Abstract
An analysis of arginine-vasopressin (AVP) V1a receptor-deficient (V1aR −/−) mice revealed that glucose homeostasis and lipid metabolism were altered in the mutant mice. Here, we used V1aR −/− mice to investigate whether the deficiency of the V1a receptor, which led to altered insulin sensitivity, affected protein metabolism. The serum 3-methylhistidine levels were increased in V1aR −/− mice under feeding conditions, indicating that proteolysis was enhanced in muscle tissue from V1aR −/− mice. Furthermore, serum amino acid profiling revealed that the amino acid levels, including glycogenic and branched-chain amino acids, were reduced in V1aR −/− mice. In addition, an alanine-loading test showed that gluconeogenesis was enhanced in V1aR −/− mice. Blood ammonia, which is a by-product of amino acid catabolism, was two times higher in V1aR −/− mice without hepatopathy under the feeding and fasting conditions than in wild-type mice. Amino acid profiling also revealed that the amino acid pattern was not typical of a urea-cycle enzymatic disorder. An ammonia tolerance test and an indocyanine green elimination test showed that V1aR −/− mice had lower ammonia clearance due to a decreased intrahepatic circulating blood volume. Metabolic acidosis, including lactic- and keto-acidosis, was not observed in V1aR −/− mice. These results provide evidence that proteolysis promotes the production of glucose in the muscles of V1aR −/− mice and that hyperammonaemia is caused by promoted protein catabolism and reduced intrahepatic blood volume. Thus, our study with V1aR −/− mice indicates that AVP plays a physiological role via the V1a receptor in regulating both protein catabolism and glucose homeostasis.
The neurohypophyseal peptide [Arg8]-vasopressin (AVP) is involved in diverse functions, including the contraction of smooth muscle, the stimulation of glycogenolysis in the liver, the modulation of corticotropin release from the pituitary, and the inhibition of diuresis (Michell et al. 1979). These physiological effects are mediated through the binding of AVP to specific membrane receptors of the target cells. AVP receptors are G protein coupled and have been divided into at least three types: V1a, V1b and V2. The V1a and V1b receptors act through phosphatidylinositol hydrolysis to mobilize intracellular Ca2+. The V2 receptor, which is associated with antidiuresis in the kidney, is linked to adenylate cyclase and the production of cAMP.
AVP is known to promote protein synthesis in several cells, such as rat mesangial cells (Wolthuis et al. 1992), vascular endothelial smooth muscle cells (Simon et al. 1995), perfused rat heart (Fukuzawa et al. 1999), rat cardiomyocytes (Xu et al. 1999; Nakamura et al. 2000), and human osteoblast-like cells (Lagumdzija et al. 2004). AVP is also reported to prevent proteolysis in the skeletal muscle by reducing the release of N-methylhistidine, a marker of myofibrillar protein degraded from skeletal muscle and cells (Goodman, 1987a; Thompson et al. 1994, 1996). It is known that these AVP actions are mediated through the V1a receptor (Fukuzawa et al. 1999; Nakamura et al. 2000; Lagumdzija et al. 2004).
In addition to its action on protein metabolism, AVP is involved in regulating glucose metabolism and homeostasis. AVP infusions lead to an increase in the circulating glucose levels (Rofe & Williamson, 1983; Spruce et al. 1985). AVP regulates the glucose level via the V1a receptor by enhancing glycogenolysis in the liver (Hems, 1977; Keppens & De Wulf, 1979) as well as via the V1b receptor by stimulating insulin and glucagon secretion from pancreatic islets (Dunning et al. 1982; Yibchok-anun & Hsu, 1998; Oshikawa et al. 2004).
We generated V1a receptor-deficient (V1aR −/−) mice, which are not lethal and have no apparent anatomical anomalies but exhibit an impairment of the spatial memory in an eight-arm radial maze (Egashira et al. 2004). Furthermore, V1aR −/− mice have a significantly lower basal blood pressure caused by a decreased blood volume, a blunted vascular response to AVP, and an impaired baroreceptor reflex (Koshimizu et al. 2006). Recently, we demonstrated that V1aR −/− mice exhibit a phenotype with the hypermetabolism of fat and insulin resistance (Hiroyama et al. 2007; Aoyagi et al. 2007). These characteristics are in part due to an interference of insulin signalling by a deficiency of the V1a receptor, which could inhibit the activation of Gs signalling to hormone-sensitive lipase (Hiroyama et al. 2007).
As protein metabolism is known to be affected by AVP stimulation and altered glucose homeostasis, for instance, in diabetes, the protein metabolism could be varied in V1aR −/− mice. In this study, we investigated the effect of a V1a-receptor deficiency on protein metabolism in V1aR −/− mice and found that mutant mice had hyperammonaemia due to promoted protein catabolism and a reduced intrahepatic blood volume.
Methods
Animals
The generation of V1a receptor-deficient (V1aR −/−) mice was previously described (Egashira et al. 2004; Koshimizu et al. 2006). The generated mice were maintained on a mixed genetic background of 129sv and C57Black/6J, and F3–5 generations were used in this study. Non-V1a receptor-deficient littermates (V1aR +/+) were used as age-matched control subjects for V1aR −/− mice. Animals were housed in microisolator cages in a pathogen-free barrier facility. V1aR +/+ and V1aR −/− mice were housed on a 12 h light/dark cycle with ad libitum access to food and water except when an experimental protocol was specified. Animals were used at 8–13 weeks of age. All data presented here were obtained from male mice. All experimentation was performed under the guidelines for the Care and Use of Laboratory Animals of the National Research Institute for Child Health and Development.
Biochemical analysis
To measure the blood ammonia level, blood (20 μl) was taken from the tail vein of mice while they were conscious in a rodent restrainer (Harvard Apparatus, Inc., MA, USA) by a siliconized capillary. The ammonia value was measured using the Amicheck meter PocketChem (ARKRAY, Japan). For the analysis of the serum amino acids 3-methylhistidine and glutamic-oxaloacetic transaminase and glutamic-pyruvic transaminase (GOT/GPT), 300 μl and 100 μl sera, respectively, were prepared from the inferior vena cava of mice anaesthetized with sodium pentobarbital (40 mg kg−1, i.p.), and mice were then killed by cervical dislocation after blood sampling. Sera were analysed using the HPLC method. The lactic acid of the serum from the inferior vena cava was measured using Determiner LA (Kyowa Medex Co., Ltd, Japan). The osmolality was measured using a cryoscopic method. The arterial blood was taken from the hearts of mice anaesthetized with sodium pentobarbital (40 mg kg−1, i.p.), and mice were then killed by cervical dislocation. The blood pH and CO2 concentration were measured with an I-STAT 200F analyser (FUSO Pharmaceutical Industries, Ltd, Osaka, Japan).
Ammonia tolerance test
Thirteen-week-old male mice were fasted for 8–12 h before the ammonia tolerance test. Ammonium chloride was given orally at 100 mg kg−1 in a 5% solution with a Teflon probe (1.5 mm (φ) × 70 mm (length), Natume, Japan). The blood ammonia levels were measured 5, 10, 15, 20 and 30 min after the oral administration of ammonium chloride using the Amicheck meter PocketChem.
Indocyanine green elimination test
Indocyanine green (ICG) was dissolved in a minimum volume of water and then diluted with PBS(–) at 0.1 mg ml−1. ICG was injected into the tail vein of 12 h-fasted mice at a dose of 0.5 mg kg−1 body weight. Blood was collected from the other side of the tail vein before and 5, 10 and 15 min after the injection. The plasma was diluted, and the absorbance was measured at 805 nm with a spectrometer (BioSpec-mini, Shimadzu). The retention rate (%) after 15 min (R15) was calculated by the 100 × ICG concentration at the collecting time/initial ICG concentration. The elimination rate (k) was calculated by 0.693/the half-life of the absorbance after 5 min (T½).
Alanine-loading test
l-Alanine (Sigma) was orally administered to mice fasted for 6–10 h at 666 mg (kg body weight)−1 with a Teflon probe. The blood glucose concentration was measured by Ascensia Dex2 (Bayer) every 30 min until 180 min.
Statistical analysis
All values are expressed as the mean ± s.e.m. Statistical analyses were performed using Student's unpaired t test or two-way analysis of variance (ANOVA) followed by Fisher's protected least significant difference (PLSD), used as a post hoc test. P < 0.05 by Student's unpaired t test was considered statistically significant.
Results
Promoted proteolysis in the muscles of V1aR −/− mice
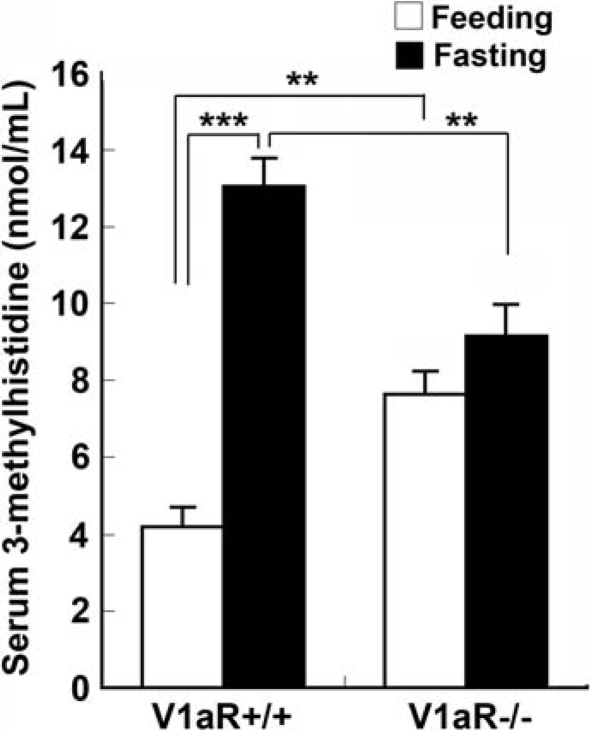
We examined serum 3-methylhistidine because 3-methylhistidine is an amino acid from actin and myosin that is not degraded or re-utilized (Young et al. 1972); therefore, its rate of accumulation in the serum should reflect its rate of release from the degradation of the myofibrils. The serum level of 3-methylhistidine in V1aR −/− mice was significantly higher than that in V1aR +/+ mice under the feeding condition (7.62 ± 0.61 nmol ml−1 in V1aR −/− mice, n = 5, and 4.2 ± 0.51 nmol ml−1 in V1aR +/+ mice, n = 5, P = 0.003) (Fig. 1), suggesting that protein degradation was promoted in V1aR −/− mice. On the other hand, the serum level of 3-methylhistidine in V1aR −/− mice was significantly lower than that in V1aR +/+ mice after fasting for 24 h (9.14 ± 0.81 nmol ml−1 in V1aR −/−, n = 5, and 13.0 ± 0.74 nmol ml−1 in V1aR +/+, n = 5, P = 0.008), but the level in V1aR −/− mice under the fasting condition tended to be higher than that under the feeding condition (7.62 ± 0.61 nmol ml−1 under the feeding condition and 9.14 ± 0.81 nmol ml−1 under the fasting condition) (Fig. 1).
Figure 1.
3-Methylhistidine levels in V1aR −/− mice To examine the serum 3-methylhistidine levels, blood was taken from the inferior vena cava of fed or 24 h-fasted mice. Serum 3-methylhistidine levels were measured using an HPLC method. Under the feeding condition, the 3-methylhistidine levels of V1aR −/− mice were significantly higher than those of V1aR +/+ mice. In contrast, the 3-methylhistidine levels of V1aR −/− mice were significantly lower than those of V1aR +/+ mice under the fasting condition. The results are means ± s.e.m. of five mice. Significance: **P < 0.01; ***P < 0.001.
Amino acid analysis of serum
To examine the metabolic disorder, the amino acid profile was determined under both feeding and fasting conditions because food intake greatly influences amino acid content. Our analysis showed that the number of most amino acids was lower in V1aR −/− mice than in V1aR +/+ mice under the feeding condition; however, there was no difference in the total number of amino acids in V1aR −/− mice and V1aR +/+ mice under the fasting condition (Table 1). The amino acid analysis also showed that the amounts of some essential amino acids, such as threonine, valine, methionine and isoleucine, were significantly decreased in V1aR −/− mice under both the fasting and feeding conditions relative to the values in V1aR +/+ mice. The amounts of ketogenic amino acids, such as leucine, isoleucine, lysine, and tyrosine, were decreased under the feeding or fasting condition in V1aR −/− mice. Of most glycogenic amino acids, such as tyrosine, serine, aspartic acid, methionine, threonine, arginine, valine, leucine and isoleucine, the amounts were also decreased under the feeding or fasting condition in V1aR −/− mice. The decreases in branched-chain amino acids, such as isoleucine, leucine and valine, were considerable in V1aR −/− mice.
Table 1.
Analysis of the amino acids of V1aR +/+ and V1aR −/− mice under the fasting and feeding conditions
| Feeding versus fasting | ||||||||
|---|---|---|---|---|---|---|---|---|
| Feeding condition | Fasting condition | P value | P value | |||||
| Amino acids | V1aR +/+ | V1aR −/− | P value | V1aR +/+ | V1aR −/− | P value | (V1aR +/+) | (V1aR −/−) |
| Aspartic acid | 14.0 ± 0.8 | 14.9 ± 0.7 | 0.418 | 8.3 ± 0.3 | 6.7 ± 0.2 | 0.008** | 0.0002*** | 0.0001*** |
| Threonine | 174.5 ± 6.8 | 127.5 ± 9.4 | 0.004** | 155.2 ± 7.6 | 125.4 ± 6.8 | 0.02* | 0.0962 | 0.8661 |
| Serine | 157.4 ± 8.3 | 121.5 ± 8.2 | 0.015* | 104.4 ± 5.1 | 108.5 ± 5.9 | 0.618 | 0.0006*** | 0.2375 |
| Asparagine | 50.6 ± 4.2 | 36.0 ± 2.2 | 0.015* | 49.5 ± 1.8 | 47.6 ± 4.3 | 0.699 | 0.8094 | 0.0452* |
| Glutamic acid | 62.7 ± 5.1 | 52.5 ± 3.7 | 0.145 | 43.3 ± 1.7 | 36.6 ± 2.3 | 0.055 | 0.0069** | 0.0069** |
| Glutamine | 576.6 ± 26.5 | 630.4 ± 28.9 | 0.207 | 529.5 ± 16.5 | 562.5 ± 33.5 | 0.403 | 0.1703 | 0.1644 |
| Proline | 111.4 ± 16.6 | 77.4 ± 8.8 | 0.108 | 69.9 ± 1.1 | 74.3 ± 3.46 | 0.267 | 0.0376* | 0.7511 |
| Glycine | 365.5 ± 20.5 | 259.2 ± 6.1 | 0.001*** | 198.8 ± 3.4 | 227.6 ± 6.24 | 0.004** | 0.0001*** | 0.0067** |
| Alanine | 440.3 ± 39.7 | 410.5 ± 49.8 | 0.652 | 279.1 ± 15.3 | 258.5 ± 14.9 | 0.363 | 0.0053** | 0.0191* |
| Citrulline | 58.6 ± 5.8 | 60.6 ± 4.3 | 0.787 | 42.0 ± 2.2 | 49.0 ± 3.1 | 0.108 | 0.0296* | 0.0605 |
| Valine | 216.1 ± 19.0 | 163.8 ± 9.4 | 0.039* | 239.8 ± 9.2 | 191.2 ± 14.1 | 0.021* | 0.2935 | 0.1455 |
| Cysteine | 4.3 ± 1.4 | 3.4 ± 0.9 | 0.607 | ND | ND | |||
| Methionine | 63.2 ± 5.5 | 42.1 ± 4.0 | 0.014* | 52.0 ± 1.59 | 44.7 ± 2.4 | 0.037* | 0.0871 | 0.5844 |
| Isoleucine | 88.5 ± 8.3 | 67.5 ± 4.4 | 0.057 | 114.3 ± 2.4 | 93.6 ± 7.7 | 0.034* | 0.0175* | 0.0187* |
| Leucine | 148.4 ± 17.5 | 116.0 ± 8.5 | 0.134 | 202.7 ± 7.0 | 163.1 ± 11.8 | 0.021* | 0.0204* | 0.012* |
| Tyrosine | 69.2 ± 4.6 | 49.8 ± 3.4 | 0.01** | 54.1 ± 0.7 | 56.68 ± 5.0 | 0.632 | 0.0124* | 0.2876 |
| Phenylalanine | 66.0 ± 5.8 | 57.4 ± 4.0 | 0.255 | 75.0 ± 1.9 | 72.76 ± 1.6 | 0.401 | 0.1764 | 0.0072** |
| Ornithine | 92.8 ± 11.6 | 55.3 ± 4.2 | 0.016* | 53.3 ± 0.7 | 64.7 ± 4.2 | 0.023* | 0.0095** | 0.1409 |
| Lysine | 324.0 ± 17.3 | 247.3 ± 16.0 | 0.012* | 236.1 ± 7.0 | 223.1 ± 6.9 | 0.226 | 0.0015** | 0.2038 |
| Arginine | 145.5 ± 9.5 | 132.0 ± 10.3 | 0.366 | 119.6 ± 6.2 | 101.5 ± 5.2 | 0.056 | 0.0528 | 0.0293* |
| Tryptophan | 56.1 ± 3.4 | 46.9 ± 3.3 | 0.086 | 38.3 ± 2.0 | 44.1 ± 2.0 | 0.086 | 0.0022** | 0.4975 |
| Histidine | 59.2 ± 2.7 | 60.8 ± 4.3 | 0.763 | 55.9 ± 2.3 | 58.2 ± 2.5 | 0.504 | 0.3768 | 0.6217 |
| Total amount | 3344 ± 138.2 | 2832 ± 113.7 | 0.021* | 2724 ± 61.2 | 2612 ± 83.2 | 0.309 | 0.0034** | 0.1569 |
| Urea | 9131.0 ± 762.2 | 8369.0 ± 500.7 | 0.428 | 11 109.1 ± 625.9 | 11 207.8 ± 1087.7 | 0.939 | 0.0798 | 0.0451* |
Values are the means ± s.e.m. (nmol ml−1). These results were obtained from five mice. ND, not detectable. Asterisks show significance(
P < 0.001
P < 0.01 and
P < 0.05).
Gluconeogenesis was promoted in V1aR −/− mice
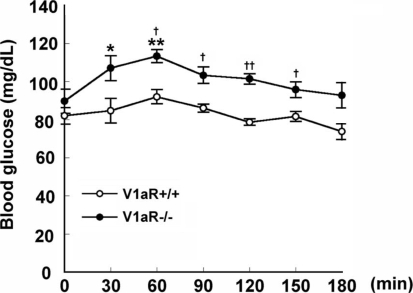
Because the degradation of most glycogenic amino acids was promoted in V1aR −/− mice, an alanine-loading test was carried out to investigate whether gluconeogenesis is promoted in V1aR −/− mice. The basal blood glucose levels were 82.0 ± 4.36 mg dl−1 and 89.7 ± 6.39 mg dl−1 in V1aR +/+ and V1aR −/− mice (n = 4, each), respectively. The blood glucose levels in V1aR −/− mice tended to be higher than those in V1aR +/+ mice 60, 90, 120 and 150 min after loading (Fig. 2), but the group comparison by two-way ANOVA followed by the post hoc test (Fisher's PLSD) revealed that there was no significant difference (F6,42 = 0.707, P = 0.6470).
Figure 2.
Gluconeogenesis in V1aR +/+ and V1aR −/− mice Alanine was orally administered to mice at a dose of 666 mg (kg body weight)−1. The blood glucose concentration was measured every 30 min until 180 min. The elevation of the blood glucose level after the administration was significantly higher in V1aR −/− mice than in V1aR +/+ mice. The results are means ± s.e.m. of four mice. Significance, versus each basal level: *P < 0.05; **P < 0.01. Significance, V1aR +/+ mice versus V1aR −/− mice: †P < 0.05; ††P < 0.01.
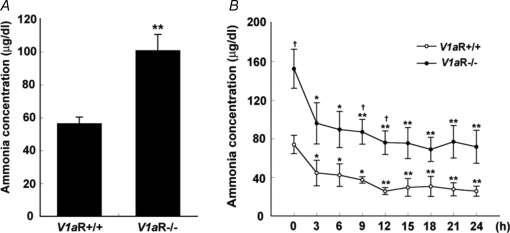
Blood ammonia
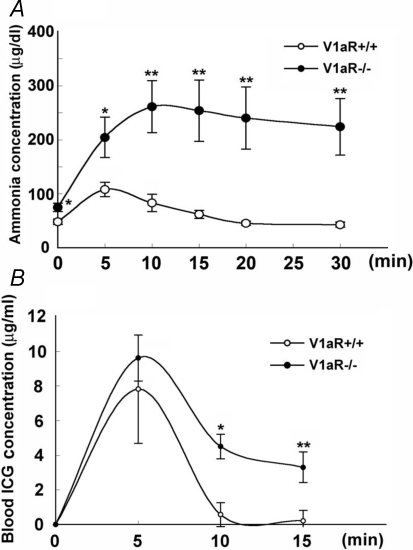
The level of blood ammonia, which is a by-product of amino acid catabolism, was examined. The blood ammonia level was 56.3 ± 3.95 μg dl−1 (daytime mean, n = 35) in V1aR +/+ mice. On the other hand, the blood ammonia level in V1aR −/− mice was 100.7 ± 9.93 μg dl−1 (daytime mean, n = 30) (Fig. 3A). To further examine the effect of food intake on the blood ammonia level, those mice were fasted for 24 h, and the ammonia levels were measured every 3 h (9:00 a.m. to 9:00 a.m.). The ammonia levels of both V1aR +/+ and V1aR −/− mice were decreased in a time-dependent manner after the onset of fasting, but the ammonia levels of V1aR −/− mice were higher than those of V1aR +/+ mice at all points (Fig. 3B). However, two-way ANOVA followed by the post hoc test (Fisher's PLSD) showed that there was no significant difference between the two groups (F8,66 = 0.229, P = 0.9842).
Figure 3.
Daytime blood ammonia levels and effect of fasting on blood ammonia levels in V1aR +/+ and V1aR −/− mice The blood ammonia levels were measured using the Amicheck meter PocketChem. The ammonia levels of V1aR −/− mice (n = 35) were significantly higher than those of V1aR +/+ mice (n = 30) under the feeding condition (A). The ammonia levels under the fasting condition (< 24 h) were compared between V1aR +/+ and V1aR −/− mice every 3 h. The ammonia level of V1aR −/− mice (n = 4) was significantly higher than that of V1aR +/+ mice (n = 6) within 24 h (B). The results are the mean ± s.e.m. of the number of mice. Significance, versus each basal level: *P < 0.05; **P < 0.01). Significance, V1aR +/+ mice versus V1aR −/− mice: †P < 0.05.
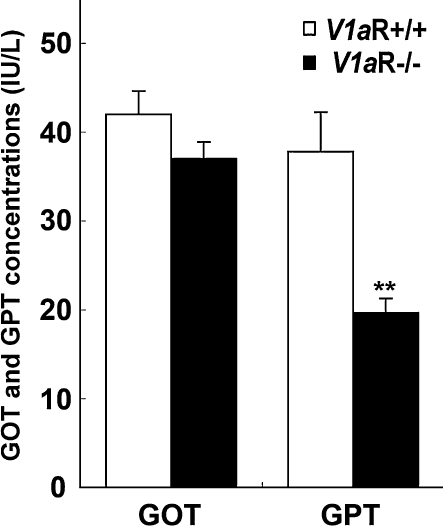
Liver dysfunction was not detected in V1aR −/− mice
To examine hepatic insufficiency and hepatopathy in V1aR −/− mice, glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) levels were measured. The GOT and GPT levels of V1aR −/− mice were within a normal range (GOT: 37.0 ± 1.86 i.u. l−1 in V1aR −/− mice and 42.0 ± 2.59 i.u. l−1 in V1aR +/+ mice, n = 9 each; GPT: 19.7 ± 1.58 i.u. l−1 in V1aR −/− mice and 37.7 ± 4.47 i.u. l−1 in V1aR +/+ mice, n = 9 each), indicating that the liver function of the V1aR −/− mice was intact (Fig. 4).
Figure 4.
Glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) levels in V1aR +/+ and V1aR −/− mice Serum was prepared from the inferior vena cava of V1aR +/+ and V1aR −/− mice and used to measure GOT and GPT levels. These levels were normal in V1aR +/+ and V1aR −/− mice. The results are the mean ± s.e.m. of nine mice. Significance, V1aR +/+ mice versus V1aR −/− mice: **P < 0.01.
Lower ammonia clearance after ammonia administration and reduced intrahepatic circulating blood volume in V1aR −/− mice
To evaluate the ammonia clearance capacity in V1aR −/− mice, an ammonia tolerance test was carried out. After 5, 10, 15, 20 and 30 min of ammonium chloride administration, blood ammonia levels were measured. In V1aR +/+ mice, the ammonia levels increased, peaking 5 min after administration, and then dropped to the basal level after 20 min. In V1aR −/− mice, the ammonia levels increased more, peaking 10 min after administration, and slowly dropped for 30 min after administration, indicating that V1aR −/− mice had decreased ammonia clearance after ammonia administration (Fig. 5A). An ICG elimination test was carried out. The ICG retention rate 15 min after injection (R15) increased more significantly in V1aR −/− mice than in V1aR +/+ mice (2.05 ± 0.006% in V1aR +/+ mice, n = 10; and 32.96 ± 0.009% in V1aR −/− mice, n = 9; P = 0.0088). The elimination rates (k) were 0.106 and 0.069 in V1aR +/+ and V1aR −/− mice, respectively (Fig. 5B).
Figure 5.
Comparison of intrahepatic blood volume in V1aR +/+ and V1aR −/− mice A, V1aR +/+ and V1aR −/− mice fasted for more than 8 h before the ammonia tolerance test. The mice were orally administered an ammonium chloride solution, and 5, 10, 15, 20 and 30 min after the administration, the blood ammonia levels were measured using the Amicheck meter PocketChem. Significance of variation: *P < 0.05; **P < 0.01. The results are the mean ± s.e.m. of six or seven mice. B, mice fasted for 12 h were injected with indocyanine green (ICG), and their blood was collected before and 5, 10 and 15 min after the injection. The ICG concentration in plasma was calculated. The results are the means ± s.e.m. of 10 or 9 mice. Significance of variation: *P < 0.05; **P < 0.01).
Comparison of serum lactic acid, ketone bodies, and arterial blood CO2 and pH level in V1aR +/+ and V1aR −/− mice
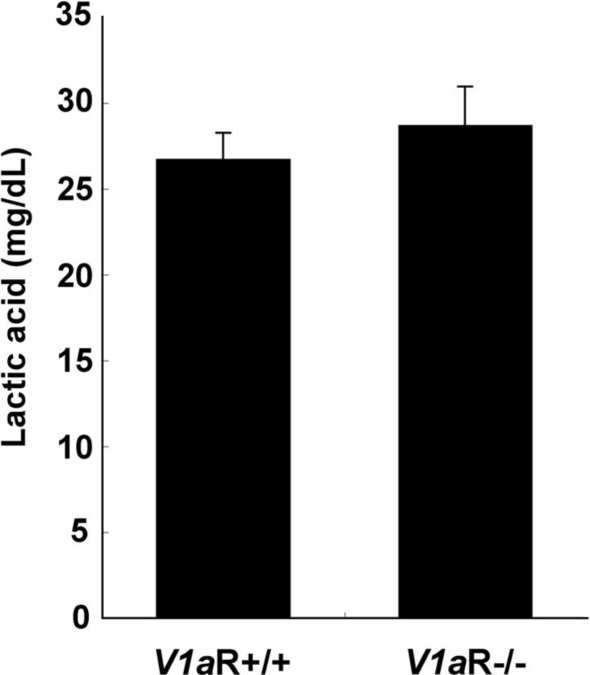
Next, we assessed whether there was metabolic acidosis in V1aR −/− mice, which could result in hyperammonaemia. To assess the possibility of lactic or ketotic acidosis in V1aR −/− mice, the lactic acid levels and total amounts of ketone bodies were examined in V1aR +/+ and V1aR −/− mice. There was no significant difference in the levels of lactic acid or ketone bodies between the two groups (lactic acid: 28.7 ± 1.50 mg dl−1 in V1aR −/− mice, n = 12, and 26.7 ± 2.24 mg dl−1 in V1aR +/+ mice, n = 12, P = 0.4777; ketone bodies: 109.8 ± 21.38 μmol l−1 in V1aR −/− mice, n = 12, and 142.4 ± 10.86 μmol l−1 in V1aR +/+ mice, n = 11, P = 0.178) (Fig. 6) (Hiroyama et al. 2007).
Figure 6.
Lactic acid levels in V1aR +/+ and V1aR −/− mice Serum was prepared from the inferior vena cava of V1aR +/+ and V1aR −/− mice. Lactic acid was measured using Determiner LA. The levels of lactic acid in V1aR +/+ and V1aR −/− mice were equivalent. The results are the mean ± s.e.m. of 12 mice.
Furthermore, the arterial blood pH in V1aR −/− mice was not different from that in V1aR +/+ mice (Table 2), indicating that there was no pH difference between those groups. The serum osmolality of V1aR −/− mice was also not different from that of V1aR +/+ mice (data not shown). Taking the lower levels of PCO2 in V1aR −/− mice into account, the metabolic acidosis in V1aR −/− mice could be compensated, but the remarkable metabolic acidosis was not observed.
Table 2.
Arterial pH and CO2 levels in V1aR +/+ and V1aR −/− mice
| Arterial pH | PCO2 (mmHg) | |
|---|---|---|
| (Feed) | ||
| V1aR +/+ | 7.35 ± 0.017 | 49.5 ± 2.33 |
| V1aR −/− | 7.36 ± 0.029 | 39.5 ± 2.98 |
| P value | 0.5978 | 0.0255* |
| n = 6 | n = 5 | |
| (Fast) | ||
| V1aR +/+ | 7.30 ± 0.02 | 47.2 ± 0.65 |
| V1aR −/− | 7.29 ± 0.05 | 38.1 ± 2.73 |
| P value | 0.9585 | 0.0849 |
| n = 5 | n = 5 |
Values are the means ± s.e.m. Asterisks show significance (
P < 0.05).
Discussion
In this study, we investigated the effect of V1a receptor deficiency on protein metabolism in V1aR −/− mice because the blockade of AVP action via the V1a receptor in the muscle by the deficiency of the receptor would influence protein metabolism. Insulin sensitivity also has been found to be impaired in V1aR −/− mice (Aoyagi et al. 2007), which could also affect protein metabolism. The 3-methylhistidine level in V1aR −/− mice was higher than that in V1aR +/+ mice under the feeding condition (Fig. 1A), indicating that protein catabolism was promoted in mutant mice. This finding is consistent with the result of the analysis of the total mass of amino acids (Table 1) as well as with previous reports that AVP prevents proteolysis by reducing the release of 3-methylhistidine (Goodman, 1987a; Thompson et al. 1994, 1996). AVP is involved in regulating protein metabolism by stimulating protein synthesis and inhibiting proteolysis (Goodman, 1987a; Wolthuis et al. 1992; Thompson et al. 1994, 1996; Simon et al. 1995; Fukuzawa et al. 1999; Xu et al. 1999; Nakamura et al. 2000; Lagumdzija et al. 2004). Therefore, the blunted signal of AVP via the V1a receptor on protein metabolism could promote protein catabolism in V1aR −/− mice. Furthermore, the phenotype of the promoted protein catabolism could be exacerbated by the accompanying insulin-resistant phenotype in V1aR −/− mice since enhanced protein breakdown has been observed in the skeletal muscle of rats with experimentally induced diabetes (Pepato et al. 1996; Bailey et al. 1997) as well as in type 1 diabetic patients (reviewed in Tessari et al. 1992).
The 3-methylhistidine level was increased in V1aR +/+ mice under the fasting condition more than under the feeding condition, perhaps due in part to the increased myofibrillar protein breakdown during fasting (Lowell et al. 1986; Goodman, 1987b). When V1aR −/− mice were compared with V1aR +/+ mice, the 3-methylhistidine level was higher in V1aR −/− mice than in V1aR +/+ mice under the feeding condition, and the increase from the fed level to the fasted level was less in V1aR −/− mice than in V1aR +/+ mice, suggesting that myofibrillar protein breakdown could be promoted in V1aR −/− mice even when they were not starving and that further promotion of the protein breakdown could hardly be achieved during fasting.
The amino acid profile suggested that the metabolism of amino acids was accelerated in V1aR −/− mice. Most glycogenic amino acids (tyrosine, serine, aspartic acid, methionine, threonine, arginine, valine, leucine and isoleucine) were decreased in V1aR −/− mice, suggesting that the glycogenic amino acid metabolism was enhanced in muscle. In particular, among those amino acids, the decreases in branched-chain amino acids (isoleucine, leucine and valine) in V1aR −/− mice indicated that the amino acid metabolism was greatly enhanced in the muscle because it is the only place where it might be used. Furthermore, the amounts of ketogenic amino acids, such as leucine, isoleucine, lysine and tyrosine, were also decreased under the feeding or fasting condition in V1aR −/− mice, suggesting that the metabolism of ketogenic amino acids was enhanced in the liver.
The total amount of amino acids decreased in V1aR +/+ mice, while the amount of urea increased, during fasting compared to during feeding, which implied that the catabolism of the amino acids was promoted during fasting in V1aR +/+ mice. On the other hand, there was no difference in the total amount of amino acids in V1aR −/− mice under the feeding and fasting conditions, whereas the urea level under the fasting condition (equal to that of V1aR +/+ mice) was higher than that under the feeding condition. This finding indicated that urea production was increased not by an increase in the ammonia level but by fasting in both V1aR +/+ and V1aR −/− mice. Urea is produced in the liver as the end-product of amino nitrogen from amino acids by the enzymes of the urea cycle such as arginase and ornithine transcarbamoylase, whose activities are known to be 30% higher under the fasting condition than the feeding condition (Schimke, 1962, Edmonds & Baker, 1987). Thus, up-regulated enzyme activity in the urea cycle could lead to an increase in the amount of urea after fasting.
We have found that proteolysis is promoted in V1a receptor-deficient mice, suggesting that the receptor could be involved in proteolytic activity in the muscle and liver. However, AVP is also reported to inhibit protein synthesis by 8% in liver cells (vom Dahl et al. 1991; Stoll et al. 1992). There is a close relationship between the extent of cell volume change and proteolytic activity in the liver (Häussinger et al. 1990). Liver cell volume is known to be an important modulator of liver cell function. Decreased cell volume promotes proteolysis, while increased cell volume suppresses proteolysis in the liver (Häussinger et al. 1990). On the other hand, AVP is known to play a crucial role in regulating cell volume as well as extracellular fluid volume (Solenov et al. 2003; Ford et al. 2005). Thus, AVP could be involved in regulating proteolytic activity in the liver by affecting cell volume. However, this action of AVP seems to be mediated via the V2 receptor in the kidney, where AVP exerts its functional role in regulating water recruitment and, thereby, cell volume (Solenov et al. 2003; Ford et al. 2005). Therefore, our finding with V1a receptor-deficient mice suggests that AVP could play a role in proteolytic activity not only via the V2 receptor, which is involved in regulating cell volume, but also via the V1a receptor. Protein synthesis via the vasopressin V1a receptor is probably accomplished with an increase in phospholipase C (PLC) activity and the mobilization of intracellular Ca2+ (Fukuzawa et al. 1999; Xu et al. 1999), although the underlying mechanism via the V1a receptor remains to be elucidated.
To investigate whether gluconeogenesis was promoted as a result of increased proteolysis in V1aR −/− mice, we carried out an alanine-loading test, during which the blood glucose levels were higher in V1aR −/− mice than in V1aR +/+ mice. This result was consistent with our findings, through a hyperinsulinaemic–euglycaemic clamp study and a histological study, that hepatic glucose production in the liver of V1aR −/− mice was enhanced and the glycogen content in the liver of V1aR −/− mice was reduced (Aoyagi et al. 2007). Furthermore, this result can explain in part why the basal level of the blood glucose in V1aR −/− mice was increased (Fig. 3). It is known that glucagon facilitates the decomposition of glycogen (Magnusson et al. 1995; Chhibber et al. 2000) and amino acids to produce glucose (Charlton et al. 1996) and that hyperglucagonaemia causes the hypermetabolism of amino acids and hyperammonaemia (Kabadi et al. 1985). Therefore, we examined the blood glucagon level, which was lower in V1aR −/− than in V1aR +/+ mice (788.7 ± 109.5 pg ml−1 in V1aR +/+ mice, n = 9, and 476.5 ± 74.5 pg ml−1 in V1aR −/− mice, n = 10, P = 0.028, authors' unpublished observations), indicating that there was no hyperglucagonaemia in V1aR −/− mice. These results might reflect the anti-proteolytic effect of AVP (Goodman, 1987a; Thompson et al. 1994, Thompson 1996).
The level of blood ammonia, which is a by-product of amino acid catabolism (Hird & Marginson, 1968), of V1aR −/− mice was significantly higher than that of V1aR +/+ mice (Fig. 4A). To investigate the effect of ammonia generated by dietary intake, we compared the blood ammonia levels during and after fasting. The results showed that the ammonia levels gradually decreased in both V1aR +/+ and V1aR −/− mice after fasting but were always higher in V1aR −/− mice than in V1aR +/+ mice (Fig. 3B). Normally, ammonia is produced from endogenous and exogenous dietary protein in the liver, kidney, gut and muscle. The gut is the major source of ammonia from dietary protein (Folin & Denis, 1912) as unabsorbed protein in the small intestine is deaminated by bacteria in the caecum (Dawson, 1978). After fasting, no exogenous dietary protein was supplied, which could have led to the reduced production of ammonia in the gut and, consequently, to the decrease in the plasma ammonia level. On the other hand, the metabolism of endogenous protein and/or amino acids is promoted in several organs such as the muscle and liver, and ammonia production from the endogenous protein could be increased during fasting. Thus, the fasting ammonia level is reflected by proteolysis of endogenous proteins rather than exogenous dietary proteins. The ammonia level in V1aR −/− mice is higher than that in V1aR +/+ mice under the fasting condition as well as under the feeding condition, suggesting that proteolysis is promoted in the muscle and liver of V1aR −/− mice compared to V1aR +/+ mice, which could be the primary cause of the increased ammonia level. In addition, the food intake of V1aR −/− mice was smaller than that of V1aR +/+ mice (Aoyagi et al. 2007).
Hyperammonaemia is usually found together with liver dysfunctions, including hepatic encephalopathy (Walker & Schenker, 1970). However, hepatopathy (increased GOT and GPT) was not observed in V1aR −/− mice (Fig. 5), suggesting that hyperammonaemia in V1aR −/− mice was not due to liver dysfunction or failure. There are a number of non-hepatic diseases or disorders which cause hyperammonaemia: genetic defects of enzymes in the urea cycle or other pathways of intermediary metabolism, organic acidurias, hyperinsulinaemic hypoglycaemia, distal renal tubular acidosis, carnitine deficiency, fatty acid oxidation defects, Reye's syndrome, portosystemic shunts, urinary diversion, urinary infection, and multiple myeloma, among others (Hawkes et al. 2001). Neither a carnitine deficiency nor a fatty acid oxidation defect was observed in V1aR −/− mice (Hiroyama et al. 2007). V1aR −/− mice were not likely to suffer from hyperinsulinaemic hypoglycaemia because the blood glucose level in V1aR −/− mice was higher than that in V1aR +/+ mice at the basal level under the fasting condition (Fig. 3). The level of ornithine, which is a key amino acid in the urea cycle, was lower in V1aR −/− mice than in V1aR +/+ mice under the fasting condition, but the arginine and citrulline levels did not significantly increase (Table 1), suggesting that the decreased ornithine level in V1aR −/− mice was not caused by the impaired activities of enzymes in the urea cycle. In addition, the blood urea levels were equivalent in both V1aR +/+ and V1aR −/− mice under either the fasting or feeding condition, emphasizing that the activity of enzymes in the urea cycle in V1aR −/− mice was not impaired. However, the decrease in branched-chain amino acids might be one of the causes of hyperammonaemia because those amino acids supply glutamic acid (Hutson et al. 2005), which is essential for ammonia consumption. Thus, the decrease in branched-chain amino acids reduces the capacity to consume ammonia, leading to hyperammonaemia.
In addition to causing hepatic disorders, the intrahepatic circulating blood volume also influences the blood ammonia level (Hawkes et al. 2001). To assess a portosystemic shunt or a small circulating blood volume in the liver, the ammonia tolerance test is useful (Conn, 1961; Gips, 1974). The result of the ammonia tolerance test showed that the rate of ammonia consumption in V1aR −/− mice was slower than that of V1aR +/+ mice (Fig. 5A), suggesting that V1aR −/− mice had a small intrahepatic circulating blood volume. Furthermore, it is known that the results of the ammonia tolerance test correlate with the plasma disappearance rate of ICG (Koyama et al. 1981), which reflects the hepatic function and the intrahepatic circulating blood volume (Koyama et al. 1981). The ICG elimination rate in V1aR −/− mice was also slower than that of V1aR +/+ mice (Fig. 5B). These results showed that the intrahepatic circulating blood volume was smaller in V1aR −/− mice, but the possibility of a portosystemic shunt remained unresolved. We previously reported that the liver weight of V1aR −/− mice was smaller than that of V1aR +/+ mice (Hiroyama et al. 2007). The smaller liver size could be explained by the small intrahepatic circulating blood volume. Furthermore, we might consider the actions of AVP on the smooth muscle cells of vessels because AVP has a vasoconstricting action on blood vessels (Altura, 1975). On the other hand, very recently, V1aR −/− mice with a reduced ANP level were reported to have a 9% lower circulating body blood volume (Koshimizu et al. 2006), supporting the idea of a decrease in the intrahepatic circulating blood volume. However, the primary mechanism responsible for the reduced circulating blood volume is uncertain.
In addition to anti-lipolysis, we here showed that AVP had an anti-proteolytic action via the V1a receptor by showing that proteolysis was promoted in V1aR −/− mice and that hyperammonaemia was induced by hyperproteolysis and the reduced intrahepatic blood volume.
Acknowledgments
This work was supported in part by research grants from the Scientific Fund of the Ministry of Education, Science, and Culture of Japan; the Ministry of Human Health and Welfare of Japan; and the Japan Health Sciences Foundation.
References
- Altura BM. Dose–response relationship for arginine vasopressin and synthetic analogs on three types of rat blood vessels: Possible evidence for regional differences in vasopressin receptor sites within a mammal. J Pharmacol Exp Ther. 1975;193:413–423. [PubMed] [Google Scholar]
- Aoyagi T, Birumachi J, Hiroyama M, Fujiwara Y, Sanbe A, Yamauchi J, Tanoue A. Alteration of glucose homeostasis in V1a vasopressin receptor-deficient mice. Endocrinology. 2007;148:2075–2084. doi: 10.1210/en.2006-1315. [DOI] [PubMed] [Google Scholar]
- Bailey JL, Price SR, England BK, Jurkovitz C, Wang X, Ding X, Mitch WE. Signals regulating accelerated muscle protein catabolism in uremia. Miner Electrolyte Metab. 1997;23:198–200. [PubMed] [Google Scholar]
- Charlton MR, Adey DB, Nair KS. Evidence for a catabolic role of glucagon during an amino acid load. J Clin Invest. 1996;98:90–99. doi: 10.1172/JCI118782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhibber VI, Soriano C, Tayek JA. Effects of low-dose and high-dose glucagon on glucose production and gluconeogenesis in humans. Metabolism. 2000;49:39–46. doi: 10.1016/s0026-0495(00)90638-3. [DOI] [PubMed] [Google Scholar]
- Conn HO. Ammonia tolerance as an index of portal-systemic collateral circulation in cirrhosis. Gastroenterology. 1961;41:97–106. [PubMed] [Google Scholar]
- Dawson AM. Regulation of blood ammonia. Gut. 1978;19:504–509. doi: 10.1136/gut.19.6.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning BE, Moltz JH, Fawcett CP. The effects of oxytocin and vasopressin on hormone release: Comparison of provocative test procedures. Am J Med. 1982;56:179–185. doi: 10.1016/0002-9343(74)90595-6. [DOI] [PubMed] [Google Scholar]
- Edmonds MS, Baker DH. Effects of fasting on tissue amino acid concentrations and urea-cycle enzymatic activities in young pigs. J Anim Sci. 1987;65:1538–1552. doi: 10.2527/jas1987.6561538x. [DOI] [PubMed] [Google Scholar]
- Egashira N, Tanoue A, Higashihara F, Mishima K, Fukue Y, Takano Y, Tsujimoto G, Iwasaki K, Fujiwara M. V1a receptor knockout mice exhibit impairment of spatial memory in an eight-arm radial maze. Neurosci Lett. 2004;356:195–198. doi: 10.1016/j.neulet.2003.11.050. [DOI] [PubMed] [Google Scholar]
- Folin O, Denis W. Protein metabolism from the standpoint of blood and tissue analysis. II. The origin and significance of the ammonia in the portal blood. J Biol Chem. 1912;11:161–167. [Google Scholar]
- Ford P, Rivarola V, Chara O, Blot-Chabaud M, Cluzeaud F, Farman N, Parisi M, Capurro C. Volume regulation in cortical collecting duct cells: role of AQP2. Biol Cell. 2005;97:687–697. doi: 10.1042/BC20040116. [DOI] [PubMed] [Google Scholar]
- Fukuzawa J, Haneda T, Kikuchi K. Arginine vasopressin increases the rate of protein synthesis in isolated perfused adult rat heart via the V1 receptor. Mol Cell Biochem. 1999;195:93–98. doi: 10.1023/a:1006980517557. [DOI] [PubMed] [Google Scholar]
- Gips CH. The rectal and oral arterial ammonia tests: a comparison. Neth J Med. 1974;17:12–19. [PubMed] [Google Scholar]
- Goodman MN. Acute alterations in sodium flux in vitro lead to decreased myofibrillar protein breakdown in rat skeletal muscle. Biochem J. 1987a;247:151–156. doi: 10.1042/bj2470151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MN. Differential effects of acute changes in cell Ca2+ concentration on myofibrillar and non-myofibrillar protein breakdown in the rat extensor digitorum longus muscle in vitro. Assessment by production of tyrosine and Nt-methylhistidine. Biochem J. 1987b;241:121–127. doi: 10.1042/bj2410121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D, Hallbrucker C, vom Dahl S, Lang F, Gerok W. Cell swelling inhibits proteolysis in perfused rat liver. Biochem J. 1990;272:239–242. doi: 10.1042/bj2720239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes ND, Thomas GA, Jurewicz A, Williams OM, Hillier CE, McQueen IN, Shortland G. Non-hepatic hyperammonaemia: An important, potentially reversible cause of encephalopathy. Postgrad Med J. 2001;77:717–722. doi: 10.1136/pmj.77.913.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems DA. Short-term hormonal control of hepatic carbohydrate and lipid catabolism. FEBS Lett. 1977;80:237–245. doi: 10.1016/0014-5793(77)80449-3. [DOI] [PubMed] [Google Scholar]
- Hird FJ, Marginson MA. The formation of ammonia from glutamine and glutamate by mitochondria from rat liver and kidney. Arch Biochem Biophys. 1968;127:718–724. doi: 10.1016/0003-9861(68)90282-8. [DOI] [PubMed] [Google Scholar]
- Hiroyama M, Aoyagi T, Fujiwara Y, Birumachi J, Shigematsu Y, Kiwaki K, Tasaki R, Endo F, Tanoue A. Hypermetabolism of fat in V1a vasopressin receptor knockout mice. Mol Endocrinol. 2007;21:247–258. doi: 10.1210/me.2006-0069. [DOI] [PubMed] [Google Scholar]
- Hutson SM, Sweatt AJ, Lanoue KF. Branched-chain amino acid metabolism: implications for establishing safe intakes. J Nutr. 2005;135:1557S–1564S. doi: 10.1093/jn/135.6.1557S. [DOI] [PubMed] [Google Scholar]
- Kabadi UM, Eisenstein AB, Konda J. Elevated plasma ammonia level in hepatic cirrhosis: role of glucagon. Gastroenterology. 1985;88:750–756. doi: 10.1016/0016-5085(85)90146-5. [DOI] [PubMed] [Google Scholar]
- Keppens S, De Wulf H. The nature of the hepatic receptors involved in vasopressin-induced glycogenolysis. Biochim Biophys Acta. 1979;588:63–69. doi: 10.1016/0304-4165(79)90371-4. [DOI] [PubMed] [Google Scholar]
- Koshimizu T, Nasa Y, Tanoue A, Oikawa R, Kawahara Y, Kiyono Y, Adachi T, Tanaka T, Kuwaki T, Mori T, Takeo S, Okamura H, Tsujimoto G. V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. Proc Natl Acad Sci U S A. 2006;103:7807–7812. doi: 10.1073/pnas.0600875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama K, Owada Y, Imaoka Y, Sato T. Oral ammonia tolerance test in patients with portal hypertension. Tohoku J Exp Med. 1981;135:93–101. doi: 10.1620/tjem.135.93. [DOI] [PubMed] [Google Scholar]
- Lagumdzija A, Bucht E, Stark A, Hulting AL, Petersson M. Arg-vasopressin increases proliferation of human osteoblast-like cells and decreases production of interleukin-6 and macrophage colony-stimulating factor. Regul Pept. 2004;121:41–48. doi: 10.1016/j.regpep.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Ruderman NB, Goodman MN. Evidence that lysosomes are not involved in the degradation of myofibrillar proteins in rat skeletal muscle. Biochem J. 1986;234:237–240. doi: 10.1042/bj2340237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson I, Rothman DL, Gerard DP, Katz LD, Shulman GI. Contribution of hepatic glycogenolysis to glucose production in humans in response to a physiological increase in plasma glucagon concentration. Diabetes. 1995;44:185–189. doi: 10.2337/diab.44.2.185. [DOI] [PubMed] [Google Scholar]
- Michell RH, Kirk CJ, Billah MM. Hormonal stimulation of phosphatidylinositol breakdown with particular reference to the hepatic effects of vasopressin. Biochem Soc Trans. 1979;7:861–865. doi: 10.1042/bst0070861. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Haneda T, Osaki J, Miyata S, Kikuchi K. Hypertrophic growth of cultured neonatal rat heart cells mediated by vasopressin V1A receptor. Eur J Pharmacol. 2000;391:39–48. doi: 10.1016/s0014-2999(99)00775-x. [DOI] [PubMed] [Google Scholar]
- Oshikawa S, Tanoue A, Koshimizu TA, Kitagawa Y, Tsujimoto G. Vasopressin stimulates insulin release from islet cells through V1b receptors: a combined pharmacological/knockout approach. Mol Pharmacol. 2004;65:623–629. doi: 10.1124/mol.65.3.623. [DOI] [PubMed] [Google Scholar]
- Pepato MT, Migliorini RH, Goldberg AL, Kettelhut IC. Role of different proteolytic pathways in degradation of muscle protein from streptozotocin-diabetic rats. Am J Physiol Endocrinol Metab. 1996;271:E340–E347. doi: 10.1152/ajpendo.1996.271.2.E340. [DOI] [PubMed] [Google Scholar]
- Rofe AM, Williamson DH. Metabolic effects of vasopressin infusion in the starved rat. Biochem J. 1983;212:231–239. doi: 10.1042/bj2120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke RT. Differential effects of fasting and protein-free diets on levels of urea cycle enzymes in rat liver. J Biol Chem. 1962;237:1921–1924. [PubMed] [Google Scholar]
- Simon JS, Baum JS, Moore SA, Kasson BG. Arginine vasopressin stimulates protein synthesis but not proliferation of cultured vascular endothelial cells. J Cardiovasc Pharmacol. 1995;25:368–375. doi: 10.1097/00005344-199503000-00004. [DOI] [PubMed] [Google Scholar]
- Solenov EI, Nesterov VV, Baturina GS, Khodus GR, Ivanova LN. Effect of dDAVP on basolateral cell surface water permeability in the outer medullary collecting duct. Eur Biophys J. 2003;32:614–619. doi: 10.1007/s00249-003-0308-9. [DOI] [PubMed] [Google Scholar]
- Spruce BA, McCulloch AJ, Burd J, Orskov H, Heaton A, Baylis PH, Alberti KG. The effect of vasopressin infusion on glucose metabolism in man. Clin Endocrinol. 1985;22:463–468. doi: 10.1111/j.1365-2265.1985.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Stoll B, Gerok W, Lang F, Häussinger D. Liver cell volume and protein synthesis. Biochem J. 1992;287:217–222. doi: 10.1042/bj2870217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari P, Biolo G, Inchiostro S, Sabadin L, Tiengo A. Insulin resistance and protein metabolism. In: Nair KH, editor. Protein Metabolism in Diabetes Mellitus. London: Smith-Gordon; 1992. pp. 181–185. [Google Scholar]
- Thompson MG, Mackie SC, Morrison KS, Thom A, Palmer RM. Stimulation of protein synthesis and phospholipase D activity by vasopressin and phorbol ester in L6 myoblasts. Biochim Biophys Acta. 1994;1224:198–204. doi: 10.1016/0167-4889(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Thompson MG, Palmer RM, Thom A, Garden K, Lobley GE, Calder G. Nτ-methylhistidine turnover in skeletal muscle cells measured by GC-MS. Am J Physiol Cell Physiol. 1996;270:C1875–C1879. doi: 10.1152/ajpcell.1996.270.6.C1875. [DOI] [PubMed] [Google Scholar]
- vom Dahl S, Hallbrucker C, Lang F, Häussinger D. Regulation of cell volume in the perfused rat liver by hormones. Biochem J. 1991;280:105–109. doi: 10.1042/bj2800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CO, Schenker S. Pathogenesis of hepatic encephalopathy – with special reference to the role of ammonia. Am J Clin Nutr. 1970;23:619–632. doi: 10.1093/ajcn/23.5.619. [DOI] [PubMed] [Google Scholar]
- Wolthuis A, Boes A, Rodemann HP, Grond J. Vasoactive agents affect growth and protein synthesis of cultured rat mesangial cells. Kidney Int. 1992;41:124–131. doi: 10.1038/ki.1992.16. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hopfber RL, McNeill JR, Gopalakrishnan V. Vasopressin accelerates protein synthesis in neonatal rat cardiomyocytes. Mol Cell Biochem. 1999;195:183–190. doi: 10.1023/a:1006961330375. [DOI] [PubMed] [Google Scholar]
- Yibchok-anun S, Hsu WH. Effects of arginine vasopressin and oxytocin on glucagon release from clonal alpha-cell line In-R1-G9: Involvement of V1b receptors. Life Sci. 1998;63:1871–1878. doi: 10.1016/s0024-3205(98)00463-9. [DOI] [PubMed] [Google Scholar]
- Young VR, Alexis SD, Baliga BS, Munro HN. Metabolism of administered 3-methylhistidine: Lack of muscle transfer of ribonucleic acid charging and quantitative excretion of 3-methylhistidine N-acetyl derivative. J Biol Chem. 1972;247:3592–3600. [PubMed] [Google Scholar]