Abstract
These experiments assessed whether the impairment in proprioceptive acuity in the hand during ‘interfering’ cutaneous stimulation could be caused by inputs from Pacinian corpuscles. The ability to detect passive movements at the proximal interphalangeal joint of the index finger was measured when vibrotactile stimuli were applied to the adjacent middle finger and thenar eminence at frequencies and amplitudes that favour activation of rapidly adapting cutaneous afferents. Inputs from Pacinian corpuscles are favoured with high-frequency vibration (300 Hz), while those from Meissner corpuscles are favoured by lower frequencies (30 Hz). Detection of movement was significantly impaired when 300 Hz (20 μm peak-to-peak amplitude) complex vibration or 300 Hz (50 μm) sinusoidal vibration was applied to the middle finger and thenar eminence. In contrast, detection of movements was not altered by low-frequency sinusoidal vibration at 30 Hz with an amplitude of 50 μm or with a larger amplitude matched in subjective intensity to the 300 Hz sinusoidal stimulus. Thus it is unlikely that the impairment in detection was due to attention being diverted by vibration of an adjacent digit. In addition, an increase in amplitude of 300 Hz vibration led to a greater impairment of movement detection, so that the impairment was graded with the input. The time taken to nominate the direction of applied movement also increased during 300 Hz but not during 30 Hz sinusoidal vibration. These findings suggest that stimuli which preferentially activate Pacinian, but not Meissner corpuscles, impair proprioceptive acuity in a movement detection task.
Joint, muscle and skin receptors discharge with joint movement and thus can contribute to proprioception. The afferent behaviour has been established in human microneurographic recordings from joint (e.g. Burke et al. 1988; Edin, 1990), muscle (e.g. Vallbo, 1974; Edin & Vallbo, 1990; Grill & Hallett, 1995) and skin afferents (e.g. Edin & Abbs, 1991; Grill & Hallett, 1995). However, the perceptual role of the different classes remains controversial. Prior to 1972, it was thought that proprioception was exclusively subserved by joint receptors, but there was evidence that most feline joint receptors responded only at the extremes of movement (Burgess & Clark, 1969), and that removal of inputs from joint receptors produced only minor proprioceptive deficits (Clark & Burgess, 1975). Studies by Goodwin et al. (1972) showed that tendon vibration that activates muscle spindle endings produced powerful illusions of joint position and movement. Studies that removed specific inputs using local anaesthesia (Clark et al. 1985) or disengagement of muscle (Gandevia & McCloskey, 1976) supported the role for muscle receptors in proprioception (Gandevia, 1996). Hence, a consensus formed that muscle spindle receptors provided the most important input for position and movement sense (e.g. Kandel et al. 2000; Smetacek & Mechsner, 2004).
The role of cutaneous receptors was initially overlooked, although clues that they may be important existed. Proprioceptive acuity decreased when cutaneous (and joint) feedback was removed by anaesthesia of digital nerves (e.g. Provins, 1958; Gandevia & McCloskey, 1976; Refshauge et al. 2003). Stronger evidence came from recent studies which showed that stretch of the skin over finger joints evoked illusions of movement (Edin & Johansson, 1995; Collins & Prochazka, 1996; Collins et al. 2000, Collins 2005). Furthermore, when muscle vibration and skin stretch were applied together, the illusory movements were augmented (Collins & Prochazka, 1996; Collins et al. 2000, Collins 2005). Thus, just as muscle vibration revealed the proprioceptive role of muscle spindle endings, so skin stretch confirmed a proprioceptive role for cutaneous receptors.
Another role for cutaneous afferents was suggested by the effects of ‘interfering’ stimuli. Contrary to the original view that cutaneous afferents ‘facilitate’ muscle signals by a central action (Gandevia & McCloskey, 1976), movement detection was not impaired after the loss of tonic inputs from skin in adjacent digits (Refshauge et al. 2003). Instead, increasing input from adjacent, but not more remote, digits with innocuous continuous electrical stimulation or repetitive brushing reduced detection of passive movements of the test digit (Refshauge et al. 2003). Thus, while tonic cutaneous inputs from adjacent digits are not required for normal movement detection, sustained enhancement of that input impairs the movement detection in the adjacent digits. This suggests an ‘interference’ or inhibitory interaction between the various proprioceptive inputs (Refshauge et al. 2003). It was reported that signals coding the properties of tactile stimuli can be masked by receptor responses to vibration, and so could lead to sensorimotor alterations (Ribot-Ciscar et al. 1989), although inhibitory interactions could also resemble the impairment by specific interfering stimuli of discrimination of cutaneous vibration in the hand (Ferrington et al. 1977). Cutaneous inputs arising from Pacinian corpuscles elevated detection thresholds and this was consistent with inhibition at synaptic relays along the sensory pathway (Ferrington et al. 1977).
The present study examined whether similar mechanisms were responsible for the proprioceptive impairment caused by interfering cutaneous stimulation. Pacinian corpuscles respond to vibration at high frequencies, 80–450 Hz (Sato, 1961; Talbot et al. 1968; LaMotte & Mountcastle, 1975), whereas rapidly adapting intradermal tactile receptors, thought to be Meissner corpuscles, respond to low frequencies, 10–80 Hz (Talbot et al. 1968). Single Pacinian afferents exert powerful actions on central neurons (McIntyre et al. 1967) and can elicit sensations of tactile vibration (Ochoa & Törebjork, 1983; Vallbo et al. 1984; Macefield et al. 1990). Based on the findings of Ferrington et al. (1977) we hypothesized that proprioceptive performance would decrease with vibrotactile input designed to engage Pacinian, but not Meissner, afferents. The interphalangeal joint of the index finger was tested because previous studies have demonstrated a role for cutaneous feedback in proprioception at this joint (Edin & Johansson, 1995; Refshauge et al. 2003).
Methods
Subjects
The proprioceptive acuity for detecting the direction of passive movements at the proximal interphalangeal joint of the index finger was measured when a vibrotactile stimulus was delivered at two locations, the tip of the adjacent middle finger or the thenar eminence. A total of 12 healthy subjects participated (5 female, 7 male; age range 22–51 years), with most involved in more than one study. Ten subjects participated in the initial study, and 8 of them then participated in the main studies. Two new subjects participated in these studies, bringing its total number to 10. All experiments conformed to the Declaration of Helsinki, subjects gave written consent to participate and procedures were approved by the ethics committee of the University of New South Wales.
Experimental setup
The right forearm and the hand were supported on a padded splint, with the wrist positioned in neutral supination–pronation and comfortable extension (∼15 deg), and the metacarpophalangeal joint of the index finger flexed to ∼45 deg (Fig. 1A). Flexion and extension movements were imposed about the proximal interphalangeal joint of the right index finger.
Figure 1.
The experimental arrangement used to impose finger movements together with waveforms and timing of the vibratory stimuli, auditory signal and decision indicators A, the proximal phalanx was stabilized with a clamp, and the middle phalanx moved by a motor. The proximal interphalangeal joint of the index finger was positioned at ∼45 deg flexion. The remaining digits, hand and wrist were stabilized in a standard position. A mechanical stimulator delivered vibrotactile stimuli at the tip of the middle finger or the thenar eminence. B, the vibration stimulus began 0.1 s before the test movement and continued until the full excursion was reached. In addition, a standard auditory signal was given during this time and during control trials (without vibration). The finger was held at its full excursion for 3 s at the end of each movement before it was restored to the rest position. Subjects gave responses of either ‘flexion’, ‘extension’ or ‘not sure’, with the decision time taken as the time between the start of the imposed movement and the response.
The initial position of the proximal interphalangeal joint was in the middle of its physiological range (∼45 deg). The middle phalanx was coupled to a linear servomotor under positional feedback, driven by a variable ramp generator and was attached via a small padded clamp (∼1.5 cm2 in contact area) over the sides of the digit (Fig. 1A). This was designed to minimize the disturbance of the skin on the dorsal and palmar surfaces of the digit. The clamp was placed 1.5 cm distal to the axis of rotation of the joint (Fig. 1A). The proximal phalanx was stabilized by a similar padded clamp so that movement was confined to the proximal interphalangeal joint (Fig. 1A). A barrier was positioned over the hand so that subjects could not see it or the apparatus. Measures of actual angular displacement and geometric calculation were used to calibrate the equipment prior to data collection.
In the main studies, vibratory stimulation with sinusoidal motion of the probe tip was delivered via a feedback-controlled mechanical stimulator (see Fig. 3B; for details see Ferrington & Rowe, 1980; Morley & Rowe, 1990; Coleman et al. 2003). However, in the initial study rectangular inputs were used to generate the vibration of 300 Hz; this produced a complex stimulus containing some components at higher frequencies (see Fig. 3A).
Figure 3.
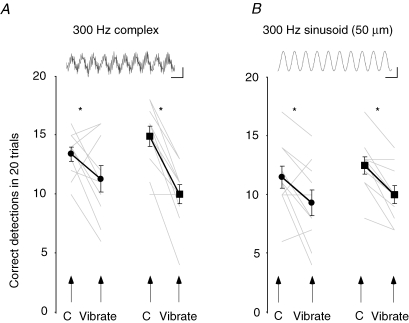
The effect of high-frequency cutaneous vibration on movement detection A, movement detection measured for each of the 10 subjects (grey lines) as the number of correct detections of the direction of movement at the proximal interphalangeal joint out of 20 trials. The black lines and symbols show the group mean ± s.e.m. There was a significant (*) decrease in the incidence of correct detection of movement direction when 300 Hz complex (20 μm) vibration was delivered to the thenar eminence (•) or to the tip of the middle finger (▪) compared with control conditions (C). The inset shows an example of the complex 300 Hz waveform (calibration: 3 ms and 10 μm). B, there was also a significant (*) decrease in the incidence of correct detection of movement direction when 300 Hz sinusoidal (50 μm) vibration was delivered to the thenar eminence (•) or to the tip of the middle finger (▪). The inset shows an example of the sinusoidal 300 Hz waveform (calibration: 3 ms and 25 μm).
The vibrotactile stimulus was delivered during the imposed movement of extension or flexion of the index finger. Vibration began 0.1 s before the movement and finished when the movement reached its full excursion (Fig. 1B). Vibration was delivered via a smooth circular probe (5 mm diameter) which was positioned to stimulate the volar aspect of the middle finger tip at the distal phalanx or the thenar eminence (Fig. 1A). In both locations, the vibrator was perpendicular to the skin surface. Each vibratory stimulus was applied on an initial skin indentation of ∼1 mm.
Standard protocol
Flexion and extension movements of ∼1–3 deg were imposed about the proximal interphalangeal joint of the index finger from the initial mid-position. Each movement was held at its full excursion for 3 s (Fig. 1B). The velocity of the imposed movement was selected as that at which the subject could detect correctly the direction of ∼60% of the control movements. For each subject this threshold velocity was found through preliminary trials and ranged from 0.3 to 1.0 deg s−1. The velocity of the reset movement back to the starting position was constant (1.25 deg s−1). Subjects received the same instructions before each session. The false positive rate (nomination of the wrong direction) was low (< 10%).
A set of test movements consisted of five extension and five flexion movements in random order. This set was repeated 8 times, four with and four without vibration. Half were completed with vibration of the middle finger and the other half with vibration of the thenar eminence (see below). In both control and vibration runs a standard auditory signal was given during the test movements so that subjects were aware when the movement was occurring (Fig. 1B).
Initial study
The first study used a stimulus of 300 Hz complex vibration (20 μm peak-to-peak amplitude). Subjects (n = 10) were asked to nominate orally the direction of movement (flexion or extension) when they were sure or ‘not sure’ when they were unsure of the direction.
Main studies
A group of 10 subjects participated in these studies. Each subject attended four sessions on separate days. During each session a different vibrotactile stimulus was tested. These comprised sinusoidal vibration at 300 Hz (50 μm peak-to-peak amplitude), 30 Hz (50 μm) and 30 Hz at an amplitude matched in perceived intensity to the 300 Hz stimulus. Unless otherwise indicated, vibration was sinusoidal. First, 300 Hz vibration (50 μm) was used to engage Pacinian corpuscles (PCs) and the 30 Hz vibration of the same amplitude was used to preferentially engage RA receptors (Meissner corpuscles) (Talbot et al. 1968; Jänig, 1971). Since, at this amplitude, 30 Hz vibration was subjectively less intense than the 300 Hz vibration, 30 Hz vibration of equal subjective intensity to the 300 Hz (50 μm) vibration was tested. This intensity was found by giving subjects successive 1 s bursts of 30 Hz and 300 Hz vibration 1 s apart. They indicated which stimulation was of greater intensity. The amplitude of the 30 Hz vibration was incrementally increased until the intensity of the two stimuli was equivalent. The amplitude of 30 Hz vibration that produced an equal subjective intensity was determined separately for the tip of the middle finger (190 ± 35 μm) and the thenar eminence (357 ± 33 μm). In the final study, 300 Hz vibration at two amplitudes (20 and 200 μm) was delivered to the middle finger only. As for the other studies, the same four trial sets were completed twice, but this time once with vibration of 200 μm amplitude and once with vibration of 20 μm amplitude.
In the main studies, subjects nominated the direction of movement using a pad with three buttons labelled ‘flexion’, ‘extension’ and ‘not sure’. They were instructed to signal the direction as soon as they were sure and to press ‘not sure’ if unsure of the direction of movement (Fig. 1B). Subjects were able to nominate the direction of movement during the movement itself or during the 3 s hold period after the movement. However, a response was recorded as ‘not sure’ if given after the index finger began to return to the initial position. Starting from the onset of the imposed movement, the time for the subject to choose a response was recorded as the ‘decision time’.
Spread of vibration
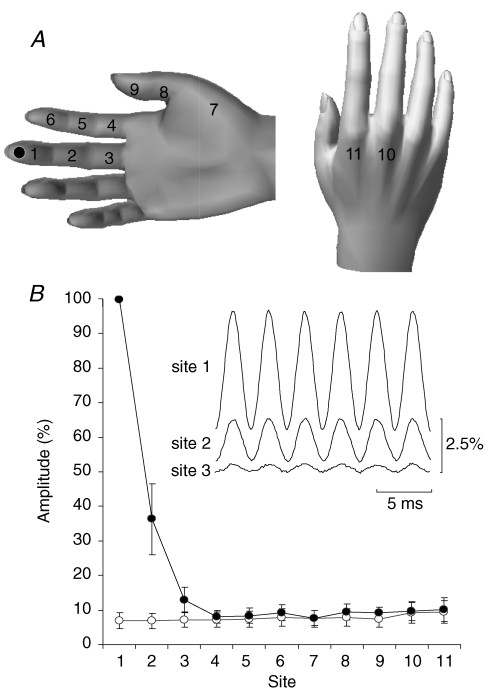
The spread of 300 Hz sinusoidal vibration (50 μm) over the hand was measured in five subjects using an accelerometer. As before, the vibration was delivered to the volar aspect of the middle finger tip at the distal phalanx with an initial skin indentation of ∼1 mm. The accelerometer was firmly attached at 11 different locations within the hand (Fig. 2A).
Figure 2.
Spread of vibration measured using an accelerometer A, spread of 300 Hz sinusoidal (50 μm) vibration was measured at 11 sites on the hand: the volar aspect of the distal (1), middle (2) and proximal (3) phalanx of the middle finger, distal (6), middle (5) and proximal (4) phalanx of the index finger, the thenar eminence (7), the volar surface of the thumb's distal (9) and proximal phalanx (8), and the bony surface of the dorsal head of the middle (10) and index (11) metacarpal. The spot signifies the tip of the middle finger where vibration was applied. B, spread of 300 Hz vibration at all 11 sites measured as a percentage of the vertical calibration (mean ± s.e.m.) of the signal recorded at the site immediately adjacent (1) to the vibrator tip on the middle finger (•) and the corresponding noise level (○). The inset shows the vibration waveform at one subject's distal (1), middle (2) and proximal (3) phalanx of the middle finger. The amplitude calibration is a percentage of movement of the vibrator probe tip.
To test the possible activation of muscle spindle endings during the 300 Hz (50 μm) stimulation, 500 bursts of vibration (3 pulses at 300 Hz, bursts 1 s apart) were applied directly to the distal tendon of the flexor carpi radialis during weak flexion of the wrist in two subjects. The probe tip was positioned about 3 cm proximal to the wrist and it indented the skin overlying the tendon by ∼1 mm. The EMG of the flexor carpi radialis was recorded with surface electrodes (amplified by 10 000; bandpass 16–1000 Hz). This arrangement was designed to maximize the likelihood that a short-latency reflex response was evoked by vibration.
Data analysis
Separate two-way repeated measures ANOVAs were performed for the number of correct detections and decision times, assessing the effects of vibration and stimulus location (middle finger/thenar eminence) for both 300 Hz and both 30 Hz stimuli. The effects of vibration on movement detection and decision time were analysed at two frequencies, 30 Hz and 300 Hz, which were matched for subjective intensity. The effect of different vibration amplitudes (20 μm and 200 μm) were also investigated for the 300 Hz vibrotactile stimulus applied to the middle finger. A paired t test was also performed for the number of incorrect responses (not sure/false positive). For all tests, significance was accepted when P < 0.05. The statistical software SPSS V. 14.0 was used.
Results
Subjects were asked to nominate the direction of passive movement applied at the proximal interphalangeal joint of the index finger when they were sure they could do so. As there was no statistical difference in the ability to detect flexion and extension movements under any condition, the data were pooled. The results for movement detection and decision time are presented separately.
Spread of vibration
The acceleration induced by 300 Hz vibration (50 μm) decreased proximally along the vibrated middle digit, and was undetectable over the neighbouring index digit, the metacarpophalangeal joint and more proximally at the other sites (Fig. 2B). The frequency of the vibration remained clearly discernable and did not degrade at proximal points along the middle digit (Fig. 2B, inset). The 30 Hz stimulus amplitude showed a similar attenuation.
When 300 Hz vibration (50 μm; 3 pulses) was applied directly over the tendon of the flexor carpi radialis during weak contraction of the wrist flexors, there was no short-latency reflex as detected in unrectified or rectified averages of responses to 500 bursts of stimulation.
Movement detection
Vibration at high frequency (300 Hz)
Detection of the direction of passively applied flexion and extension movements at the index finger was poorer when a complex 300 Hz (20 μm) vibration was delivered to the tip of the middle finger or to the thenar eminence (P = 0.001) (Fig. 3A). This impairment in detection of the direction of the applied movements was greater with vibration at the middle finger (24.5 ± 3.6% decrease in detection) compared with the thenar eminence (10.5 ± 5.4% decrease; P = 0.046). In addition, the decrease in detection with middle finger stimulation was a consistent finding, being present in 9 of the 10 subjects.
Because the vibration used in the initial study was not purely sinusoidal and contained some components at frequencies higher than 300 Hz (Fig. 3A, inset), in the main studies we used a special stimulator which produced pure sinusoidal motion of the probe (Fig. 3B, inset; see Methods). This achieves more selective activation of specific classes of cutaneous receptor (e.g. Talbot et al. 1968; Bystrzycka et al. 1977; Ferrington et al. 1977).
When 300 Hz vibration at 50 μm (chosen to activate PC afferents) was applied to the middle finger or the thenar eminence, detection of movements at the index finger was also significantly impaired (P = 0.002; Fig. 3B). The decrease in movement detection was similar when vibration was applied to the middle finger (12.5 ± 2.5%) and thenar eminence (11 ± 4.6%; P = 0.78).
We also determined whether the impairment in movement detection with 300 Hz sinusoidal vibration applied to the nearby skin was greater with higher amplitudes of the stimulus. Vibration of the middle finger (at 300 Hz) at amplitudes of 20 and 200 μm led to considerable impairment in movement detection of the index finger (6.5 ± 5.0 and 20.5 ± 3.0% decrease, respectively), though this impairment was only significant at the 200 μm amplitude (P = 0.002; Fig. 4).
Figure 4.
The effect of the amplitude of 300 Hz sinusoidal cutaneous vibration on movement detection Movement detection at the proximal interphalangeal joint was measured as the number of correct responses (mean ± s.e.m.) out of 20 trials. The group average (black lines) and individual data (grey lines) are shown. There was a significant (*) decrease in the perception of movement when 200 μm amplitude of 300 Hz sinusoidal vibration was delivered to the middle finger. The decrease was significantly bigger with the 200 μm stimulus.
The impairment in movement detection with high-frequency vibration could be due to subjects being ‘not sure’ whether a flexion or extension movement occurred, or because of erroneous (false positive) judgements. For both the 300 Hz complex stimuli and the sinusoidal stimuli the proportion of ‘not sure’ responses increased significantly (P = 0.001 and P = 0.008, respectively), but the proportion of false positive judgements did not (P = 0.6 and P = 0.5, respectively).
Vibration at low frequency (30 Hz)
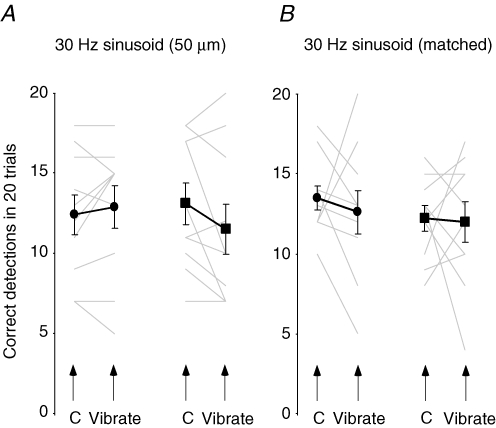
In contrast to high-frequency vibration, 30 Hz (50 μm) chosen to activate Meissner afferents had no effect on movement detection at the index finger when delivered to the middle finger or thenar eminence (P = 0.43; Fig. 5A).
Figure 5.
The effect of low-frequency cutaneous vibration on movement detection A, proprioceptive acuity at the proximal interphalangeal joint measured as the number of correct detections (mean ± s.e.m.) out of 20 trials. The group average (black lines) and individual data (grey lines) are both shown. There was no difference in the perception of movement when 30 Hz (50 μm) vibration was delivered to the thenar eminence (•) or to the tip of the middle finger (▪) compared with control conditions. B, there was also no difference in perception of movement when 30 Hz vibration matched in subjective intensity to 300 Hz (50 μm) vibration was delivered to either site.
As cutaneous vibration at 300 Hz is experienced as more intense than 30 Hz vibration at the same amplitude (e.g. Verrillo et al. 1966; confirmed here), it is possible that the greater effect of the 300 Hz vibrotactile stimulus on the detection of finger movement might be attributable to it being more distracting for the subjects. To assess this, the study was repeated, but the amplitude of the 30 Hz vibration was increased (∼5-fold) so that it was subjectively of equal intensity to the 300 Hz (50 μm) vibration. However, this 30 Hz vibration which was matched in subjective intensity, again had no effect on movement detection when applied either to the middle finger or thenar eminence (P = 0.64; Fig. 5B). Thus, it is unlikely that the reduced detection during the 300 Hz vibration was due simply to altered attention.
Decision times
The time taken to decide the direction of the applied movement (‘decision time’) was also used to assess the difficulty of the proprioceptive task. We reasoned that if vibration to adjacent digits made subjects take longer to nominate with certainty the direction of applied movement, proprioceptive acuity had deteriorated. There were trials in which subjects could detect that movement had occurred, but could not nominate its direction. Depending on the angular velocity at which the subject detected the direction of ∼60% of trial movements, subjects had a window of 9.7–13 s to decide on the direction of movement from its start (6.7–10 s during the movement, plus 3 s when the joint was maintained at full excursion). The decision times were usually ∼6–9 s for the detection of the correct direction of movements. The average decision time for detecting movement was 7.2 ± 0.4 s without vibration and this increased to 8.0 ± 0.4 s during 300 Hz vibration (P = 0.007).
In Fig. 6, changes in the decision times for correct detections are plotted for trials with and without vibration. Decision times were prolonged during vibration of the middle finger (7.2% increase) and the thenar eminence (10.5% increase) when 300 Hz (50 μm) vibration was applied compared with when the stimuli were absent (P = 0.007; Fig. 6). When 300 Hz vibration at amplitudes of 20 and 200 μm was applied to the middle finger, the decision times again increased (by 4.9 and 10.9%, respectively; P = 0.036).
Figure 6.
Decision times for correct detections of the direction of passive movement The decision time was measured from the onset of the movement until the direction of movement was correctly detected (mean ± s.e.m.). Low-frequency 30 Hz (50 μm) vibration had no significant effect on decision time when applied to either the thenar eminence (•) or the tip of the middle finger (▪). However, 30 Hz vibration of equal subjective intensity to 300 Hz (50 μm) vibration led to small but significant (*) increases in decision times at both sites. Stimulation with 300 Hz (50 μm) vibration at the thenar eminence and middle finger led to longer decision times. Similarly, 300 Hz sinusoidal vibration at amplitudes of 20 μm and 200 μm increased decision times. This increase was larger with the larger amplitudes of vibration. The highest 200 μm vibration value (59%) is shown in brackets.
In contrast to the 300 Hz (50 μm) stimulus, decision times were not altered by 30 Hz (50 μm) vibration on either the middle finger or the thenar eminence (P = 0.63; Fig. 6). However, when the 30 Hz vibrotactile stimuli of matched intensity to 300 Hz was used, decision times were increased when stimulation was applied to the middle finger (4.7% increase) and thenar eminence (5.5% increase) (Fig. 6). These increases in detection time were lower than with 300 Hz stimuli, but were of borderline significance (P = 0.049).
When subjects were ‘not sure’ or made false positive judgements, the decision times (∼9–11 s) were longer on average than those for correct responses (∼6–9 s). However, for these incorrect detections there were no significant changes in decision times with any intervention.
Discussion
The detection of the direction of passive movements applied to the index finger was impaired when adjacent digits received vibrotactile stimuli at high frequency (300 Hz). In contrast, low-frequency vibration (30 Hz) did not impair detection even when its amplitude had been increased (more than 5-fold) so that its perceived intensity matched that of the 300 Hz (50 μm) stimulus. Thus, the impairment from the high-frequency stimuli is unlikely to be due to attention being diverted by the stimulation of the adjacent digits, but rather due to the selective effect of the high-frequency stimuli. Distraction is also unlikely as other observations have shown that interference stimuli applied more remotely in the hand or to other parts of the body do not impair performance in a similar task (Refshauge et al. 2003). Also, higher amplitudes of 300 Hz vibrotactile stimuli caused increased disturbances to movement detection and decision times, showing the effect was graded with the input.
The parameters for the applied vibration were selected to activate preferentially particular classes of cutaneous receptor. This was guided by studies in primates (Sato, 1961; Talbot et al. 1968) and human subjects (Mountcastle et al. 1972; LaMotte & Mountcastle, 1975; Bolanowski et al. 1988) which had shown that PC afferents respond to focal stimuli from 10 to 1000 Hz, but they are most sensitive to frequencies of ∼250–550 Hz. An amplitude of 50 μm was selected for the main experiments as this amplitude was considered sufficient to activate a population of PCs. Within the optimal frequency range, ∼10 μm is the lowest vibratory amplitude to engage the most sensitive PC afferents (Talbot et al. 1968). The vibration transmitted through the finger to the proximal phalanx attenuated markedly, and was undetectable over the neighbouring index digit, the metacarpophalangeal joint and more proximally (Fig. 2). There was also no evidence of a change in its dominant frequency. Therefore, it is likely that the 300 Hz vibration activated mainly PCs in the distal and middle phalanges of the vibrated finger. The small amplitudes and high frequency of vibration are unlikely to have produced major activation of other cutaneous receptors.
Correlative neural and psychophysical studies indicate that at vibration frequencies above 20 Hz, the slowly adapting cutaneous afferents are less sensitive than other classes and are unlikely to contribute to vibrotactile sensibility (e.g. Talbot et al. 1968; Bolanowski et al. 1988; Bolanowski et al. 1994). Meissner corpuscles are also unlikely to be entrained by 300 Hz stimulation. They are distinguished from PC afferents by their much smaller receptive fields and by being most sensitive to vibratory stimuli around 40–60 Hz (e.g. Talbot et al. 1968; Jänig, 1971; Ferrington & Rowe, 1980).
It seems quite unlikely that muscle spindle endings account for the vibration-induced interference in proprioceptive capacity as there was also no evidence of a short-latency tendon jerk when the flexor carpi radialis tendon was indented and stimulated directly with bursts of 300 Hz vibration (50 μm). This strongly suggests that muscle spindles endings, which are highly sensitive to vibration (Brown et al. 1967), were not engaged by the stimulation. In addition, there were no signs of a tonic vibration reflex, or any reported illusory movements during the proprioceptive experiment. Also, the amplitude of skin vibration that was used (50 μm) was very much lower than that commonly used to evoke illusions of movement when applied directly over tendons (0.5–2 mm) (e.g. Goodwin et al. 1972; Roll & Vedel, 1982; Collins & Prochazka, 1996).
A 300 Hz vibration with a complex non-sinusoidal waveform was used to establish that the interference phenomenon was present with high-frequency stimulation. Although smaller in peak-to-peak amplitude (20 μm), this non-sinusoidal stimulus was more intense than the sinusoidal 300 Hz vibration, presumably due to the higher frequency components in the stimulus (Fig. 3). It led to greater disturbance in movement detection when applied to the tip of the middle finger compared with stimulation over the thenar eminence. This difference was not obvious for the sinusoidal vibration at 300 Hz. It may reflect greater spread of the highest frequency components along the finger. The mechanical impedance of the fingers is lower than the palm of the hand for high-frequency vibration (Dong et al. 2005), hence the complex stimulus may activate more PCs via the finger tip, leading to a greater disturbance in proprioception.
When high-frequency (300 Hz) vibration was used as an interfering stimulus it took subjects longer to detect correctly the direction of movements. Presumably subjects were unsure of the direction of movement and they took longer to make a final decision (by ∼700 ms). Decision times provided an extra measure for gauging subjective judgements. For example, there was a significant rise in decision time with 30 Hz vibration of matched intensity to 300 Hz vibration (which required the amplitude of vibration to be increased) (Fig. 6), although the overall disturbance was not sufficient to affect the number of correct detections (Fig. 5B). Although PCs are optimally sensitive to cutaneous vibration around 300 Hz, they may be activated at low frequencies, for example at 30 Hz, if high amplitudes are used (Talbot et al. 1968). Thus, the present results do not reveal whether this effect was due to increased numbers of PC afferents activated at the higher amplitude of low-frequency vibration, increased recruitment of other rapidly adapting inputs or whether other critical inputs were activated by the high-amplitude vibration.
The impairment in proprioception was graded with the input. Detection accuracy decreased and detection times increased as the amplitude of 300 Hz sinusoidal vibration increased. One explanation is that when receptor thresholds vary widely, as occurs for PC afferents (Mountcastle et al. 1972; Freeman & Johnson, 1982), an increase in the amplitude of vibration increases the population response as the volume in which the vibratory field exceeds the threshold of the most sensitive afferents increases, and less sensitive afferents are recruited. The discharge rate of recruited afferents also increases (Johnson, 1974).
Our findings extend the findings of Refshauge et al. (2003) who found that increasing cutaneous input with a stroking stimulus to digits adjacent to the test finger impaired movement detection. Additional input from adjacent digits may effectively add ‘noise’ to the neural circuits involved in movement detection (Refshauge et al. 2003). In some circumstances noise can enhance the detection of weak signals in sensory systems, via a mechanism known as stochastic resonance (e.g. Cordo et al. 1996; Collins et al. 2003). For example, the detection of subthreshold tactile stimuli can be enhanced by adding mechanical noise which would activate PCs and other rapidly adapting receptors (Collins et al. 1997; Gravelle et al. 2002). In contrast, adding noise through the activation of PCs led to impaired detection of movements in our studies. This is probably because all the interfering stimuli were suprathreshold, and at these levels the enhancement due to stochastic resonance is abolished (Collins et al. 1997).
Our findings, together with those of Refshauge et al. (2003), make it probable that there are interactions among the various proprioceptive inputs evoked by finger movements. Ferrington et al. (1977) showed that a stimulus of 300 Hz, but not of 30 Hz, at the thenar eminence elevated detection thresholds for detection of vibration of the index finger over a wide range of frequencies (10–450 Hz). These results were interpreted as PC-induced inhibition of input from all classes of vibration-sensitive cutaneous receptors. Our results are consistent with PC involvement not only in cutaneous sensation, but also in movement detection. However, whether PCs inhibit only the cutaneous input to proprioception or also the muscle and joint afferent contribution cannot be deduced from the present study.
The emerging view, supported by a combination of psychophysical and microneurographical data (e.g. Collins et al. 2000, Collins 2005; Edin, 2004), is that cutaneous feedback provides proprioceptive information that is integrated with that from muscle spindles to provide judgement of joint position and movement. Our result implies that there is convergence between skin (PC) and proprioceptive projections along the somatosensory pathway. This could be between skin and muscle afferents or among the cutaneous inputs to proprioception. One possible location where this may occur is the dorsal column nuclei as it contains prominent inputs from vibration-sensitive receptors (e.g. Bystrzycka et al. 1977; Douglas et al. 1978; Ferrington & Rowe, 1982; Connor et al. 1984; see also Hummelsheim et al. 1985). Suppression at the thalamic or cortical level is also possible, although there is no definite data for humans.
In conclusion, sustained enhancement of high-frequency cutaneous input to digits adjacent to the moving digit impaired movement detection. However, this proprioceptive interference was not observed for low-frequency vibration. The impairment in movement detection is graded with the input. Taken together, these findings suggest that stimuli that preferentially activate cutaneous PC afferents may produce significant impairment in proprioceptive acuity.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia. We are most grateful to Professor Mark Rowe for use of the stimulator and for comments on the manuscript.
References
- Bolanowski SJ, Gescheider GA, Verrillo RT. Hairy skin: psychophysical channels and their physiological substrates. Somatosensory Motor Res. 1994;11:279–290. doi: 10.3109/08990229409051395. [DOI] [PubMed] [Google Scholar]
- Bolanowski SJ, Jr, Gescheider GA, Verrillo RT, Checkosky CM. Four channels mediate the mechanical aspects of touch. J Acoust Soc Am. 1988;84:1680–1694. doi: 10.1121/1.397184. [DOI] [PubMed] [Google Scholar]
- Brown MC, Engberg I, Matthews PB. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967;192:773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P, Clark F. Characteristics of knee joint receptors in the cat. J Physiol. 1969;203:317–335. doi: 10.1113/jphysiol.1969.sp008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, Macefield G. Responses to passive movement of receptors in joint, skin and muscle of the human hand. J Physiol. 1988;402:347–361. doi: 10.1113/jphysiol.1988.sp017208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrzycka E, Nail BS, Rowe M. Inhibition of cuneate neurones: its afferent source and influence on dynamically sensitive ‘tactile’ neurones. J Physiol. 1977;268:251–270. doi: 10.1113/jphysiol.1977.sp011856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark FJ, Burgess PR. Slowly adapting receptors in cat knee joint: can they signal joint angle? J Neurophysiol. 1975;38:1448–1463. doi: 10.1152/jn.1975.38.6.1448. [DOI] [PubMed] [Google Scholar]
- Clark FJ, Burgess RC, Chapin JW, Lipscomb WT. Role of intramuscular receptors in the awareness of limb position. J Neurophysiol. 1985;54:1529–1540. doi: 10.1152/jn.1985.54.6.1529. [DOI] [PubMed] [Google Scholar]
- Coleman GT, Zhang H-Q, Rowe MJ. Transmission security for single kinesthetic afferent fibers of joint origin and their target cuneate neurons in the cat. J Neurosci. 2003;23:2980–2992. doi: 10.1523/JNEUROSCI.23-07-02980.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Prochazka A. Movement illusions evoked by ensemble cutaneous input from the dorsum of the human hand. J Physiol. 1996;496:857–871. doi: 10.1113/jphysiol.1996.sp021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Gandevia SC. Sensory integration in the perception of movements at the human metacarpophalangeal joint. J Physiol. 2000;529:505–515. doi: 10.1111/j.1469-7793.2000.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Todd G, Gandevia SC. Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol. 2005;94:1699–1706. doi: 10.1152/jn.00191.2005. [DOI] [PubMed] [Google Scholar]
- Collins JJ, Imhoff TT, Grigg P. Noise-mediated enhancements and decrements in human tactile sensation. Phys Rev E. 1997;56:923–926. [Google Scholar]
- Collins JJ, Priplata AA, Gravelle DC, Niemi J, Harry J, Lipsitz LA. Noise-enhanced human sensorimotor function. IEEE Engineering Med Biol Magazine. 2003;22:76–83. doi: 10.1109/memb.2003.1195700. [DOI] [PubMed] [Google Scholar]
- Connor KM, Ferrington DG, Rowe MJ. Tactile sensory coding during development: signaling capacities of neurons in kitten dorsal column nuclei. J Neurophysiol. 1984;52:86–98. doi: 10.1152/jn.1984.52.1.86. [DOI] [PubMed] [Google Scholar]
- Cordo P, Inglis JT, Verschueren S, Collins JJ, Merfeld DM, Rosenblum S, Buckley S, Moss F. Noise in human muscle spindles. Nature. 1996;383:769–770. doi: 10.1038/383769a0. [DOI] [PubMed] [Google Scholar]
- Dong RG, Wu JZ, McDowell TW, Welcome DE, Schopper AW. Distribution of mechanical impedance at the fingers and the palm of the human hand. J Biomech. 2005;38:1165–1175. doi: 10.1016/j.jbiomech.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Douglas PR, Ferrington DG, Rowe M. Coding of information about tactile stimuli by neurones of the cuneate nucleus. J Physiol. 1978;285:493–513. doi: 10.1113/jphysiol.1978.sp012585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB. Finger joint movement sensitivity of non-cutaneous mechanoreceptor afferents in the human radial nerve. Exp Brain Res. 1990;82:417–422. doi: 10.1007/BF00231261. [DOI] [PubMed] [Google Scholar]
- Edin BB. Quantitative analyses of dynamic strain sensitivity in human skin mechanoreceptors. J Neurophysiol. 2004;92:3233–3243. doi: 10.1152/jn.00628.2004. [DOI] [PubMed] [Google Scholar]
- Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol. 1991;65:657–670. doi: 10.1152/jn.1991.65.3.657. [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. J Physiol. 1995;487:243–251. doi: 10.1113/jphysiol.1995.sp020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, Vallbo ÅB. Dynamic response of human muscle spindle afferents to stretch. J Neurophysiol. 1990;63:1297–1306. doi: 10.1152/jn.1990.63.6.1297. [DOI] [PubMed] [Google Scholar]
- Ferrington DG, Nail BS, Rowe M. Human tactile detection thresholds: modification by inputs from specific tactile receptor classes. J Physiol. 1977;272:415–433. doi: 10.1113/jphysiol.1977.sp012052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington DG, Rowe MJ. Functional capacities of tactile afferent fibres in neonatal kittens. J Physiol. 1980;307:335–353. doi: 10.1113/jphysiol.1980.sp013438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington DG, Rowe MJ. Specificity of connections and tactile coding capacities in cuneate nucleus of the neonatal kitten. J Neurophysiol. 1982;47:622–640. doi: 10.1152/jn.1982.47.4.622. [DOI] [PubMed] [Google Scholar]
- Freeman A, Johnson K. Cutaneous mechanoreceptors in macaque monkey: temporal discharge patterns evoked by vibration, and a receptor model. J Physiol. 1982;323:21–41. doi: 10.1113/jphysiol.1982.sp014059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Handbook of Physiology Exercise: Regulation and Intergration of Multiple Systems. Bethesda, MD: American Physiological Society; 1996. Kinesthesia: roles for afferent signals and motor commands; pp. 128–172. [Google Scholar]
- Gandevia SC, McCloskey DI. Joint sense, muscle sense, and their combination as position sense, measured at the distal interphalangeal joint of the middle finger. J Physiol. 1976;260:387–407. doi: 10.1113/jphysiol.1976.sp011521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. Proprioceptive illusions induced by muscle vibration: contribution by muscle spindles to perception? Science. 1972;175:1382–1384. doi: 10.1126/science.175.4028.1382. [DOI] [PubMed] [Google Scholar]
- Gravelle DC, Laughton CA, Dhruv NT, Katdare KD, Niemi JB, Lipsitz LA, Collins JJ. Noise-enhanced balance control in older adults. Neuroreport. 2002;13:1853–1856. doi: 10.1097/00001756-200210280-00004. [DOI] [PubMed] [Google Scholar]
- Grill SE, Hallett M. Velocity sensitivity of human muscle spindle afferents and slowly adapting type II cutaneous mechanoreceptors. J Physiol. 1995;489:593–602. doi: 10.1113/jphysiol.1995.sp021075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummelsheim H, Wiesendanger R, Wiesendanger M, Bianchetti M. The projection of low-threshold muscle afferents of the forelimb to the main and external cuneate nuclei of the monkey. Neuroscience. 1985;16:979–987. doi: 10.1016/0306-4522(85)90110-1. [DOI] [PubMed] [Google Scholar]
- Jänig W. Morphology of rapidly and slowly adapting mechanoreceptors in the hairless skin of the cat's hind foot. Brain Res. 1971;28:217–231. doi: 10.1016/0006-8993(71)90656-1. [DOI] [PubMed] [Google Scholar]
- Johnson KO. Reconstruction of population response to a vibratory stimulus in quickly adapting mechanoreceptive afferent fiber population innervating glabrous skin of the monkey. J Neurophysiol. 1974;37:48–72. doi: 10.1152/jn.1974.37.1.48. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessel TM. Principles of Neural Science. 4. New York: McGraw-Hill; 2000. [Google Scholar]
- LaMotte RH, Mountcastle VB. Capacities of humans and monkeys to discriminate vibratory stimuli of different frequency and amplitude: a correlation between neural events and psychological measurements. J Neurophysiol. 1975;38:539–559. doi: 10.1152/jn.1975.38.3.539. [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC, Burke D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. J Physiol. 1990;429:113–129. doi: 10.1113/jphysiol.1990.sp018247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre AK, Holman ME, Veale JL. Cortical responses to impulses from single Pacinian corpuscles in the cat's hind limb. Exp Brain Res. 1967;4:243–255. doi: 10.1007/BF00248025. [DOI] [PubMed] [Google Scholar]
- Morley JW, Rowe MJ. Perceived pitch of vibrotactile stimuli: effects of vibration amplitude, and implications for vibration frequency coding. J Physiol. 1990;431:403–416. doi: 10.1113/jphysiol.1990.sp018336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB, LaMotte RH, Carli G. Detection thresholds for stimuli in humans and monkeys: comparison with threshold events in mechanoreceptive afferent nerve fibers innervating the monkey hand. J Neurophysiol. 1972;35:122–136. doi: 10.1152/jn.1972.35.1.122. [DOI] [PubMed] [Google Scholar]
- Ochoa J, Törebjork E. Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervating the human hand. J Physiol. 1983;342:633–654. doi: 10.1113/jphysiol.1983.sp014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provins K. The effect of peripheral nerve block on the appreciation and execution of finger movements. J Physiol. 1958;143:55–67. doi: 10.1113/jphysiol.1958.sp006043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refshauge KM, Collins DF, Gandevia SC. The detection of human finger movement is not facilitated by input from receptors in adjacent digits. J Physiol. 2003;551:371–377. doi: 10.1113/jphysiol.2003.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Vedel JP, Roll JP. Vibration sensitivity of slowly and rapidly adapting cutaneous mechanoreceptors in the human foot and leg. Neurosci Lett. 1989;104:130–135. doi: 10.1016/0304-3940(89)90342-x. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res. 1982;47:177–190. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- Sato M. Response of Pacinian corpuscles to sinusoidal vibration. J Physiol. 1961;159:391–409. doi: 10.1113/jphysiol.1961.sp006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smetacek V, Mechsner F. Making sense. Nature. 2004;432:21. doi: 10.1038/432021a. [DOI] [PubMed] [Google Scholar]
- Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968;31:301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- Vallbo ÅB. Afferent discharge from human muscle spindles in non-contracting muscles. Steady state impulse frequency as a function of joint angle. Acta Physiol Scand. 1974;90:303–318. doi: 10.1111/j.1748-1716.1974.tb05593.x. [DOI] [PubMed] [Google Scholar]
- Vallbo ÅB, Olsson KA, Westberg KG, Clark FJ. Microstimulation of single tactile afferents from the human hand. Sensory attributes related to unit type and properties of receptive fields. Brain. 1984;107:727–749. doi: 10.1093/brain/107.3.727. [DOI] [PubMed] [Google Scholar]
- Varrillo RT. Vibrotactile thresholds for hairy skin. Journal of Experimental Psychology. 1966;72:47–50. doi: 10.1037/h0023321. [DOI] [PubMed] [Google Scholar]