Abstract
A novel R133W β-tropomyosin (β-Tm) mutation, associated with muscle weakness and distal limb deformities, has recently been identified in a woman and her daughter. The muscle weakness was not accompanied by progressive muscle wasting or histopathological abnormalities in tibialis anterior muscle biopsy specimens. The aim of the present study was to explore the mechanisms underlying the impaired muscle function in patients with the β-Tm mutation. Maximum force normalized to fibre cross-sectional area (specific force, SF), maximum velocity of unloaded shortening (V0), apparent rate constant of force redevelopment (ktr) and force–pCa relationship were evaluated in single chemically skinned muscle fibres from the two patients carrying the β-Tm mutation and from healthy control subjects. Significant differences in regulation of muscle contraction were observed in the type I fibres: a lower SF (P < 0.05) and ktr (P < 0.01), and a faster V0 (P < 0.05). The force–pCa relationship did not differ between patient and control fibres, indicating an unaltered Ca2+ activation of contractile proteins. Collectively, these results indicate a slower cross-bridge attachment rate and a faster detachment rate caused by the R133W β-Tm mutation. It is suggested that the R133W β-Tm mutation induces alteration in myosin–actin kinetics causing a reduced number of myosin molecules in the strong actin-binding state, resulting in overall muscle weakness in the absence of muscle wasting.
Striated muscle tropomyosin (Tm) is an integral component of the thin filament. It forms a α-helical coiled-coil dimer which binds to actin and the troponin (Tn) complex. Tm coils around the actin filament and spans seven actin monomers for every Tn complex. Tm is expressed in most mammalian cell types and there are multiple Tm isoforms coded by four different human Tm genes: TPM1, TPM2, TPM3 and TPM4 (Lees-Miller & Helfman, 1991; Michele & Metzger, 2000; Perry, 2001). The isoform diversity is related to alternative splicing, alternative promoters, and differential RNA processing, resulting in specific striated muscle, smooth muscle, and non-muscle isoforms. In adult human skeletal muscle, there are three major tropomyosin isoforms, α-Tm, β-Tm and γ-Tm, which are encoded by the TPM1, TPM2 and TPM3 genes, respectively. The muscle isoform encoded by TPM1 is predominantly expressed in cardiac and skeletal muscle cells expressing the fast (type II) myosin heavy chain (MyHC) isoform. TPM2 is mainly expressed in the β/slow (type I) MyHC isoform and to some extent in fibres expressing the fast MyHC isoform and in cardiac muscle cells. TPM3 is predominantly expressed in the heart and to some extent in skeletal muscle fibres expressing the β/slow MyHC isoform (Pieples & Wieczorek, 2000).
Tm plays an integral role in the regulation of muscle contraction, as it ‘relays’ the information from the Ca2+ sensor, the Tn complex, to the actin–myosin interactions, i.e. the cross-bridges. However, Tm is believed to be involved in the regulation not only by modulating the propagation of Ca2+ signals along the thin filament, i.e. by controlling Ca2+ sensitivity and cooperativity (Brandt et al. 1987; Schachat et al. 1987), but also by modulating actin–myosin cross-bridge cycle kinetics by altering the rate of movement of myosin from an attached non-force-generating state to states that generate force (. Homsher et al. 2003). In this manner Tm is thought to control both the rate of force generation and the steady-state level of force (Brenner, 1988).
A number of mutations in the TPM1, TPM2 and TPM3 genes have been reported to cause cardiac and skeletal myopathies (Michele & Metzger, 2000; Clarkson et al. 2004; Chang & Potter, 2005). Mutations in TPM1, which encodes the α-Tm, are associated with familial hypertrophic cardiomyopathies with no skeletal myopathies (Michele & Metzger, 2000; Chang & Potter, 2005). On the other hand, a mutation described in TPM3, which encodes γ-Tm, is associated with a nemaline myopathy, i.e. skeletal myopathy with limb abnormalities and contractures (Michele & Metzger, 2000). Mutations in TPM2 have been associated with nemaline myopathy and distal arthrogryposis, i.e. distal limb deformities (Donner et al. 2002; Sung et al. 2003). A novel Tm mutation resulting from a defect on the TPM2 gene has been recently identified in a woman and her daughter (R133W) (Tajsharghi et al. 2007). This R133W β-Tm mutation was associated with muscle weakness without progressive muscle wasting or histopathological signs of muscle fibre atrophy, together with distal arthrogryposis type 2B, with mainly distal limb involvement, typically clenched fists at birth, overlapping fingers, camptodactyly, ulnar deviation of fingers, and positional foot deformities (Tajsharghi et al. 2007).
This study was conducted to investigate the effects of the R133W β-Tm mutation on the regulation of contraction at the single muscle fibre level in an attempt to explain the muscle weakness in patients carrying this mutation. It was hypothesized that the β-Tm mutation has a significant impact on both (1) Ca2+ sensitivity and cooperativity, and (2) actin–myosin interactions. Studies were therefore made of force–pCa curves, maximal force normalized to cross-sectional area, apparent rate constant of force redevelopment (release–restretch procedure), and maximum velocity of unloaded shortening (slack test procedure) in skinned single muscle fibre segments from the mother and daughter carrying the R133W β-Tm mutation and from six control subjects. Because of fibre-type-specific expression of Tm isoforms, muscle cells were characterized according to their MyHC isoform expression, and the function of specific MyHC isoforms extracted from single muscle fibres was studied in the patients and controls using a single muscle fibre in vitro motility assay. The results from this study show that this specific mutation does not affect the Ca2+ sensitivity, but that it has a significant impact on the force-generation capacity as a consequence of altered actin–myosin kinetics. The results have been presented in abstract forms elsewhere (Ochala et al. 2006a,Ochala 2006b).
Methods
Subjects
The study was conducted on two patients, i.e. a mother aged 65 years, and her daughter aged 28 years, with muscle weakness and distal arthrogryposis type 2B, and carrying an Arg133Trp β-Tm mutation, R133W, in exon 4. Six healthy subjects of ages 26–67 years, with no history of neuromuscular disease, served as controls. The genotype analysis of the two patients carrying the novel β-Tm mutation, associated with muscle weakness and distal arthrogryposis, was carried out elsewhere (Tajsharghi et al. 2007). Informed consent was obtained from the patients and control subjects enrolled in the present study. A local ethics committee at Sahlgrenska University approved the protocol and the experiments were carried out according to the guidelines of the Declaration of Helsinki. At birth, the two patients carrying the β-Tm mutation presented mainly distal joint contractures. At the time of the investigation, both had weakness in proximal and distal muscles. Cardiac investigation was normal, including left ventricular dimensions and systolic function analysed with the echo-Doppler technique. The clinical history and findings have been summarized by Tajsharghi et al. (2007). No other family members were affected.
Muscle biopsy samples and muscle fibre membrane permeabilization
Fresh muscle biopsy specimens were obtained from the tibialis anterior muscle under local anaesthesia, and they were dissected into two parts. One part was frozen in liquid nitrogen-chilled propane and stored at −80°C, and the other was placed in relaxing solution at 4°C, and bundles of ∼50 fibres were dissected free and then tied with surgical silk to glass capillary tubes at slightly stretched lengths. The muscle bundles were then treated with skinning solution (relaxing solution containing glycerol, 50:50 v/v) for 24 h at 4°C, after which they were transferred to −20°C. In addition, the muscle bundles were treated with sucrose, a cryoprotectant, within 1–2 weeks for long-term storage (Frontera & Larsson, 1997). Muscle bundles were detached from the capillary tubes and snap frozen in liquid nitrogen-chilled propane and stored at −80°C.
Single muscle fibre experimental procedure
On the day of an experiment, a fibre segment 1–2 mm long was left exposed to the solution between connectors leading to a force transducer (model 400A; Aurora Scientific, Ontario, Canada) and a lever arm system (model 308B; Aurora Scientific) (Moss, 1979; Larsson & Moss, 1993). The total compliance of the attachment system was carefully checked and remained similar for all the single muscle fibres tested (5 ± 0.5% of the fibre length). The apparatus was mounted on the stage of an inverted microscope (model IX70; Olympus). While the fibre segments were in relaxing solution, the sarcomere length was set to 2.75–2.85 μm by adjusting the overall segment length. The sarcomere length was controlled during the experiments using a high-speed video analysis system (model 901A HVSL; Aurora Scientific). If at the end of the experiment the sarcomere length of a single muscle fibre had changed by >5% of the initial sarcomere length, the data of this single fibre were not analysed. The diameter of the fibre segment between the connectors was also measured through the microscope at a magnification of ×320 with an image analysis system prior to the mechanical experiments. Fibre depth was measured by recording the vertical displacement of the microscope nosepiece while focusing on the top and bottom surfaces of the fibre. The focusing control of the microscope was used as a micrometer. Fibre cross-sectional area (CSA) was calculated from the diameter and depth, assuming an elliptical circumference, and was corrected for the 20% swelling that is known to occur during skinning (Moss, 1979).
Relaxing and activating solutions contained (mm): 4 MgATP, 1 free Mg2+, 20 imidazole, 7 EGTA, 14.5 creatine phosphate, and KCl to adjust the ionic strength to 180 mm. The pH was adjusted to 7.0. The concentrations of free Ca2+ were 10−9 M (relaxing solution) and 10−4.5 M (maximum activating solution) and are expressed as pCa (i.e. −log[Ca2+]). Apparent stability constants for Ca2+-EGTA were corrected for temperature (15°C) and ionic strength (180 mm). The computer program of Fabiato (1988) was used to calculate the concentrations of each metal, ligand, and metal–ligand complex.
At 15°C, immediately preceding each activation, the fibre was immersed for 10–20 s in a solution with a reduced Ca2+-EGTA buffering capacity. This solution was identical to the relaxing solution except that the EGTA concentration was reduced to 0.5 mm, which results in more rapid attainment of steady force during subsequent activation. Maximal isometric force was calculated as the difference between the total force in activating solution (pCa 4.5) and the resting force measured in the same segment while in the relaxing solution. Maximal isometric force (F0) was adjusted for CSA, i.e. specific force (SF).
Ca2+-sensitivity measurements
The single muscle fibres were exposed to nine solutions with varying Ca2+ concentrations (pCa 6.3–4.5) to determine the force versus pCa relationship. Computer software programs (SigmaPlot 5.0 and Origin 6.1 professional software; Jandel Scientific) were used to fit the force versus pCa curve for each fibre to the following Hill equation:
Ca2+ sensitivity (Ca50) was evaluated on the basis of the Ca2+ concentration producing half-maximal force (pCa50 = −log[Ca50]). The cooperativity of Ca2+ activation (Hill coefficient, nH) was evaluated on the basis of the steepness of the curve from nH.
Release–restretch procedure
At pCa 4.5, once steady-state isometric force was reached, a 20% release of the original fibre length was rapidly introduced (within 1–2 ms) at one end of the fibre, resulting in a rapid reduction of force to near zero. This was followed by a brief period of unloaded shortening (20 ms) after which the preparation was quickly restretched to its original length and the force was recovered to its original steady-state value. As previously described (Brenner & Eisenberg, 1986), the apparent rate constant of force redevelopment (ktr) was estimated by linear transformation of the half-time of force redevelopment (t½) (. Regnier et al. 1998):
Slack test procedure
At pCa 4.5, once steady-state isometric force was reached, nine releases of various amplitudes were rapidly introduced (within 1–2 ms) at one end of the fibre (Edman, 1979). Releases were applied at different amplitudes ranging from 7 to 13% of the fibre length. The fibre was re-extended between releases while relaxed in order to minimize changes in sarcomere length. During the slack test, the time required to take up the imposed release was measured from the onset of the length step to the beginning of the tension redevelopment. A straight line was fitted to a plot of release length versus time, using least-squares regression. The slope of the line divided by the fibre segment length was recorded as maximum unloaded shortening velocity for that fibre segment (V0).
Myosin extraction and in vitro motility assay
The unregulated actin used was purified from rabbit skeletal muscle essentially as described earlier (Pardee & Spudich, 1982) and was fluorescently labelled with rhodamine-phalloidin (Molecular Probes). The single fibre in vitro motility system has been described in detail elsewhere (Hook et al. 1999; Hook & Larsson, 2000). In brief, a short muscle fibre segment was placed on a glass slide between two strips of grease, and a nitrocellulose-coated coverslip was placed on top, creating a flow cell with a volume of ∼5 μl. Myosin was extracted from the fibre segment by addition of a high-salt buffer (0.5 m KCl, 25 mm Hepes [pH 7.6], 4 mm MgCl2, 4 mm EGTA, 2 mm ATP, and 1% 2-mercaptoethanol). After 30 min incubation on ice, a low-salt buffer (25 mm KCl, 25 mm Hepes [pH 7.6], 4 mm MgCl2, 1 mm EGTA, and 1% 2-mercaptoethanol) was applied, followed by bovine serum albumin (1 mg ml−1). Non-functional myosin molecules were blocked with fragmentized F-actin, and rhodamine-phalloidin-labelled actin filaments were subsequently infused into the flow cell, followed by motility buffer (2 mm ATP, 0.1 mg ml−1 glucose oxidase, 23 μg ml−1 catalase, 2.5 mg ml−1 glucose, and 0.4% methyl cellulose, in low-salt buffer) to initiate movement. The pH of the buffers was adjusted with KOH, and the final ionic strength of the motility buffer was 71 mm. The flow cell was placed on the stage of an inverted epifluorescence microscope (model IX 70; Olympus) and thermostatically controlled at 25°C. Actin movements were filmed with an image-intensified SIT camera (SIT 66; DAGE-MIT) and recorded on tape with a video-cassette recorder. From each single fibre preparation, 20 actin filaments moving at constant speed in an orientated motion were selected for speed analysis. Recordings and analysis were only performed from preparations in which >90% of the filaments moved bidirectionally. A filament was tracked from the centre of mass, and its speed of movement was calculated from 20 frames at an acquisition rate of five frames or 1 frame s−1, depending on the fibre type, using an image-analysis package (OPTIMAS 6.0). The mean speed of the 20 filaments was calculated. Since the s.d. in this group of filaments was small (between 10 and 15% of the mean), the average speed was taken as representative for the muscle fibre (Vf).
After the mechanical measurements, each fibre was placed in urea buffer (120 g urea, 38 g thiourea, 70 ml H2O, 25 g mixed bed resin, 2.89 g dithiothreitol, 1.51 g Trizma base, 7.5 g SDS, 0.004% bromophenol blue) in a plastic microcentrifuge tube and stored at −80°C.
Protein isoform determination and quantification
The MyHC isoform composition of single fibres was determined by 6% SDS-PAGE. The acrylamide concentration was 4% (w/v) in the stacking gel and 6% in the running gel, and the gel matrix included 30% glycerol. Sample loads were kept small (equivalent to ∼0.05 mm of fibre segment) to improve the resolution of the MyHC bands (types I, IIa and IIx). Electrophoresis was performed at 120 V for 24 h with a Tris-glycine electrode buffer (pH 8.3) at 15°C (SE 600 vertical slab gel unit; Hoefer Scientific Instruments). The gels were silver stained and subsequently scanned in a soft laser densitometer (Molecular Dynamics) with a high spatial resolution (50 μm pixel spacing) and 4096 optical density levels (Greaser et al. 1988).
Tm isoform expression by single muscle fibres was determined by 12% SDS-PAGE. The acrylamide concentration was 4% (w/v) in the stacking gel and 12% in the running gel, and the gel matrix included 10% glycerol. The gels were stained with Coomassie blue (0.5 g Brilliant Blue, 225 ml MeOH, 225 ml distilled H2O, and 50 ml acetic acid). The relative contents of α- and β-Tm isoforms for each single muscle fibre were then calculated from the densitometric scanning (see above).
Statistical analysis
Data are presented as means ± s.d. Sigma Stat software was used to generate descriptive statistics. Given the small number of hybrid (type I/IIa and IIa/IIx) and pure type IIx fibres observed in these experiments, comparisons were limited to type I and IIa. In addition, there is type I muscle fibre predominance in human tibialis anterior muscle. Thus, some statistical analyses were only carried out on muscle fibres expressing the type I MyHC isoform. When type I and IIa data were available for a parameter, one-way ANOVA was performed, and in cases where the data did not meet the criteria of normality (Kolmogorov–Smirnov test, P < 0.05), the Kruskal–Wallis one-way ANOVA on ranks was applied. When differences were significant, post hoc analysis was performed by Tukey's test or by Dunn's method, respectively. When only type I data were available for a parameter, unpaired Student's t test was used, and in cases where the data did not meet the criteria of normality, the nonparametric Mann–Whitney rank-sum test was applied. Otherwise, linear regression analysis for slack-test determination and non-linear regression for force–pCa curve fitting were performed and regression parameters were considered significantly different from zero at P < 0.05.
Results
After permeabilization, single muscle fibres from tibialis anterior biopsy specimens were isolated and mounted for analysis of contractile properties. A total of 69 type I and 16 type IIa fibres from the patients carrying the β-Tm mutation and 56 type I and 36 type IIa fibres from the six control subjects were tested. The mean size of the muscle fibres did not differ between the patients and controls. The mean size of the type I muscle fibres was 2630 ± 1060 μm2 in the patients and 2410 ± 880 μm2 in the controls. The type IIa fibre size was 2850 ± 830 μm2 in the patients and 2600 ± 960 μm2 in the controls.
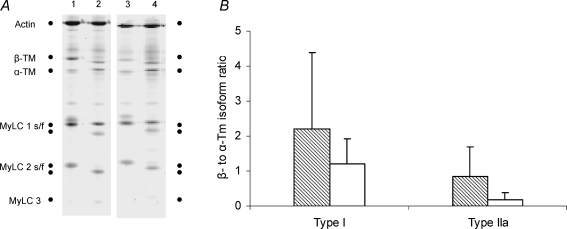
The α- to β-Tm isoform ratio varied significantly between individual cells and a higher ratio was confirmed in muscle fibres expressing the type I than the IIa MyHC isoforms in both patients and controls (P < 0.05). The mean α- to β-Tm isoform ratios were lower in the patients than in controls, but did not differ statistically. Among type I fibres the ratio was 1.20 ± 0.71 in β-Tm mutated fibres (n = 28) and 2.21 ± 2.18 in the controls (n = 22). The corresponding ratios among the type IIa fibres were 0.19 ± 0.20 (n = 8) and 0.84 ± 0.85 (n = 8), respectively (Fig. 1).
Figure 1.
α- and β-tropomyosin isoform expression A, electrophoretic separation of myosin heavy chain (MyHC), actin, and α- and β-tropomyosin (Tm) isoforms in single muscle fibres expressing type I (lanes 1 and 3) and IIa (lanes 2 and 4) MyHC isoforms from controls (lanes 1 and 2) and patients (lanes 3 and 4). B, β- to α-Tm isoform ratio in single muscle fibres from patients carrying the β-Tm mutation (open bar: type I fibres, n = 28; type IIa fibres, n = 8) and control subjects (hatched bar: type I fibres, n = 22; type IIa fibres, n = 8). Values are means ± s.d.
Ca2+ sensitivity
The Ca2+ sensitivity and cooperativity of the type I fibres, evaluated by force–pCa relationships, were unrelated to the mutation (Fig. 2). Ca2+ sensitivity was assessed on the basis of the Ca2+ concentration producing half-maximal force. Mean pCa50 was 5.37 ± 0.32 in fibres carrying the β-Tm mutation (n = 19) and 5.49 ± 0.41 in control fibres (n = 20). The cooperativity of Ca2+ activation was evaluated on the basis of the steepness of the curve from the Hill coefficient. Mean nH was 1.78 ± 0.29 in β-Tm-mutated fibres (n = 19) and 1.70 ± 0.31 in control fibres (n = 20).
Figure 2.
Force–pCa relationships Force–pCa curves of type I fibres from patients carrying the β-Tm mutation (•, n = 19) and control subjects (○, n = 20). All values are means.
Specific force, force redevelopment and maximum velocity of unloaded shortening
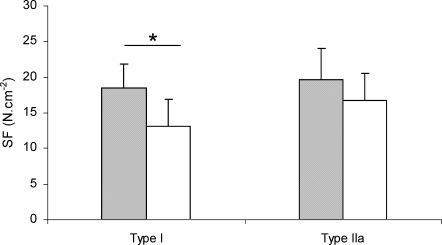
Maximum isometric force normalized to fibre cross-sectional area (SF) was lower in type I fibres containing the β-Tm mutation than in those without the mutation (−29%), i.e. mean SF was 13.11 ± 4.30 N cm−2 in β-Tm mutated fibres (n = 51) and 18.45 ± 3.34 N cm−2 in control fibres (n = 45; Fig. 3; P < 0.05). In type IIa fibres, SF did not differ between patients (16.70 ± 3.80 N cm−2; n = 10) and controls (19.70 ± 4.38 N cm−2; n = 29).
Figure 3.
Specific force SF, specific force. Comparison between fibres from patients carrying the β-Tm mutation (open bar: type I fibres, n = 51; type IIa fibres, n = 10) and those from control subjects (filled bar: type I fibres, n = 45; type IIa fibres, n = 29). Values are means ± s.d.*Statistically significant difference.
The apparent rate constant of force redevelopment (ktr) was 60% lower in type I fibres expressing the β-Tm mutation compared with control fibres; i.e. the mean ktr value was 8.45 ± 2.26 s−1 in β-Tm-mutated fibres (n = 10) and 21.26 ± 2.79 s−1 in control fibres (n = 10; Fig. 4; P < 0.01).
Figure 4.
Apparent rate constant of force redevelopment (ktr) A, comparison between type I fibres from patients carrying the β-Tm mutation (open bar, n = 10) and control subjects (hatched bar, n = 10). Values are means ± s.d.*Statistically significant difference. B, typical experimental force signal recording from a type I fibre from a control subject (ktr 17.33 s−1). C, typical experimental force signal recording from a patient carrying the β-Tm mutation (ktr 7.70 s−1). The scale bar denotes 400 ms. A 20% release of original fibre length rapidly introduced at one end of the fibre reduces the proportion of available cross-bridges that are attached from approximately 80% in the isometric fibre to 20% (force drops to zero). Dissociation of the remaining cross-bridges is accomplished by rapidly re-extending overall muscle length to the initial value. Coincident with this re-stretch, force transiently increased because of positive straining of attached cross-bridges. These positively strained cross-bridges rapidly dissociated since the imposed length change is much greater than estimates of the working distance of a cross-bridge.
The maximum velocity of unloaded shortening (V0) was 40% faster in type I fibres carrying the β-Tm mutation than in control fibres, i.e. mean V0 was 1.25 ± 0.30 ML s−1 (muscle length per second) in β-Tm-mutated fibres (n = 32) and 0.81 ± 0.40 ML s−1 in controls (n = 25; Fig. 5; P < 0.05). In muscle cells expressing the type IIa MyHC isoform, V0 did not differ between fibres expressing the β-Tm mutation (2.06 ± 0.30 ML s−1; n = 8) and controls (1.80 ± 0.30 ML s−1; n = 14).
Figure 5.
Maximum unloaded shortening velocity (V0) A, comparison between fibres from patients carrying the β-Tm mutation (open bar: type I fibres, n = 32; type IIa fibres, n = 8) and from control subjects (hatched bar: type I fibres, n = 25, type IIa fibres, n = 14). Values are means ± s.d.*Statistically significant difference. B, typical experimental force signal recording from a type I fibre from a control subject (12% slack, fibre length 1890 μm, V0 0.65 ML s−1 (muscle length per second)). C, typical experimental force signal recording from a patient carrying the β-Tm mutation (12% slack, fibre length 1800 μm, V0 1.27 ML s−1). The scale bar denotes 500 ms. When a release is introduced at one end of the fibre, force abruptly falls to zero, and the fibre is permitted to shorten under unloaded conditions.
In vitro motility speed
There is a close relationship between speed (Vf) of unregulated actin filaments propelled by myosin extracted from single muscle fibre segment, and V0 of the same muscle fibre or fibre type (Larsson et al. 1999; Hook & Larsson, 2000). We therefore compared Vf propelled by myosin extracted from type I and IIa muscle fibres from patients carrying the β-Tm mutation and that by myosin extracted from control type I fibres. Vf did not differ when type I and IIa myosins were extracted from the fibres containing the β-Tm mutation (type I, n = 18: 0.74 ± 0.14 μm s−1; type IIa, n = 6: 2.63 ± 0.23 μm s−1) compared with control fibres (type I, n = 11: 0.72 ± 0.11 μm s−1; type IIa, n = 7: 2.57 ± 0.34 μm s−1), indicating that the observed difference in shortening velocity at the single muscle fibre level was not caused by an alteration of myosin function.
Discussion
In this study, we have explored contractile properties of single human muscle fibres carrying an Arg133Trp β-Tm mutation in the central portion of the Tm segment (Fig. 6). This mutation did not affect the Ca2+ sensitivity and cooperativity, but it had a significant impact on actin–myosin interactions, with consequences for force generation and contractile speed. These results suggest that the β-Tm mutation has a negative action, which is likely to be a mechanism underlying the muscle weakness in the patients carrying this mutation. Significant changes were only observed in muscle fibres expressing the type I MyHC isoform, i.e. the muscle cells with the highest relative content of β-Tm. However, a trend toward the same modifications was noted for type IIa fibres expressing a lower content of β-Tm (Billeter et al. 1981; Salviati et al. 1983).
Figure 6.
The β-Tm mutation TnT binds Tm in an overall antiparallel manner; i.e. its C-terminal region is positioned near residue 190 of Tm, and the N-terminal tail of TnT extends towards the C terminus of Tm, overlapping the head to tail joint of Tm as well as 10–30 residues of the N terminus of the next molecule along the filament. The present β-TM mutation is located on residue 133.
Surprisingly, Ca2+ sensitivity and cooperativity were not disrupted by the mutation in the present experiment, even though Tm is believed to be involved in ‘relaying’ the information from the Ca2+ sensor, the Tn complex, by modulating the propagation of Ca2+ signals along the thin filament (Brandt et al. 1987; Schachat et al. 1987). These results do not conform with those for other Tm mutations. For example, Glu180Gly and Asp175Asn α- and γ-Tm mutations inducing hypertrophic cardiomyopathy increased Ca2+ sensitivity (Bottinelli et al. 1998; Wernicke et al. 2004), whereas a Met9Arg γ-Tm mutation causing a nemaline myopathy resulted in slightly decreased Ca2+ sensitivity (Michele et al. 1999). We propose that the lack of an effect of the present Arg133Trp β-Tm mutation on Ca2+ sensitivity is related to its location in a region of the Tm molecule that is not directly associated with the Tn complex Ca2+-binding sites (Fig. 6), in contrast to the Glu180Gly, Asp175Asn and Met9Arg α- and γ-Tm mutations (Bottinelli et al. 1998; Michele et al. 1999; Wernicke et al. 2004). In human skeletal muscle, the critical interactions between Tm and TnT take place between Tm residues 0–30 and 190–284 (Li et al. 2002). The present β-Tm mutation is 60 residues away from the nearest potential TnT-anchoring region (Fig. 6).
Usually, Tm alternates between three positions: the blocked, closed and open states (Poole et al. 1995; Vibert et al. 1997). In the absence of Ca2+, Tm is in a position on the outer domain of actin that sterically hinders the docking of cross-bridges – the blocked state. Full activation by reversal of steric blocking involves two additional thin filament states, requiring successive Tm movements away from the blocked configuration. Ca2+ bindings to the Tn complex cause Tm movement toward the inner domain of actin, exposing sites that allow weak binding of myosin heads while still inhibiting isomerization to the strong binding state (Vibert et al. 1997). Following this change, Tm still covers an essential part of the site, leaving it inaccessible to myosin heads – the closed state. Weak-to-strong myosin binding transition induces a second Tm shift, permitting cooperative binding of additional myosin heads by exposing entire neighbouring sites, fully activating the thin filament – the open state (Lehman et al. 2000).
Muscle cells carrying α-Tm mutations positioned on residues near the C- and N-terminal regions, such as the Glu180Gly, Asp175Asn or Met9Arg mutations, maintain their force-generation capacity (Bottinelli et al. 1998; Michele et al. 1999; Wernicke et al. 2004). The present Arg133Trp β-Tm mutation situated in the central portion of the Tm segment (Fig. 6) had a significant impact on SF, indicating the crucial role of this region for myosin–actin interactions. This is supported by the lower ktr and faster V0 during maximal Ca2+ activation in muscle cells expressing the Arg133Trp β-Tm mutation.
The apparent rate constant of force redevelopment (ktr) reflects the cross-bridge cycle turnover rate and the rate of cross-bridge attachment is considered to be the rate-limiting step, i.e. the rate of transition from the weakly bound non-force-generating cross-bridge state to the strongly bound force-generating state (Brenner, 1988; Metzger et al. 1989). The exact mechanisms by which the β-Tm mutation slows the cross-bridge attachment rate are not known, but it may be speculated that the mutation affects Tm movement toward the inner domain of actin and the transition from the blocked state to the closed and open states (Clemmens et al. 2005). This would disrupt attachment and/or transition of myosin heads from the weakly to the strongly bound force-generating state, decreasing the cross-bridge attachment rate. The maximum velocity of unloaded shortening (V0), on the other hand, is primarily determined by the enzymatic properties of myosin, i.e. MyHC isoform expression (Siemankowski & White, 1984). However, in vitro motility measurements (Vf) did not reveal any changes in the function of myosin extracted from muscle cells expressing a high content of the β-Tm isoform, suggesting that the changes in contractile properties at the single cell level are primarily caused by the mutated Tm. The mechanisms by which the β-Tm mutation might affect the cross-bridge detachment rate are not known, but we suggest that the mutation modifies Tm movement from the open to the blocked state, slowing myosin attachment and promoting dissociation of myosin heads from the actin filament; thereby increasing the cross-bridge detachment rate. The combination of a decreased apparent rate of force redevelopment and an increased maximum unloaded shortening velocity in muscle fibres carrying the β-Tm mutation suggest a shortened cross-bridge duty cycle, i.e. a shorter time spent by myosin heads in a strongly bound force-generating state, resulting in a decreased specific force. The significant impact of the β-Tm mutation indicates that the Tm isoform diversity observed in normal skeletal muscle cells may confer a significant modulatory effect on the dominant regulatory influence of MyHC isoform expression on contractile speed and force generation.
Although the β- to α-Tm isoform ratios were not statistically affected by the mutation, trends towards lower ratios were visible in both type I and IIa fibres expressing the Arg133Trp β-Tm mutation (Fig. 1). This can potentially result in an increased effect of α-Tm on function in muscle cells carrying the present mutation. An overexpression of β-Tm is known to induce an increase in Ca2+ sensitivity (Palmiter et al. 1996; Wolska et al. 1999) and a decrease in specific force (Wolska et al. 1999). Then, a trend towards a greater proportion of α-Tm, as in the patients carrying the Arg133Trp β-Tm mutation, would be expected to reduce Ca2+ sensitivity and improve specific force. Three main explanations may emphasize the discrepancies observed between the previous experiments and the present study: (1) species differences (mouse versus human), (2) differences between mouse cardiac myocytes and human skeletal muscle fibres, (3) range of variations in the data. Further, it cannot be completely ruled out that some of the reported changes could be caused by some unidentified factors other than the β-Tm mutation in the two affected family members, although this appears to be less likely.
In conclusion, the present study addresses the effects of a novel β-Tm mutation on the regulation of skeletal muscle contraction in an attempt to unravel the mechanisms underlying the muscle weakness in patients carrying this mutation. The β-Tm mutation causes changes in contractile characteristics at the single muscle fibre level, such as a decrease in the force-generating capacity and in the apparent rate constant of force redevelopment, and an increase in the maximum velocity of unloaded shortening, at a maximal Ca2+ concentration, confirming a negative action of the mutation. The altered rate constant for force redevelopment and contractile speeds imply a direct effect of the mutation on the actin–myosin cross-bridge duty cycle, resulting in a decreased force production per cross-bridge and overall muscle weakness.
Acknowledgments
This study was supported by grants from the Swedish Institute and Agence Française contre les Myopathies to J.O., from the Agence Française contre les Myopathies and Swedish Research Council (07122) to A.O., from the Linnéa and Josef Carlssons Foundation to E.K., and from the Swedish Research Council (08651), Agence Française contre les Myopathies, Cancer Foundation and National Institutes of Health (AR045627, AR047318) to L.L. We are grateful to Yvette Hedström and Ann-Marie Gustafsson for excellent technical assistance. We thank the patients and control subjects for making this work possible.
References
- Billeter R, Heizmann CW, Reist U, Howald H, Jenny E. α- and β-tropomyosin in typed single fibers of human skeletal muscle. FEBS Lett. 1981;132:133–136. doi: 10.1016/0014-5793(81)80446-2. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Coviello DA, Redwood CS, Pellegrino MA, Maron BJ, Spirito P, Watkins H, Reggiani C. A mutant tropomyosin that causes hypertrophic cardiomyopathy is expressed in vivo and associated with an increased calcium sensitivity. Circ Res. 1998;82:106–115. doi: 10.1161/01.res.82.1.106. [DOI] [PubMed] [Google Scholar]
- Brandt PW, Diamond MS, Rutchik JS, Schachat FH. Co-operative interactions between troponin–tropomyosin units extend the length of the thin filament in skeletal muscle. J Mol Biol. 1987;195:885–896. doi: 10.1016/0022-2836(87)90492-x. [DOI] [PubMed] [Google Scholar]
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci U S A. 1988;85:3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B, Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci U S A. 1986;83:3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AN, Potter JD. Sarcomeric protein mutations in dilated cardiomyopathy. Heart Fail Rev. 2005;10:225–235. doi: 10.1007/s10741-005-5252-6. [DOI] [PubMed] [Google Scholar]
- Clarkson E, Costa CF, Machesky LM. Congenital myopathies: diseases of the actin cytoskeleton. J Pathol. 2004;204:407–417. doi: 10.1002/path.1648. [DOI] [PubMed] [Google Scholar]
- Clemmens EW, Entezari M, Martyn DA, Regnier M. Different effects of cardiac versus skeletal muscle regulatory proteins on in vitro measures of actin filament speed and force. J Physiol. 2005;566:737–746. doi: 10.1113/jphysiol.2005.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K, Ollikainen M, Ridanpaa M, Christen HJ, Goebel HH, de Visser M, Pelin K, Wallgren-Pettersson C. Mutations in the β-tropomyosin (TPM2) gene – a rare cause of nemaline myopathy. Neuromuscul Disord. 2002;12:151–158. doi: 10.1016/s0960-8966(01)00252-8. [DOI] [PubMed] [Google Scholar]
- Edman KA. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Larsson L. Contractile studies of single human skeletal muscle fibers: a comparison of different muscles, permeabilization procedures, and storage techniques. Muscle Nerve. 1997;20:948–952. doi: 10.1002/(sici)1097-4598(199708)20:8<948::aid-mus3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Greaser ML, Moss RL, Reiser PJ. Variations in contractile properties of rabbit single muscle fibres in relation to troponin T isoforms and myosin light chains. J Physiol. 1988;406:85–98. doi: 10.1113/jphysiol.1988.sp017370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E, Nili M, Chen IY, Tobacman LS. Regulatory proteins alter nucleotide binding to acto-myosin of sliding filaments in motility assays. Biophys J. 2003;85:1046–1052. doi: 10.1016/S0006-3495(03)74543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook P, Larsson L. Actomyosin interactions in a novel single muscle fiber in vitro motility assay. J Muscle Res Cell Motil. 2000;21:357–365. doi: 10.1023/a:1005614212575. [DOI] [PubMed] [Google Scholar]
- Hook P, Li X, Sleep J, Hughes S, Larsson L. In vitro motility speed of slow myosin extracted from single soleus fibres from young and old rats. J Physiol. 1999;520:463–471. doi: 10.1111/j.1469-7793.1999.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Hook P, Pircher P. Regulation of human muscle contraction at the cellular and molecular levels. Ital J Neurol Sci. 1999;20:413–422. doi: 10.1007/s100720050061. [DOI] [PubMed] [Google Scholar]
- Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller JP, Helfman DM. The molecular basis for tropomyosin isoform diversity. Bioessays. 1991;13:429–437. doi: 10.1002/bies.950130902. [DOI] [PubMed] [Google Scholar]
- Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, Geeves MA, Van Eyk JE, Tobacman LS, Craig R. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol. 2000;302:593–606. doi: 10.1006/jmbi.2000.4080. [DOI] [PubMed] [Google Scholar]
- Li Y, Mui S, Brown JH, Strand J, Reshetnikova L, Tobacman LS, Cohen C. The crystal structure of the C-terminal fragment of striated-muscle α-tropomyosin reveals a key troponin T recognition site. Proc Natl Acad Sci U S A. 2002;99:7378–7383. doi: 10.1073/pnas.102179999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Greaser ML, Moss RL. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Implications for twitch potentiation in intact muscle. J Gen Physiol. 1989;93:855–883. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Albayya FP, Metzger JM. A nemaline myopathy mutation in α-tropomyosin causes defective regulation of striated muscle force production. J Clin Invest. 1999;104:1575–1581. doi: 10.1172/JCI7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Metzger JM. Physiological consequences of tropomyosin mutations associated with cardiac and skeletal myopathies. J Mol Med. 2000;78:543–553. doi: 10.1007/s001090000161. [DOI] [PubMed] [Google Scholar]
- Moss RL. Sarcomere length–tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochala J, Kimber E, Li M, Larsson L. A mutant tropomyosin that causes muscle weakness and distal arthrogryposis is associated with a defective regulation of muscle contraction. Eleventh International Congress of the World Muscle Society Congress; 4–7 October 2006; Bruges, Belgium. p. 688. (abstract P.P. 50 4) [Google Scholar]
- Ochala J, Kimber E, Tajsharghi H, Tulinius M, Oldfors A, Larsson L. New Directions in Biology and Disease of Skeletal Muscle. Dallas, Texas: 2006b. Regulation of muscle contraction in a novel myopathy associated with a mutation in the beta-tropomyosin (TPM2) gene; p. 58. 23–26 April, 2006, (abstract 92) [Google Scholar]
- Palmiter KA, Kitada Y, Muthuchamy M, Wieczorek DF, Solaro RJ. Exchange of β- for α-tropomyosin in hearts of transgenic mice induces changes in thin filament response to Ca2+, strong cross-bridge binding, and protein phosphorylation. J Biol Chem. 1996;271:11611–11614. doi: 10.1074/jbc.271.20.11611. [DOI] [PubMed] [Google Scholar]
- Pardee JD, Spudich JA. Purification of muscle actin. Methods Cell Biol. 1982;24:271–289. doi: 10.1016/s0091-679x(08)60661-5. [DOI] [PubMed] [Google Scholar]
- Perry SV. Vertebrate tropomyosin: distribution, properties and function. J Muscle Res Cell Motil. 2001;22:5–49. doi: 10.1023/a:1010303732441. [DOI] [PubMed] [Google Scholar]
- Pieples K, Wieczorek DF. Tropomyosin 3 increases striated muscle isoform diversity. Biochemistry. 2000;39:8291–8297. doi: 10.1021/bi000047x. [DOI] [PubMed] [Google Scholar]
- Poole V, Evans G, Rosenbaum G, Lorenz M, Holmes K. The effect of cross-bridges on the calcium sensitivity of the structural change of the regulated thin filament. Biophys J. 1995;68:365. [Google Scholar]
- Regnier M, Martyn DA, Chase PB. Calcium regulation of tension redevelopment kinetics with 2-deoxy-ATP or low [ATP] in rabbit skeletal muscle. Biophys J. 1998;74:2005–2015. doi: 10.1016/S0006-3495(98)77907-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salviati G, Betto R, Danieli Betto D. Polymorphism of myofibrillar proteins of rabbit skeletal-muscle fibers. An electrophoretic study of single fibers. Biochem J. 1983;207:261–272. doi: 10.1042/bj2070261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachat FH, Diamond MS, Brandt PW. Effect of different troponin T-tropomyosin combinations on thin filament activation. J Mol Biol. 1987;198:551–554. doi: 10.1016/0022-2836(87)90300-7. [DOI] [PubMed] [Google Scholar]
- Siemankowski RF, White HD. Kinetics of the interaction between actin, ADP, and cardiac myosin-S1. J Biol Chem. 1984;259:5045–5053. [PubMed] [Google Scholar]
- Sung SS, Brassington AM, Krakowiak PA, Carey JC, Jorde LB, Bamshad M. Mutations in TNNT3 cause multiple congenital contractures: a second locus for distal arthrogryposis type 2B. Am J Hum Genet. 2003;73:212–214. doi: 10.1086/376418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajsharghi H, Kimber E, Holmgren D, Tulinius M, Oldfors A. Distal arthrogryposis and muscle weakness associated with a β–tropomyosin mutation. Neurology. 2007;68:772–775. doi: 10.1212/01.wnl.0000256339.40667.fb. [DOI] [PubMed] [Google Scholar]
- Vibert P, Craig R, Lehman W. Steric-model for activation of muscle thin filaments. J Mol Biol. 1997;266:8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- Wernicke D, Thiel C, Duja-Isac CM, Essin KV, Spindler M, Nunez DJ, Plehm R, Wessel N, Hammes A, Edwards RJ, Lippoldt A, Zacharias U, Stromer H, Neubauer S, Davies MJ, Morano I, Thierfelder L. α–Tropomyosin mutations Asp (175) Asn and Glu (180) Gly affect cardiac function in transgenic rats in different ways. Am J Physiol Regul Integr Comp Physiol. 2004;287:R685–R695. doi: 10.1152/ajpregu.00620.2003. [DOI] [PubMed] [Google Scholar]
- Wolska BM, Keller RS, Evans CC, Palmiter KA, Phillips RM, Muthuchamy M, Oehlenschlager J, Wieczorek DF, de Tombe PP, Solaro RJ. Correlation between myofilament response to Ca2+ and altered dynamics of contraction and relaxation in transgenic cardiac cells that express β-tropomyosin. Circ Res. 1999;84:745–751. doi: 10.1161/01.res.84.7.745. [DOI] [PubMed] [Google Scholar]