine not only exhibited a decrease in amplitude, but a decrease in the slope of the diastolic rise (Fig. 2C). A 38% and 83% reduction in the rise time was determined in ESdCs at day 9 (n = 5) and day 17 (n = 3), respectively, during superfusion with caffeine. This is consistent with a decrease in the transient frequency in the presence of caffeine (Fig. 3E). The data support the idea that although RyR-mediated Ca2+ release is not an absolute prerequisite for the maintenance of spontaneous activity, it increasingly contributes to the diastolic rise of the Ca2+ transient and thereby modulates the frequency.
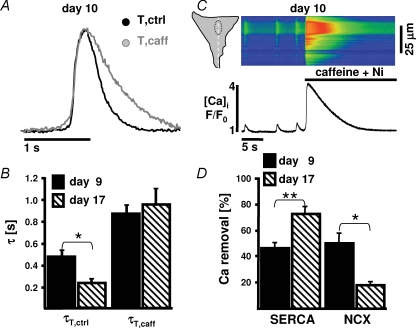
Ca2+ extrusion mechanisms in ESdCs
The minor contribution of the RyR-mediated Ca2+ release to ECC in neonatal cardiomyocytes and ESdCs also suggests a minor role of the intracellular Ca2+ stores for the termination of the Ca2+ transient (Escobar et al. 2004). To determine the role of SERCA-mediated Ca2+ reuptake into the SR and of Ca2+ extrusion via NCX in the termination of the Ca2+ transient, we analysed the time constants of decay for twitch Ca2+ transients in control conditions (τT,ctrl), for twitch transients in the presence of caffeine (10 mm) (τT,caff) (Fig. 4A and B), and for the caffeine-induced Ca2+ transient (τcaff). τT,ctrl significantly decreased during differentiation from day 9 (0.47 ± 0.06 s) to day 17 (0.23 ± 0.03 s; P < 0.05) but no significant developmental change could be detected in τT,caff (day 9, 0.86 ± 0.07 s; day 17, 0.95 ± 0.14 s, P > 0.5; Fig. 4B). We quantified the contribution of SERCA and NCX to the removal of Ca2+ by using the approach described by Fowler et al. (2005) where the contribution of SERCA is described as (ktwitch−kcaffeine)/ktwitch and the contribution of NCX as (kcaffeine−kcaffeine+Ni)/ktwitch (where k is rate constant; 1/τ). Ca2+ transients induced in the presence of nickel ([Ni+]o = 10 mm) had a prolonged time constant of decay (τcaff+Ni; Fig. 4C) due to inhibition of NCX. The analysis reveals that in 9 day old ESdCs, 46.5 ± 5% of Ca2+ extrusion is due to Ca2+ reuptake into caffeine-sensitive intracellular stores whereas 50.0 ± 8% is extruded via NCX (n = 6). This relation shifted in ESdCs at day 17, where 72 ± 5% of Ca2+ is taken up into intracellular stores and only 17 ± 2% is extruded by NCX (n = 9; Fig. 4D). The data support the notion that during developmental differentiation of ESdCs, intracellular Ca2+ stores gain importance for Ca2+ release during ECC and Ca2+ reuptake during the termination of the Ca2+ transient.
Figure 4.
Changing contribution of Ca2+ extrusion and Ca2+ reuptake to the Ca2+ transient decay A, whole-cell Ca2+ transients recorded under control conditions and in the presence of caffeine were normalized and superimposed to visualize the change in the time course of Ca2+ removal. B, the time constants of decay exhibited a significant developmental decrease (*P < 0.05). Superfusion with caffeine in both cases significantly increased the time-dependent decay of Ca2+ but abolished differences in time constant (τ)T,caff between day 9 and day 17. C, confocal linescan image and spatially averaged [Ca2+]i. The time constant of decay of the caffeine transient is further prolonged in the presence of nickel (10 mm). D, estimated contribution of sarco(endo)plasmic Ca2+ ATPase and Na+–Ca2+ exchanger to the removal of Ca2+ after a twitch transient in ESdCs at day 9 (n = 6) and day 17 (n = 9). The increased contribution from SERCA and decreased contribution from NCX was significant from day 9 to day 17 (**P < 0.005; *P < 0.05).
Spontaneous activity in early ESdCs depends on IP3R-mediated Ca2+ release
Experiments in early embryonic heart showed that spontaneous activity depends on the presence of [Ca2+]o. However, in previous experiments on early ESdCs the relevance of IP3R-mediated Ca2+ release, and the independence of spontaneous Ca2+ transients from voltage-activated Ca2+ influx was proposed (Viatchenko-Karpinski et al. 1999; Mery et al. 2005). We aimed to determine the mechanism by which whole-cell Ca2+ transients are generated in ESdCs. Previously, a diastolic rise in intracellular Ca2+ has been postulated to promote spontaneous activity in atrial pacemaker cells (Huser et al. 2000; Bogdanov et al. 2001). To determine whether there is a correlation between the frequency of spontaneous Ca2+ transients and the diastolic rise of Ca2+, we buffered Ca2+ with the membrane-permeable Ca2+ chelator BAPTA/AM (1 μm; Fig. 5A) After 5 min of superfusion, basal [Ca2+]i was decreased by 18.4 ± 1.6% together with a decrease in the frequency of spontaneous Ca2+ transients by 29 ± 5% (Fig. 5B; n = 2) indicating that [Ca2+]i can modulate the frequency of the transients.
Figure 5.
Spontaneous activity in ESdCs is maintained by Ca2+ release from intracellular stores A, spatially averaged changes of [Ca2+]i in an ESdC at 11 days during superfusion with the membrane-permeable Ca2+ chelator BAPTA/AM (1 μm). B, superfusion with BAPTA/AM (5 min) resulted in a significant decrease in the frequency of spontaneous whole-cell Ca2+ transients (n = 2). C, spatially averaged changes of [Ca2+]i in ESdCs with the SERCA inhibitor thapsigargin (1 μm). In a total of three experiments, thapsigargin significantly decreased the decay of the spontaneous Ca2+ transients (at 5 min) and resulted ultimately (8 min) in a block of spontaneous whole-cell transients. Cessation of Ca2+ transients coincided with a depletion of the intracellular stores indicated by the lack of caffeine-induced Ca2+ transients.
Changes in the [Ca2+]i can be due to Ca2+ influx through voltage-dependent Ca2+ channels and/or Ca2+ release from intracellular Ca2+ stores. To determine whether the rise in [Ca2+]i originates from intracellular Ca2+ stores, we superfused spontaneously active cells with the SERCA inhibitor thapsigargin (1 μm) (Thastrup et al. 1990). Figure 5C shows a representative experiment. During the first period of superfusion (∼5 min), spontaneous activity continues in the presence of thapsigargin although a prolonged decay time of the Ca2+ transient could be observed. During prolonged application, Ca2+ transient frequency decreased and the transients ultimately ceased. At this point, the average increase in basal [Ca2+]i was 5% (n = 3). After 8 min superfusion with thapsigargin and cessation of spontaneous activity, application of caffeine did not result in a Ca2+ transient. The experiments show that spontaneous Ca2+ transient frequency is modulated by the [Ca2+]i and that the Ca2+ release from intracellular stores plays a role in the initiation of the spontaneous Ca2+ transients.
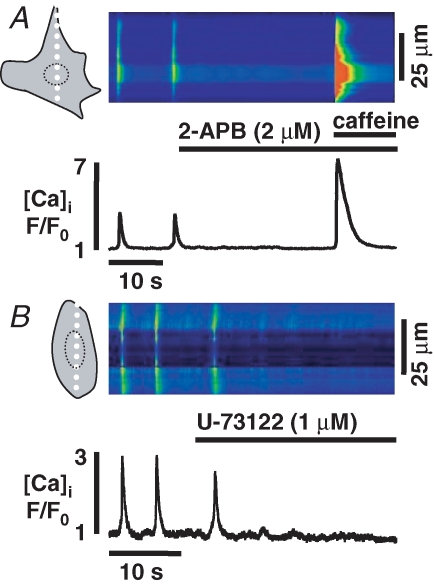
As spontaneous activity continues in the presence of caffeine we determined the relevance of IP3R-mediated Ca2+ release by superfusion of spontaneously active ESdCs with the IP3R blocker 2-aminoethoxydiphenyl borate (2-APB; 2 μm) (Wilcox et al. 1998; Bootman et al. 2002). In 26 out of 26 experiments the application of 2-APB resulted in a suppression (Fig. 6A) of the spontaneous whole-cell Ca2+ transients. Application of caffeine in the presence of 2-APB still resulted in a Ca2+ transient with an amplitude not significantly different from control conditions (Fig. 10A). The data suggest that the cessation of spontaneous activity was not due to a depletion of intracellular Ca2+ stores, or block of RyR-mediated Ca2+ release. To test whether 2-APB blocks voltage-gated Ca2+ entry (Bootman et al. 2002), we evaluated the effect of the 2-APB concentration used on field stimulation-induced Ca2+ transients in rat ventricular myocytes. Amplitude and duration of Ca2+ transients remained unchanged, supporting the hypothesis that block of spontaneous activity in ESdCs did not result from block of Ca2+ influx through voltage-dependent Ca2+ channels (data not shown).
Figure 6.
Whole-cell Ca2+ transients require IP3R-mediated Ca2+ release Confocal linescan images and spatially averaged [Ca2+]i in ESdCs superfused with the IP3R blocker 2-APB (2 μm) (A) or the PLC inhibitor U73122 (1 μm) (B). Suppression of IP3-mediated signalling in both cases resulted in a suppression of spontaneous activity which in the case of 2-APB was not due to a depletion of Ca2+ from intracellular stores, as indicated by the caffeine-induced Ca2+ transient
Figure 10.
Role of Ca2+ influx on the loading of Ca2+ stores and ESdCs spark and beating frequency A, caffeine transient amplitude (TAcaff) is not changed in the presence of 2-APB (2 μm) and nifedipine (10 μm) (ESdCs day 10). B, T-type Ca2+ channel blocker nickel (30 μm) only caused a depression of Ca2+ transient frequency (n = 4). C, Ca2+ spark frequency, as an indicator of the trigger of spontaneous whole-cell Ca2+ transients, remains unaffected in Ca2+-free solution (n = 2; P > 0.2) or in the presence of nifedipine (n = 2; P > 0.7).
Ca2+ transient frequency in ESdCs is modulated by PLC-mediated IP3 production
Receptor-stimulated increase in IP3 production depends on the activation of phospholipase C (PLC) where the hydrolysis of phosphatidylinositol (4,5) bisphospate results in the production of the second messengers IP3 and diacylglycerol. Superfusion of ESdCs with the PLC inhibitor U73122 (1 μm) (Bootman et al. 2002) reversibly blocked spontaneous whole-cell Ca2+ transients (n = 6; Fig. 6B). By contrast, receptor-mediated stimulation of IP3 production by the α-adrenergic receptor agonist phenylephrine (PE; 10 μm, n = 3) or endothelin-1 (ET-1; 100 nm, n = 4) had a positive chronotropic effect on spontaneous Ca2+ transients (Fig. 7A and B). PE and ET-1 both increase PLC activity via a G-protein (Gq)-dependent mechanism. In both cases the positive chronotropic effect was reversed in the presence of U73122 (1 μm; Fig. 7A and B). Block of the ET-1-induced positive chronotropic response by 2-APB (n = 3; Fig. 7C) underlines the relevance of IP3R-mediated Ca2+ release in the translation of this signalling pathway. To determine whether ET-1 triggers IP3-mediated Ca2+ release, we analysed its effect on basal [Ca2+]i when spontaneous activity was suppressed by the application of tetracaine (1 mm) in Ca2+-free solution; at this concentration tetracaine not only blocks RyR-mediated Ca2+ release but also exhibits a non-specific effect on Ca2+ influx through voltage-dependent Ca2+ channels (Carmeliet et al. 1986). As shown in Fig. 7D, ET-1 increases basal Ca2+ in ESdCs under these conditions by 15.8 ± 0.03% (n = 4; Fig. 7E). The data support the notion that the positive chronotropic effect of ET-1 is mediated by IP3R-mediated Ca2+ release.
Figure 7.
Stimulation of IP3 production has a positive chronotropic effect on spontaneous Ca2+ transients in ESdCs A, phenylephrine (10 μm)-induced increase in Ca2+ transient frequency (n = 5) is reversed upon simultaneous superfusion with U73122 (1 μm; n = 2). B and C, ET-1 (100 nm) has a significant positive chronotropic effect (n = 4) on spontaneous Ca2+ transients that is reversed by superfusion with U73122 (B) (n = 2) or 2-APB (C) (n = 3). D, spatially averaged change of [Ca2+]i induced by the application of ET-1 while spontaneous Ca2+ transients are suppressed by superfusion of the cells with tetracaine (1 mm) in Ca2+-free solution. E, ET-1 raised basal [Ca2+] by 15.8 ± 0.03% (n = 4) in comparison to the control in the presence of tetracaine plus Ca2+-free solution.
RyR channels amplify the IP3R-mediated Ca2+ release
In ESdCs of all developmental stages, we detected spontaneous spatially restricted Ca2+ release events (Fig. 8A; and see Fig. 1A and B). The events had an amplitude (ΔF/F0) of 2.1 ± 0.2, a width of 2.3 ± 0.18 μm and a duration of 31.4 ± 3.5 ms at half-maximal amplitude (Fig. 8G). The data are in good agreement with the parameters recorded previously for RyR-mediated Ca2+ sparks in ESdCs (Sauer et al. 2001). To test the hypothesis that IP3R-mediated Ca2+ release is amplified by CICR from RyR channels, we determined whether the spark frequency is influenced by PLC activity or IP3R-mediated Ca2+ release. Under control conditions, superfusion of spontaneously active ESdCs with the PLC inhibitor U73122 resulted in a significant decrease of the spark frequency (Fig. 8B and H). By contrast, the spark frequency increased upon stimulation with ET-1 (Fig. 8C and H). Again, this effect can be reversed by 2-APB (Fig. 8D and H). The experimental results support the hypothesis that IP3R-mediated Ca2+ release can trigger RyR-mediated Ca2+ sparks by CICR. However, sparks did not seem to be a prerequisite for spontaneous Ca2+ transients. We also analysed Ca2+ release events that were recorded in the presence of caffeine (10 mm) when presumably RyR-operated stores are depleted. Figure 8F shows such a release event. The release events in the presence of caffeine had an amplitude (ΔF/F0) of 1.15 ± 0.2, a half-maximal width of 2.03 ± 0.22 μm and a duration of 39.27 ± 2 ms. The release events detected under these conditions exhibited a significantly decreased amplitude (Fig. 8G).
Figure 8.
RyR-mediated Ca2+ sparks are promoted by IP3-dependent Ca2+ release A, confocal linescan image and (bottom) local subcellular changes in [Ca2]i (F/F0 averaged over the area indicated by the arrow on the left of the linescan). B, the frequency of sparks was suppressed by application of the PLC inhibitor U73122 (1 μm; n = 2) and C, increased when IP3 production was stimulated by ET-1 (100 nm; n = 2). D, the effect of ET-1 was reversible upon application of the IP3R blocker 2-APB (n = 2). The data are summarized in H. E and F, confocal linescan images depicting a spatially restricted Ca2+ release event in the presence of caffeine (10 mm). G, release events in the presence of caffeine exhibit a significant decrease in the amplitude (*P < 0.002). In comparison to sparks they tend to have a similar width (FWHM) and an increased duration (FDHM); however, this difference did not reach significance in our analysis.
Translation of IP3R-mediated Ca2+ release into whole-cell Ca2+ transients
Several studies have addressed the role of Ca2+ release from intracellular stores in the generation of spontaneous activity (Huser et al. 2000; Bogdanov et al. 2001; Lipsius et al. 2001). In these experimental models, it is proposed that localized Ca2+ release from intracellular stores can contribute in part to the diastolic depolarization due to activation of NCX, a further depolarization of the membrane potential and subsequent activation of voltage-dependent Ca2+ channels. We evaluated the role of Ca2+ influx by superfusing spontaneously active ESdCs with nominally Ca2+-free solution. As demonstrated in Fig. 9A, spontaneous Ca2+ transients in ESdCs at day 11 ceased immediately after exposure of the cells to Ca2+-free solution (n = 3). The lack of spontaneous Ca2+ transients was not due to depletion of the intracellular stores as indicated by the caffeine-induced Ca2+ transient after cessation of spontaneous activity. In addition, spontaneous transients resumed immediately with smaller amplitude.
Figure 9.
Whole-cell Ca2+ transients in ESdCs depend on Ca2+ influx through L-type Ca2+ channels A, confocal linescan images and spatially averaged changes in [Ca2+]i of ESdCs during superfusion with Ca2+-free solution or in the presence of L-type Ca2+ channel blocker nifedipine (B and C). Superfusion with Ca2+-free solution (n = 4) or nifedipine (10 μm; n = 14) resulted in a block of spontaneous whole-cell Ca2+ transients. The spontaneous activity did not cease in Ca2+-free solution because of the depletion of the intracellular stores as indicated by the caffeine-induced Ca2+ transient.
To determine the mechanism of Ca2+ influx we superfused spontaneously active cells with nifedipine (10 μm; n = 14) or nickel (30 μm; n = 4), which are blockers of the voltage-dependent L-type and T-type Ca2+ channels, respectively. Figure 9B and C shows representative examples of spontaneously active ESdCs at day 9 and 14. Application of nifedipine resulted in a block of spontaneous activity. Also in these cases, RyR-regulated intracellular stores were not depleted (Fig. 10A). In contrast to the block of ICa,L, nickel had only a small attenuating effect on the Ca2+ transient frequency (Fig. 10B). The experimental results support the hypothesis that depolarization-dependent Ca2+ influx through L-type Ca2+ channels is not essential for maintaining spontaneous whole-cell Ca2+ transients in ESdCs.
IP3R-mediated Ca2+ release events or puffs are smaller in amplitude than Ca2+ sparks and have a prolonged rise time and duration (Bootman et al. 1997; Zima & Blatter, 2004). Our experimental results suggest that localized RyR-mediated Ca2+ release events or sparks are promoted by the stimulation of IP3R-mediated Ca2+ release. Therefore we used the more frequent RyR-mediated sparks as a parameter to determine whether IP3R-mediated Ca2+ release persists even in the absence of whole-cell Ca2+ transients. For this purpose we analysed the spark frequency during superfusion of ESdCs with Ca2+-free solution (n = 2) or nifedipine (10 μm; n = 2). As shown in Fig. 10C neither in Ca-free conditions nor in the presence of nifedipine, a significant decrease in spark frequency in comparison to control conditions could be detected, although whole-cell Ca2+ transients ceased. The data support the hypothesis that IP3R-mediated Ca2+ release and therefore the trigger for the spontaneous activity of the ESdCs persists even in the absence of Ca2+ transients.
Because our experimental results indicate that Ca2+ influx is required for whole-cell Ca2+ transients, we tested the hypothesis that, in contrast to previous reports (Viatchenko-Karpinski et al. 1999), membrane depolarization is required for spontaneous activity in ESdCs. For this purpose we depolarized ESdCs with a high-K+ ([K+]o, 140 mm) solution. Superfusion with high- K+ solution resulted in an initial Ca2+ transient after which oscillatory changes of [Ca2+]i were maintained over time (Fig. 11A). In comparison to the whole-cell Ca2+ transients recorded in the cell prior to the change of the extracellular solution, these oscillations had increased amplitudes, a reduced frequency, and a prolonged upstroke velocity (Fig. 11C). The increased rise time of 129.6 ± 19 ms (n = 2) in comparison to 41.2 ± 2.4 ms (n = 10) in transients in control solutions resulted from the non-instantaneous increase of [Ca2+]i throughout the cell reflecting a propagating Ca2+ wave rather than a Ca2+ transient (Schlotthauer & Bers, 2000). The data indicate that Ca2+ signals recorded in ESdCs under depolarizing conditions are distinctly different from spontaneous whole-cell Ca2+ transients which we could not identify in high-K+ solution.
Figure 11.
Spontaneous whole-cell Ca2+ transients are replaced by Ca2+ waves when changes of the plasma membrane potential are suppressed A, superfusion of a spontaneously beating ESdC with depolarizing high-K+ solution resulted in the suppression of whole-cell Ca2+ transients but lead to the appearance of Ca2+ waves. B, confocal linescan image of an electrically coupled cell pair and the F/F0 plot averaged over the entire width of the lower ESdC. Superfusion of the cell with low-Na+ solution suppressed whole-cell Ca2+ transients (*) that were synchronized between the two adjacent cells. Spontaneous release events transitioned into Ca2+ waves (•) that were confined to the boundaries of the individual cell. C, Ca2+ waves in the presence of LiCl-containing (n = 5) and KCl-containing solution (n = 1) could be distinguished from whole-cell Ca2+ transients under control conditions (n = 3) because of their significantly reduced upstroke velocity (P < 0.05).
To determine whether NCX is the mechanism by which intracellular Ca2+ release is translated into a depolarization of the membrane potential, we superfused the cells with a low-Na+ solution. Superfusion with low-Na+ resulted in the instantaneous arrest of spontaneous Ca2+ transients; however, Ca2+ release events were observed that transitioned into intracellular Ca2+ waves (n = 5). The experiment in Fig. 11B was obtained from a cell pair and exhibits the difference between the two Ca2+ signals. Whole-cell Ca2+ transients (asterisk in Fig. 11B) were synchronized between the two cells with a propagation delay of less than 4.4 ms. This indicates that Ca2+ transient synchronization is supported by the intercellular propagation of an action potential. In contrast, the localized release events or Ca2+ waves (circle in Fig. 11B;) that appeared in low-Na+ solution ([Na+]o, 12 mm), were restricted to the perimeter of an individual cell underlining the fact that intracellular Ca2+ release was not translated into a membrane depolarization and subsequent intercellular action potential propagation. Intracellular Ca2+ waves in low-Na+ solution were not blocked by 2-APB (data not shown) indicating that they do not depend on the release of Ca2+ through IP3Rs. Our experimental results indicate that spontaneous intercellularly propagating whole-cell Ca2+ transients depend on a depolarization of Vm potentially brought about by NCX, and subsequent voltage-dependent Ca2+ influx. In the absence of IP3R-initiated depolarization, RyR-mediated Ca2+ release results in Ca2+ waves with properties distinct from the pacemaker mechanism.
Discussion
In this study we present experimental evidence that the developmental changes of spontaneous whole-cell Ca2+ transients in ESdCs are explained by an increased contribution of RyR-mediated Ca2+ release and a shift of the Ca2+ removal mechanisms from Ca2+ extrusion by NCX to Ca2+ reuptake into intracellular stores by SERCA. We further present evidence that spontaneous whole-cell Ca2+ transients in ESdCs are initiated by IP3-mediated Ca2+ release which is translated into whole-cell Ca2+ transients by a NCX-mediated depolarization and subsequent induction of voltage-dependent Ca2+ influx through L-type channels.
In adult cardiac myocytes, the whole-cell Ca2+ transient is initiated by Ca2+ influx through voltage-dependent L-type Ca2+ channels; however, the major increase of [Ca2+]i depends on the subsequent CICR from RyR-operated intracellular Ca2+ stores (Fabiato, 1985a,Fabiato, 1985b). The contribution of Ca2+ release from the SR for twitch transients constitutes 90% in adult mouse ventricular myocytes (Li et al. 1998), whereas in whole heart experiments with neonatal rat myocytes a contribution as low as 5% has been described (Escobar et al. 2004). In ESdCs at day 17 the twitch transient amplitude decreased by 70% in the presence of caffeine which correlates well with the observation that 72% of the Ca2+ removal after a twitch is contributed by SERCA. The data represent a significant increase in the relevance of RyRs and SERCA during ESdC differentiation. One reason for the low contribution of RyRs to the Ca2+ transient could be the lack of functional coupling between voltage-dependent Ca2+ influx and the RyR channel release site (Franzini-Armstrong et al. 2005). In ESdCs, early RyR channel expression and its developmental up-regulation has been described (Yang et al. 2002). These data support our experimental results indicating an age-dependent increase of the caffeine transient amplitude. As RyR-mediated Ca2+ release also contributes to the diastolic increase in [Ca2+]i, its up-regulation could also explain the developmental increases in the frequency of the spontaneous transients.
Although IP3Rs are present in adult ventricular muscle (Bare et al. 2005), their Ca2+ release seems to contribute only to a small degree to the excitation-induced Ca2+ transient (Proven et al. 2006). Our experimental results support the hypothesis that spontaneous whole-cell Ca2+ transients in ESdCs depend on IP3 production and IP3R-mediated Ca2+ release (see Figs 6A and B, and 8). This is in agreement with previous reports that in ESdCs spontaneous whole-cell Ca2+ transients are inhibited by the IP3R blocker xestospongin and by IP3R down-regulation via IP3R type1 antisense cDNA (Mery et al. 2005). Here we show that promoting (ET-1 and PE) or suppressing (U73122) IP3 production in ESdCs changes the chronotropic response of the spontaneous whole-cell Ca2+ transients. The reported interdependence of IP3 production and RyR-mediated Ca2+ sparks was previously described in adult atrial myocytes of cat and mice where ET-1 increased the spark frequency by IP3R-mediated Ca2+ release (Zima & Blatter, 2004; Li et al. 2005). Spontaneous spatially restricted Ca2+ release events from IP3R channels known as blips or puffs differ from Ca2+ sparks by their amplitude and kinetics (Bootman et al. 1997; Zima & Blatter, 2004). The events are difficult to distinguish when they appear simultaneously. Although we can detect the ET-1-mediated increase in basal [Ca2+]i, the appearance of individual release events during suppression of Ca2+ sparks by caffeine were rare and had a lower amplitude and longer duration than Ca2+ sparks (see Fig. 8G).
Ca2+ release from intracellular stores has been postulated previously as a mechanism contributing to spontaneous activity (Viatchenko-Karpinski et al. 1999; Huser et al. 2000; Bogdanov et al. 2001). In contrast to ESdCs (see Figs 2 and 3) (Yang et al. 2002), in these cases RyR channels were described as the source for the increase in diastolic calcium. Continuing spontaneous activity in the presence of ryanodine was recorded in sinus nodal cells (Boyett et al. 2003); however, voltage-activated ion channels are proposed as the driving force for the diastolic depolarization. In ESdCs the presence of voltage-dependent ion channels, such as If, ICa,L and ICa,T, has been described but neither block of If nor block of ICa,T in early ESdCs suppressed their spontaneous activity (Zhang et al. 2003; Mery et al. 2005). Our results show a cessation of spontaneous whole-cell Ca2+ transients upon block of ICa,L at all developmental stages (Fig. 9B and C). This result is contrary to prior studies where spontaneous Ca2+ transients in early ESdCs were described to be independent of voltage-dependent Ca2+ influx. We propose that the spontaneous whole-cell Ca2+ transients under depolarizing conditions represent Ca2+ waves (see Fig. 11) rather than whole-cell Ca2+ transients, and are based on CICR that originates as a result of Ca2+ overload of the intracellular stores rather than IP3R-mediated Ca2+ release that promotes spontaneous activity.
The mechanisms that promote the oscillatory changes in spontaneous Ca2+ release are still under debate. In latent pacemaker cells the depolarization-induced Ca2+ influx through T-type Ca2+ channels was proposed as a potential mechanism (Huser et al. 2000). In rabbit SA nodal cells, however, the rhythmic increase in RyR release events seems to be promoted by protein kinase A-modulated filling of the intracellular stores (Bogdanov et al. 2001; Vinogradova et al. 2005; Vinogradova et al. 2006). In comparison to our study and to reports from embryonic cardiomyocytes (Koushik et al. 2001), SR-dependent diastolic increase of [Ca2+]i promotes the depolarization of the plasma membrane by NCX activation (Fig. 9 and 11). The subsequent activation of ICa,L then leads to the rapid upstroke of the Ca2+ transients. In addition, recent studies on guinea-pig cardiomyocytes have suggested a contribution of NCX to the pacemaker activity as a result of a Na+ leak current (Sanders et al. 2006).
It remains to be determined where the IP3R-mediated Ca2+ release occurs to promote sufficient depolarization of Vm to trigger the voltage-dependent Ca2+ influx. Release events appear to be sparse and no distinct spatial location could yet be determined. All three IP3R subtypes (1–3) are expressed in undifferentiated embryonic stem cells (Kapur et al. 2006) and embryonic cardiomyocytes (Rosemblit et al. 1999; Jaconi et al. 2000; Puceat & Jaconi, 2005). Immunostaining revealed that IP3R-1 is most prominent within the nuclear envelope (Jaconi et al. 2000; Mery et al. 2005). However, one could speculate that a subsarcolemmal location of the Ca2+ release site would be more efficient to promote plasma membrane depolarization. Our experiments demonstrate that spontaneous activity can continue even after depletion of RyR-sensitive stores by caffeine (Fig. 3A and B). Although the fact that spark activity strongly depends on IP3 production indicates a close proximity of the two release channels, the continuation of spontaneous activity in the presence of caffeine would indicate a functional separation of IP3R- and RyR-controlled stores. In adult ventricular myocytes, the interconnectivity of the store system is supported by experiments showing refilling of stores through intra-SR diffusion (Wu & Bers, 2006). Future experiments will need to address the spatial distribution and the functional relation of IP3R- and RyR-controlled Ca2+ stores.
Conclusion
Our experimental results identify a developmental change in the functional contribution of RyR-mediated Ca2+ release to spontaneous whole-cell Ca2+ transients in ESdCs and provide evidence that the pacemaker mechanism in early ESdCs depends on IP3R-mediated Ca2+ release that is translated into a depolarization of Vm and subsequent voltage-dependent Ca2+ influx by ICa,L. The Ca2+ handling mechanisms in ESdCs therefore represent a model of how IP3R-mediated Ca2+ release can modulate spontaneous activity in cardiomyocytes or promote the appearance of arrhythmic activity.
Acknowledgments
The authors would like to thank Dr L. J. Field (Krannert Institute of Cardiology, Indiana University School of Medicine, Indianapolis) for providing the αMHC–neomycin construct. This work was supported by grants from the American Heart Association (AHA) (AHA 0330393Z) to K.B. and the Potts Foundation Loyola University Chicago (RFC 11086) to K.B.
References
- Abi-Gerges N, Ji GJ, Lu ZJ, Fischmeister R, Hescheler J, Fleischmann BK. Functional expression and regulation of the hyperpolarization activated non-selective cation current in embryonic stem cell-derived cardiomyocytes. J Physiol. 2000;523:377–389. doi: 10.1111/j.1469-7793.2000.t01-2-00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banach K, Halbach MD, Hu P, Hescheler J, Egert U. Development of electrical activity in cardiac myocyte aggregates derived from mouse embryonic stem cells. Am J Physiol Heart Circ Physiol. 2003;284:H2114–H2123. doi: 10.1152/ajpheart.01106.2001. [DOI] [PubMed] [Google Scholar]
- Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2005;280:15912–15920. doi: 10.1074/jbc.M414212200. [DOI] [PubMed] [Google Scholar]
- Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na+-Ca 2+ exchanger: molecular partners in pacemaker regulation. Circ Res. 2001;88:1254–1258. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res. 2002;91:189–201. doi: 10.1161/01.res.0000027865.61704.32. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- Bootman M, Niggli E, Berridge M, Lipp P. Imaging the hierarchical Ca2+ signalling system in HeLa cells. J Physiol. 1997;499:307–314. doi: 10.1113/jphysiol.1997.sp021928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett MR, Dobrzynski H, Lancaster MK, Jones SA, Honjo H, Kodama I. Sophisticated architecture is required for the sinoatrial node to perform its normal pacemaker function. J Cardiovasc Electrophysiol. 2003;14:104–106. doi: 10.1046/j.1540-8167.2003.02307.x. [DOI] [PubMed] [Google Scholar]
- Carmeliet E, Morad M, Van der Heyden G, Vereecke J. Electrophysiological effects of tetracaine in single guinea-pig ventricular myocytes. J Physiol. 1986;376:143–161. doi: 10.1113/jphysiol.1986.sp016146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs LL, Martin BL, Schroder EA, Keller BB, Delisle BP, Satin J. Identification of the T-type calcium channel (Cav3.1d) in developing mouse heart. Circ Res. 2001;88:403–407. doi: 10.1161/01.res.88.4.403. [DOI] [PubMed] [Google Scholar]
- Escobar AL, Ribeiro-Costa R, Villalba-Galea C, Zoghbi ME, Perez CG, Mejia-Alvarez R. Developmental changes of intracellular Ca2+ transients in beating rat hearts. Am J Physiol Heart Circ Physiol. 2004;286:H971–H978. doi: 10.1152/ajpheart.00308.2003. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985a;85:247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985b;85:291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijnvandraat AC, van Ginneken AC, de Boer PA, Ruijter JM, Christoffels VM, Moorman AF, Lekanne DRH. Cardiomyocytes derived from embryonic stem cells resemble cardiomyocytes of the embryonic heart tube. Cardiovasc Res. 2003;58:399–409. doi: 10.1016/s0008-6363(03)00282-7. [DOI] [PubMed] [Google Scholar]
- Fowler MR, Naz JR, Graham MD, Bru-Mercier G, Harrison SM, Orchard CH. Decreased Ca2+ extrusion via Na+/Ca2+ exchange in epicardial left ventricular myocytes during compensated hypertrophy. Am J Physiol Heart Circ Physiol. 2005;288:H2431–H2438. doi: 10.1152/ajpheart.01069.2004. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F, Tijskens P. The assembly of calcium release units in cardiac muscle. Ann N Y Acad Sci. 2005;1047:76–85. doi: 10.1196/annals.1341.007. [DOI] [PubMed] [Google Scholar]
- Fu JD, Li J, Tweedie D, Yu HM, Chen L, Wang R, Riordon DR, Brugh SA, Wang SQ, Boheler KR, Yang HT. Crucial role of the sarcoplasmic reticulum in the developmental regulation of Ca2+ transients and contraction in cardiomyocytes derived from embryonic stem cells. FASEB J. 2006;20:181–183. doi: 10.1096/fj.05-4501fje. [DOI] [PubMed] [Google Scholar]
- Fujii S, Hirota A, Kamino K. Optical recording of development of electrical activity in embryonic chick heart during early phases of cardiogenesis. J Physiol. 1981a;311:147–160. doi: 10.1113/jphysiol.1981.sp013578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Hirota A, Kamino K. Optical indications of pace-maker potential and rhythm generation in early embryonic chick heart. J Physiol. 1981b;312:253–263. doi: 10.1113/jphysiol.1981.sp013627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorza L, Vettore S, Tessaro A, Sorrentino V, Vitadello M. Regional and age-related differences in mRNA composition of intracellular Ca2+-release channels of rat cardiac myocytes. J Mol Cell Cardiol. 1997;29:1023–1036. doi: 10.1006/jmcc.1996.0346. [DOI] [PubMed] [Google Scholar]
- Gryshchenko O, Fischer IR, Dittrich M, Viatchenko-Karpinski S, Soest J, Bohm-Pinger MM, Igelmund P, Fleischmann BK, Hescheler J. Role of ATP-dependent K+ channels in the electrical excitability of early embryonic stem cell-derived cardiomyocytes. J Cell Sci. 1999;112:2903–2912. doi: 10.1242/jcs.112.17.2903. [DOI] [PubMed] [Google Scholar]
- Gryshchenko O, Lu ZJ, Fleischmann BK, Hescheler J. Outwards currents in embryonic stem cell-derived cardiomyocytes. Pflugers Arch. 2000;439:798–807. doi: 10.1007/s004249900196. [DOI] [PubMed] [Google Scholar]
- He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- Hescheler J, Wartenberg M, Fleischmann BK, Banach K, Acker H, Sauer H. Embryonic stem cells as a model for the physiological analysis of the cardiovascular system. Methods Mol Biol. 2002;185:169–187. doi: 10.1385/1-59259-241-4:169. [DOI] [PubMed] [Google Scholar]
- Huser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol. 2000;524:415–422. doi: 10.1111/j.1469-7793.2000.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaconi M, Bony C, Richards SM, Terzic A, Arnaudeau S, Vassort G, Puceat M. Inositol 1,4,5-trisphosphate directs Ca2+ flow between mitochondria and the endoplasmic/sarcoplasmic reticulum: a role in regulating cardiac autonomic Ca2+ spiking. Mol Biol Cell. 2000;11:1845–1858. doi: 10.1091/mbc.11.5.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji GJ, Fleischmann BK, Bloch W, Feelisch M, Andressen C, Addicks K, Hescheler J. Regulation of the L-type Ca2+ channel during cardiomyogenesis: switch from NO to adenylyl cyclase-mediated inhibition. FASEB J. 1999;13:313–324. doi: 10.1096/fasebj.13.2.313. [DOI] [PubMed] [Google Scholar]
- Kapur N, Mignery G, Banach K. Cell cycle dependent calcium oscillations in mouse embryonic stem cells. Am J Physiol Cell Physiol. 2006 doi: 10.1152/ajpcell.00181.2006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Klockner U, Lee JH, Cribbs LL, Daud A, Hescheler J, Pereverzev A, Perez-Reyes E, Schneider T. Comparison of the Ca2+ currents induced by expression of three cloned alpha1 subunits, alpha1G, alpha1H and alpha1I, of low-voltage-activated T-type Ca2+ channels. Eur J Neurosci. 1999;11:4171–4178. doi: 10.1046/j.1460-9568.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolossov E, Lu Z, Drobinskaya I, Gassanov N, Duan Y, Sauer H, Manzke O, Bloch W, Bohlen H, Hescheler J, Fleischmann BK. Identification and characterization of embryonic stem cell-derived pacemaker and atrial cardiomyocytes. FASEB J. 2005;19:577–579. doi: 10.1096/fj.03-1451fje. [DOI] [PubMed] [Google Scholar]
- Komuro H, Hirota A, Yada T, Sakai T, Fujii S, Kamino K. Effects of calcium on electrical propagation in early embryonic precontractile heart as revealed by multiple-site optical recording of action potentials. J Gen Physiol. 1985;85:365–382. doi: 10.1085/jgp.85.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL, Conway SJ. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J. 2001;15:1209–1211. doi: 10.1096/fj.00-0696fje. [DOI] [PubMed] [Google Scholar]
- Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Physiol Heart Circ Physiol. 1998;274:H1335–H1347. doi: 10.1152/ajpheart.1998.274.4.H1335. [DOI] [PubMed] [Google Scholar]
- Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate (IP3)-receptor type 2-deficient mice. Circ Res. 2005;96:1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c. [DOI] [PubMed] [Google Scholar]
- Lipsius SL, Huser J, Blatter LA. Intracellular Ca2+ release sparks atrial pacemaker activity. News Physiol Sci. 2001;16:101–106. doi: 10.1152/physiologyonline.2001.16.3.101. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Ji GJ, Wobus AM, Fleischmann BK, Hescheler J. Establishment of beta-adrenergic modulation of L-type Ca2+ current in the early stages of cardiomyocyte development. Circ Res. 1999;84:136–145. doi: 10.1161/01.res.84.2.136. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Rohwedel J, Hescheler J, Wobus AM. Embryonic stem cells differentiate in vitro into cardiomyocytes representing sinusnodal, atrial and ventricular cell types. Mech Dev. 1993;44:41–50. doi: 10.1016/0925-4773(93)90015-p. [DOI] [PubMed] [Google Scholar]
- Mery A, Aimond F, Menard C, Mikoshiba K, Michalak M, Puceat M. Initiation of embryonic cardiac pacemaker activity by inositol 1,4,5-trisphosphate-dependent calcium signaling. Mol Biol Cell. 2005;16:2414–2423. doi: 10.1091/mbc.E04-10-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer N, Jaconi M, Landopoulou A, Fort P, Puceat M. A fluorescent reporter gene as a marker for ventricular specification in ES-derived cardiac cells. FEBS Lett. 2000;478:151–158. doi: 10.1016/s0014-5793(00)01839-1. [DOI] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita N, Bielinska M, Wilson DB. Cardiomyocyte differentiation by GATA-4-deficient embryonic stem cells. Development. 1997;124:3755–3764. doi: 10.1242/dev.124.19.3755. [DOI] [PubMed] [Google Scholar]
- Perez CG, Copello JA, Li Y, Karko KL, Gomez L, Ramos-Franco J, Fill M, Escobar AL, Mejia-Alvarez R. Ryanodine receptor function in newborn rat heart. Am J Physiol Heart Circ Physiol. 2005;288:H2527–H2540. doi: 10.1152/ajpheart.00188.2004. [DOI] [PubMed] [Google Scholar]
- Proven A, Roderick HL, Conway SJ, Berridge MJ, Horton JK, Capper SJ, Bootman MD. Inositol 1,4,5-trisphosphate supports the arrhythmogenic action of endothelin-1 on ventricular cardiac myocytes. J Cell Sci. 2006;119:3363–3375. doi: 10.1242/jcs.03073. [DOI] [PubMed] [Google Scholar]
- Puceat M, Jaconi M. Ca2+ signalling in cardiogenesis. Cell Calcium. 2005;38:383–389. doi: 10.1016/j.ceca.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Puceat M, Travo P, Quinn MT, Fort P. A dual role of the GTPase Rac in cardiac differentiation of stem cells. Mol Biol Cell. 2003;14:2781–2792. doi: 10.1091/mbc.E02-09-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RB, Yu H, Chang F, Cohen IS. Developmental change in the voltage-dependence of the pacemaker current, if, in rat ventricle cells. Pflugers Arch. 1997;433:533–535. doi: 10.1007/s004240050309. [DOI] [PubMed] [Google Scholar]
- Rosemblit N, Moschella MC, Ondriasa E, Gutstein DE, Ondrias K, Marks AR. Intracellular calcium release channel expression during embryogenesis. Dev Biol. 1999;206:163–177. doi: 10.1006/dbio.1998.9120. [DOI] [PubMed] [Google Scholar]
- Sakai T, Fujii S, Hirota A, Kamino K. Optical evidence for calcium-action potentials in early embryonic precontractile chick heart using a potential-sensitive dye. J Membr Biol. 1983;72:205–212. doi: 10.1007/BF01870587. [DOI] [PubMed] [Google Scholar]
- Sanders L, Rakovic S, Lowe M, Mattick PA, Terrar DA. Fundamental importance of Na+-Ca2+ exchange for the pacemaking mechanism in guinea-pig sino-atrial node. J Physiol. 2006;571:639–649. doi: 10.1113/jphysiol.2005.100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Sperelakis N. Hyperpolarization-activated inward current in embryonic chick cardiac myocytes: developmental changes and modulation by isoproterenol and carbachol. Eur J Pharmacol. 1993;240:283–290. doi: 10.1016/0014-2999(93)90910-a. [DOI] [PubMed] [Google Scholar]
- Sauer H, Theben T, Hescheler J, Lindner M, Brandt MC, Wartenberg M. Characteristics of calcium sparks in cardiomyocytes derived from embryonic stem cells. Am J Physiol Heart Circ Physiol. 2001;281:H411–H421. doi: 10.1152/ajpheart.2001.281.1.H411. [DOI] [PubMed] [Google Scholar]
- Schlotthauer K, Bers DM. Sarcoplasmic reticulum Ca2+ release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ Res. 2000;87:774–780. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- Sheehan KA, Blatter LA. Regulation of junctional and non-junctional sarcoplasmic reticulum calcium release in excitation-contraction coupling in cat atrial myocytes. J Physiol. 2003;546:119–135. doi: 10.1113/jphysiol.2002.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom TB, Field LJ, Ruediger M. Allogeneic stem cells, clinical transplantation and the origins of regenerative medicine. Curr Opin Immunol. 2002;14:601–605. doi: 10.1016/s0952-7915(02)00387-4. [DOI] [PubMed] [Google Scholar]
- Takemura H, Yasui K, Opthof T, Niwa N, Horiba M, Shimizu A, Lee JK, Honjo H, Kamiya K, Ueda Y, Kodama I. Subtype switching of L-Type Ca2+ channel from Cav1.3 to Cav1.2 in embryonic murine ventricle. Circ J. 2005;69:1405–1411. doi: 10.1253/circj.69.1405. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viatchenko-Karpinski S, Fleischmann BK, Liu Q, Sauer H, Gryshchenko O, Ji GJ, Hescheler J. Intracellular Ca2+ oscillations drive spontaneous contractions in cardiomyocytes during early development. Proc Natl Acad Sci U S A. 1999;96:8259–8264. doi: 10.1073/pnas.96.14.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98:505–514. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Maltsev VA, Bogdanov KY, Lyashkov AE, Lakatta EG. Rhythmic Ca2+ oscillations drive sinoatrial nodal cell pacemaker function to make the heart tick. Ann N Y Acad Sci. 2005;1047:138–156. doi: 10.1196/annals.1341.013. [DOI] [PubMed] [Google Scholar]
- Wilcox RA, Primrose WU, Nahorski SR, Challiss RA. New developments in the molecular pharmacology of the myo-inositol 1,4,5-trisphosphate receptor. Trends Pharmacol Sci. 1998;19:467–475. doi: 10.1016/s0165-6147(98)01260-7. [DOI] [PubMed] [Google Scholar]
- Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–678. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- Wu X, Bers DM. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ Res. 2006;99:283–291. doi: 10.1161/01.RES.0000233386.02708.72. [DOI] [PubMed] [Google Scholar]
- Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HT, Tweedie D, Wang S, Guia A, Vinogradova T, Bogdanov K, Allen PD, Stern MD, Lakatta EG, Boheler KR. The ryanodine receptor modulates the spontaneous beating rate of cardiomyocytes during development. Proc Natl Acad Sci U S A. 2002;99:9225–9230. doi: 10.1073/pnas.142651999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YM, Shang L, Hartzell C, Narlow M, Cribbs L, Dudley SC., Jr Characterization and regulation of T-type Ca2+ channels in embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2770–H2779. doi: 10.1152/ajpheart.01114.2002. [DOI] [PubMed] [Google Scholar]
- Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol. 2004;555:607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]